An Update on Planned Oocyte Cryopreservation (POC) in Italy: Medical, Epidemiological and Legal Consideration
Abstract
:1. Introduction
2. Methods
3. Legal Approach to Planned Oocyte Cryopreservation in Italy
4. Assisted Reproductive Technology in Italy
5. POC as a Medical Treatment for Age-Related Fertility Preservation in Italy
5.1. POC and ICSI Efficiency and Safety
- Ovarian stimulation and oocyte retrieval;
- Cryopreservation and storage;
- Thawing, fertilization of oocytes and implantation.
5.1.1. Ovarian Stimulation and Oocyte Retrieval
5.1.2. Cryopreservation and Storage
5.1.3. Thawing, Fertilization of Oocytes and Implantation
5.2. Oocyte and Pregnant Women Age as Determinants of ICSI Performance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Italian National Institute of Statistics (ISTAT). Natalità e Fecondità della Popolazione Residente. 2019. Available online: https://www.istat.it/it/archivio/251937 (accessed on 5 July 2021).
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef] [Green Version]
- Kohler, H.P.; Billari, F.C.; Ortega, J.A. The emergence of lowest-low fertility in Europe during the 1990s. Popul. Dev. Rev. 2002, 28, 641–680. [Google Scholar] [CrossRef]
- Gustafsson, S. Optimal age at motherhood. Theoretical and empirical consideration on postponement of maternity in Europe. J. Popul. Econ. 2001, 14, 225–247. [Google Scholar] [CrossRef]
- Ye, A.; Yan, S.; Huang, K.; Mao, L.; Ge, X.; Weng, T.; Zuo, A.; Tao, X.; Tao, F. Maternal intelligence quotient and motor development in early childhood: The mediating role of mother’s education. J. Paediatr. Child Health 2019, 55, 87–94. [Google Scholar] [CrossRef]
- McDonald, P. Gender Equity in Theories of Fertility Transition. Popul. Dev. Rev. 2000, 26, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Caltabiano, M.; Castiglioni, M.; Rosina, A. Lowest-low fertility: Signs of a recovery in Italy? Demogr. Res. 2009, 21, 681–718. [Google Scholar] [CrossRef] [Green Version]
- Adserà, A. Changing fertility rates in developed countries. The impact of labor market institutions. J. Popul. Econ. 2004, 17, 17–43. [Google Scholar] [CrossRef]
- Del Boca, D. The Impact of Child Care Costs and Availability on Mothers’ Labor Supply; ImPRovE Working Paper 15/04; Herman Deleeck Centre for Social Policy, University of Antwerp: Antwerpen, Belgium, 2015. [Google Scholar]
- Blundell, R.; Duncan, A.; Mccrae, J.; Meghir, C. The Labour Market Impact of the Working Families’ Tax Credit. Fisc. Stud. 2000, 21, 75–103. [Google Scholar] [CrossRef]
- Del Boca, D. The Effect of Child Care and Part Time Opportunities on Participation and Fertility of Italian Women. J. Popul. Econ. 2002, 15, 549–573. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.; Lutz, W.; Testa, M.R. The emergence of sub-replacement family size ideals in Europe. Popul. Res. Policy Rev. 2003, 22, 479–496. [Google Scholar] [CrossRef]
- Testa, M.R. Childbearing preferences and family issues in Europe: Evidence from the Eurobarometer 2006 survey. Vienna Yearb. Popul. Res. 2007, 5, 357–379. [Google Scholar] [CrossRef] [Green Version]
- Nasab, S.; Ulin, L.; Nkele, C.; Shah, J.; Abdallah, M.E.; Sibai, B.M. Elective egg freezing: What is the vision of women around the globe? Future Sci. OA 2020, 6, FSO468. [Google Scholar] [CrossRef] [Green Version]
- Tozzo, P.; Fassina, A.; Nespeca, P.; Spigarolo, G.; Caenazzo, L. Understanding social oocyte freezing in Italy: A scoping survey on university female students’ awareness and attitudes. Life Sci. Soc. Policy 2019, 15, 3. [Google Scholar] [CrossRef]
- The Italian Assisted Reproductive Technology Register (IARTR). Executive Summary for 2018. Available online: https://www.iss.it/documents/20126/0/EXECUTIVE+SUMMARY+of+ART+in+ITALY+-+Activity+2018_.pdf/06d0b225-ac13-9be0-c20c-c94dfb6e3547?t=1617357530502 (accessed on 28 November 2021).
- Italian Parliament. Rules on Medically Assisted Procreation. Law no. 40/2004. Available online: https://www.ieb-eib.org/ancien-site/pdf/loi-pma-italie-english.pdf (accessed on 5 July 2021).
- Attività del Consiglio di Stato e del CGARS. 2019. Available online: https://www.sipotra.it/wp-content/uploads/2020/02/ATTIVIT%C3%80-DEL-CONSIGLIO-DI-STATO-E-DEL-CGARS-2019.pdf (accessed on 28 November 2021).
- Consiglio di Stato, Sentenza N. 7343/2020, È Illegittima la Delibera che Prevede una Disciplina Differente per la Procreazione Medicalmente Assistita (PMA) Eterologa, a Carico del Servizio Sanitario Regionale, Rispetto a Quella Omologa. Available online: https://www.federalismi.it/nv14/articolo-documento.cfm?Artid=44757 (accessed on 28 November 2021).
- Italian Constitutional Court. Decision no. 96/2015. Available online: https://www.cortecostituzionale.it/actionSchedaPronuncia.do?anno=2015&numero=96 (accessed on 28 November 2021).
- Italian Ministry of Health. Linee Guida Contenenti le Indicazioni delle Procedure e delle Tecniche di Procreazione Medicalmente Assistita. Decree 01/07/2015, no. 161. Available online: https://www.salute.gov.it/imgs/C_17_notizie_2148_listaFile_itemName_0_file.pdf (accessed on 5 July 2021).
- ESHRE Working Group on Oocyte Cryopreservation in Europe; Shenfield, F.; de Mouzon, J.; Scaravelli, G.; Kupka, M.; Ferraretti, A.P.; Prados, F.J.; Goossens, V. Oocyte and ovarian tissue cryopreservation in European countries: Statutory background, practice, storage and use. Hum. Reprod. Open 2017, 2017, hox003. [Google Scholar] [CrossRef] [Green Version]
- Calhaz-Jorge, C.; De Geyter, C.H.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. Survey on ART and IUI: Legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. Open 2020, 2020, hoz044. [Google Scholar] [CrossRef]
- Italian Constitutional Court. Decision no 162/2014, from 10/06/2014. Available online: https://www.cortecostituzionale.it/actionSchedaPronuncia.do?anno=2014&numero=162 (accessed on 28 November 2021).
- Case of S.H. and Others v. Austria. Application no. 57813/00, 2011. Available online: https://hudoc.echr.coe.int/eng#{%22dmdocnumber%22:[%22865856%22],%22itemid%22:[%22001-98048%22]} (accessed on 28 November 2021).
- Case of Evans v. The United Kingdom, Application no. 6339/05, 2002. Available online: https://hudoc.echr.coe.int/eng#{%22itemid%22:[%22001-80046%22]} (accessed on 28 November 2021).
- Italian Constitutional Court. Decision no. 167/1999, from 10/05/1999. Available online: https://www.cortecostituzionale.it/actionSchedaPronuncia.do?anno=1999&numero=167#:~:text=disciplina%20l’ipotesi%20di%20costituzione,del%20fondo%20e%20non%20ampliabile (accessed on 28 November 2021).
- Italian Constitutional Court. Decision no. 251/2008, from 04/07/2008. Available online: https://www.cortecostituzionale.it/actionSchedaPronuncia.do?anno=2008&numero=251 (accessed on 28 November 2021).
- Italian Constitutional Court. Decision no. 113/2004, from 06/04/2004. Available online: https://www.cortecostituzionale.it/actionSchedaPronuncia.do?param_ecli=ECLI:IT:COST:2004:113 (accessed on 28 November 2021).
- Riezzo, I.; Neri, M.; Bello, S.; Pomara, C.; Turillazzi, E. Italian law on medically assisted reproduction: Do women’s autonomy and health matter? BMC Womens Health 2016, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Cippitani, R. Ethical issues and law-making power: How European case law has rewritten Italian law on medically assisted reproduction. Monash Bioeth. Rev. 2019, 37, 46–67. [Google Scholar] [CrossRef]
- Italian Constitutional Court. Decision no. 282/2002. Available online: https://www.forumcostituzionale.it/wordpress/images/stories/pdf/old_pdf/997.pdf (accessed on 28 November 2021).
- Martinelli, L.; Busatta, L.; Galvagni, L.; Piciocchi, C. Social egg freezing: A reproductive chance or smoke and mirrors? Croat. Med. J. 2015, 4, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Levi Setti, P.E.; Borini, A.; Patrizio, P.; Bolli, S.; Vigiliano, V.; De Luca, R.; Scaravelli, G. ART results with frozen oocytes: Data from the Italian ART registry (2005–2013). J. Assist. Reprod. Genet. 2016, 33, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Tozzo, P.; Caenazzo, L. The Skeleton in the Closet: Faults and Strengths of Public Versus Private Genetic Biobanks. Biomolecules 2020, 10, 1273. [Google Scholar] [CrossRef]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borovecki, A.; Tozzo, P.; Cerri, N.; Caenazzo, L. Social egg freezing under public health perspective: Just a medical reality or a womn’s right? An ethical case analysis. J. Public Health Res. 2018, 7, 1484. [Google Scholar] [CrossRef] [PubMed]
- Tozzo, P. Oocyte Biobanks: Old Assumptions and New Challenges. Biotech 2021, 10, 2–4. [Google Scholar] [CrossRef]
- Benagiano, G.; Filippi, V.; Sgargi, S.; Gianaroli, L. Italian Constitutional Court removes the prohibition on gamete donation in Italy. Reprod. Biomed. Online 2014, 29, 662–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripodina, C. Il “diritto” a procreare artificialmente in Italia: Una storia emblematica tra legislatore, giudici e corti. Biolaw J. 2014, 2, 68. [Google Scholar]
- Wilding, M.; Coppola, G.; De Icco, F.; Arenare, L.; Di Matteo, L.; Dale, B. Maternal non-Mendelian inheritance of a reduced lifespan? A hypothesis. J. Assist. Reprod. Genet. 2014, 31, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Faddy, M.J.; Gosden, R.G.; Gougeon, A.; Richardson, S.J.; Nelson, J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992, 7, 1342–1346. [Google Scholar] [CrossRef]
- Fish, E.; Shahrokh, D.; Bagot, R.; Caldji, C.; Bredy, T.; Szyf, M.; Meaney, M.J. Epigenetic programming of stress responses through variations in maternal care. Ann. N. Y. Acad. Sci. 2004, 1036, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Wilding, M.; Dale, B.; Marino, M.; Di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef]
- Wilding, M.; Di Matteo, L.; Dale, B. The maternal age effect: A hypothesis based on oxidative phosphorylation. Zygote 2005, 13, 317–323. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Jacobs, P. Trisomy in man. Annu. Rev. Genet. 1984, 18, 69–97. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Saso, S.; Mania, A.; Smith, J.R.; Serhal, P.; Ben-Nagi, J. The dawn of a new ice age: Social egg freezing, Nordic Federation of Societies of Obstetrics and Gynecology. Acta Obstet. Gynecol. Scand. 2018, 97, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.K.; Mutton, D.E.; Alberman, E. Revised estimates of the maternal age specific live birth prevalence of Down’s syndrome. J. Med. Screen. 2002, 9, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Johnston, M.; Richings, N.M.; Leung, A.; Sakkas, D.; Catt, S. A major increase in oocyte cryopreservation cycles in the USA, Australia and New Zealand since 2010 is highlighted by younger women but a need for standardized data collection. Hum. Reprod. 2021, 36, 624–635. [Google Scholar] [CrossRef]
- Tran, M. Apple and Facebook Offer to Freeze Eggs for Female Employees. The Guardian. 15 October 2014. Available online: https://www.theguardian.com/technology/2014/oct/15/apple-facebook-offer-freeze-eggs-female-employees (accessed on 28 November 2021).
- Goold, I.; Savulescu, J. In favour of freezing eggs for non-medical reasons. Bioethics 2009, 23, 47–58. [Google Scholar] [CrossRef]
- Bayliss, F. Left out in the cold: Arguments against non-medical oocyte cryopreservation. J. Obstet. Gynaecol. Can. 2015, 37, 64–67. [Google Scholar] [CrossRef]
- Gonzalez, C.; Boada, M.; Devesa, M.; Veiga, A. Concise Review: Fertility Preservation: An Update. Stem Cells Transl. Med. 2012, 1, 668–672. [Google Scholar] [CrossRef]
- Kuwayama, M.; Vajita, G.; Kato, O.; Leibo, S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Katayama, K.P.; Stehlik, J.; Kuwayama, M.; Kato, O.; Stehlik, E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil. Steril. 2003, 80, 223–224. [Google Scholar] [CrossRef]
- Antinori, M.; Licata, E.; Dani, G.; Cerusico, F.; Versaci, C.; Antinori, S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod. Biomed. Online 2007, 14, 72–79. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Q.; Li, L.; Cong, L.; Zhang, Z.; Wei, Z.; Zhou, P. Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil. Steril. 2009, 92, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Fadini, R.; Brambillasca, F.; Mignini Renzini, M.; Merola, M.; Comi, R.; De Ponti, E.; Dal Canto, M.B. Human oocyte cryopreservation: Comparison between slow and ultrarapid methods. Reprod. Biomed. Online 2009, 19, 171–180. [Google Scholar] [CrossRef]
- Smith, G.D.; Serafini, P.C.; Fioravanti, J.; Yadid, I.; Coslovsky, M.; Hassun, P.; Alegretti, J.R.; Motta, E.L. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil. Steril. 2010, 94, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Kazem, R.; Thompson, L.A.; Srikantharajah, A.; Laing, M.A.; Hamilton, M.P.R.; Templeton, A. Cryopreservation of human oocytes and fertilization by two techniques: In vitro fertilization and intracytoplasmic sperm injection. Hum. Reprod. 1995, 10, 2650–2654. [Google Scholar] [CrossRef]
- American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil. Steril. 2008, 90, S241–S246. [Google Scholar] [CrossRef]
- Crawford, S.; Boulet, S.L.; Kawwass, J.F.; Jamieson, D.J.; Kissin, D.M. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, 2013. Fertil. Steril. 2017, 107, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Cobo, A.; Kuwayama, M.; Perez, S.; Ruiz, A.; Pellicer, A.; Remohí, J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil. Steril. 2008, 89, 1657–1664. [Google Scholar] [CrossRef]
- European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Wyns, C.; Bergh, C.; Calhaz-Jorge, C.; De Geyter, C.; Kupka, M.S.; Motrenko, T.; Rugescu, I.; Smeenk, J.; Tandler-Schneider, A.; et al. ART in Europe, 2016: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoaa032. [Google Scholar]
- Levi Setti, P.E.; Albani, E.; Morenghi, E.; Morreale, G.; Delle Piane, L.; Scaravelli, G. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil. Steril. 2013, 100, 396–401. [Google Scholar] [CrossRef]
- Chian, R.; Huang, J.Y.I.; Tan, S.L.; Lucena, E.; Saa, A.; Rojas, A.; Castello, N.L.; Amador, M.I.G.; Sarmiento, J.E.M. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod. Biomed. Online 2008, 16, 608–610. [Google Scholar] [CrossRef]
- Noyes, N.; Porcu, E.; Borini, A. Over 900 cryopreservation babies born with no apparent increase in congenital anomalies. Reprod. Biomed. Online 2009, 18, 769–776. [Google Scholar] [CrossRef]
- Cobo, A.; Serra, V.; Garrido, N.; Olmo, I.; Pellicer, A.; Remohi, J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil. Steril. 2014, 102, 1006–1015. [Google Scholar] [CrossRef]
- Cobo, A.; Remohí, J.; Chang, C.; Nagy, Z.P. Oocyte cryopreservation for donor egg banking. Reprod. Biomed. Online 2011, 23, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobo, A.; Meseguer, M.; Remohí, J.; Pellicer, A. Use of cryo-banked oocytes in an ovum donation programme: A prospective, randomized, controlled, clinical trial. Hum. Reprod. 2010, 25, 2239–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gefenas, E.; Dranseika, V.; Serepkaite, J.; Cekanauskaite, A.; Caenazzo, L.; Gordijn, B.; Pegoraro, R.; Yuko, E. Turning residual human biological materials into research collections: Playing with consent. J. Med. Ethics 2012, 38, 351–355. [Google Scholar] [CrossRef]
- De Melo-Martin, I.; Cholst, I.N. Researching human oocyte cryopreservation: Ethical issues. Fertil. Steril. 2008, 89, 523–528. [Google Scholar] [CrossRef]
- Mertes, H.; Pennings, G.; Dondorp, W.; De Wert, G. Implications of oocyte cryostorage for the practice of oocyte donation. Hum. Reprod. 2012, 27, 2886–2893. [Google Scholar] [CrossRef] [Green Version]
- Homburg, R.; Van Der Veen, F.; Silber, S.J. Oocyte vitrification—Women’s emancipation set in stone. Fertil. Steril. 2009, 91, 1319–1320. [Google Scholar] [CrossRef]
- Sauer, M.V.; Kavic, S.M. Oocyte and embryo donation. 2006: Reviewing two decades of innovation and controversy. Reprod. Biomed. Online 2006, 12, 153–162. [Google Scholar] [CrossRef]
- Sauer, M.; Paulson, R.; Lobo, R. Reversing the Natural Decline in Human Fertility an Extended Clinical Trial of Oocyte Donation to Women of Advanced Reproductive Age. J. Am. Med. Assoc. 1992, 268, 1275–1279. [Google Scholar] [CrossRef]
- Ziadeh, S.; Yahaya, A. Pregnancy outcome at age 40 and older. Arch. Gynecol. Obstet. 2001, 26, 30–33. [Google Scholar] [CrossRef]
- Jacobsson, B.; Ladfors, L.; Milsom, I. Advanced Maternal Age and Adverse Perinatal Outcome. Obstet. Gynecol. 2004, 104, 727–733. [Google Scholar] [CrossRef]
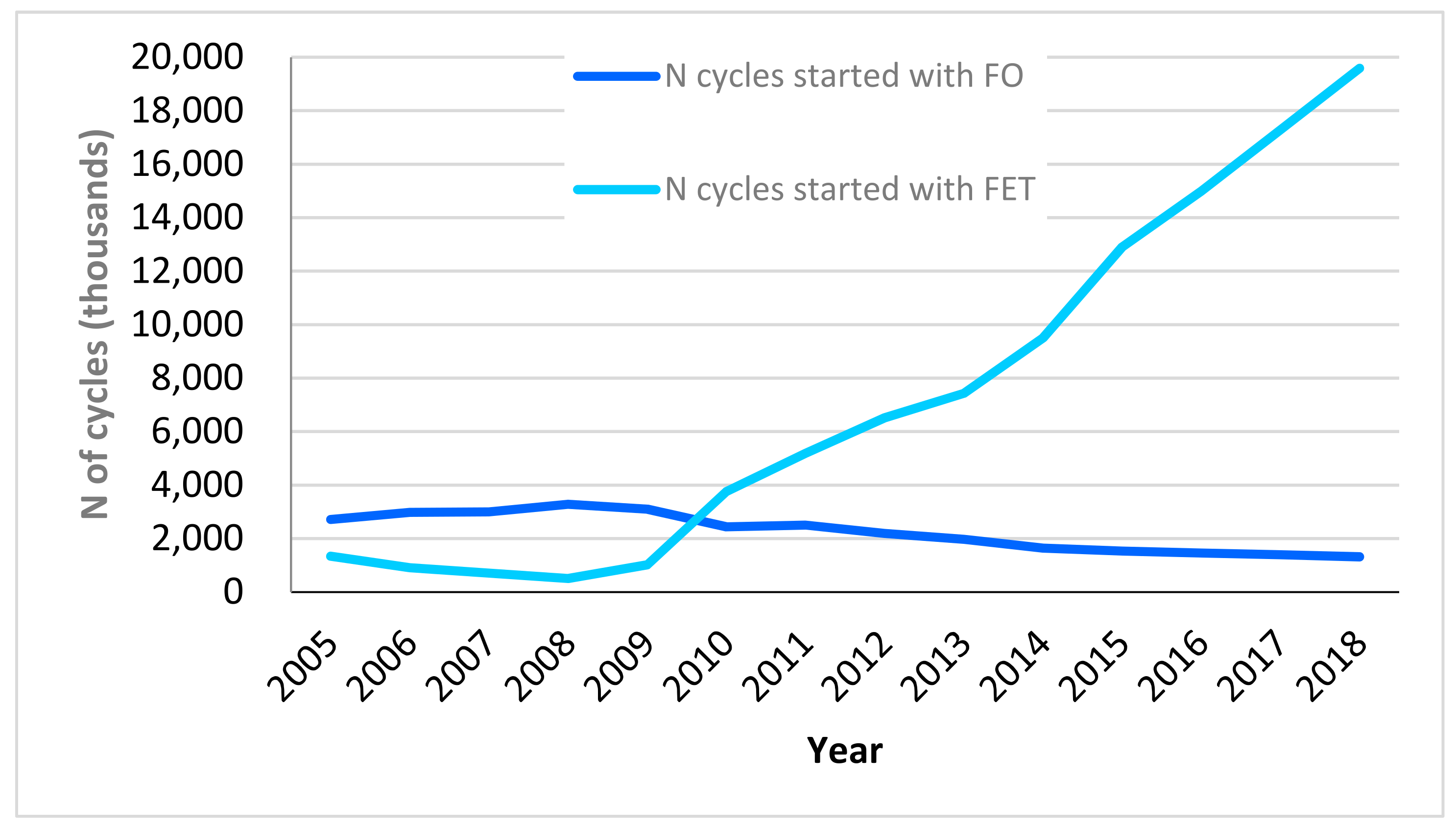
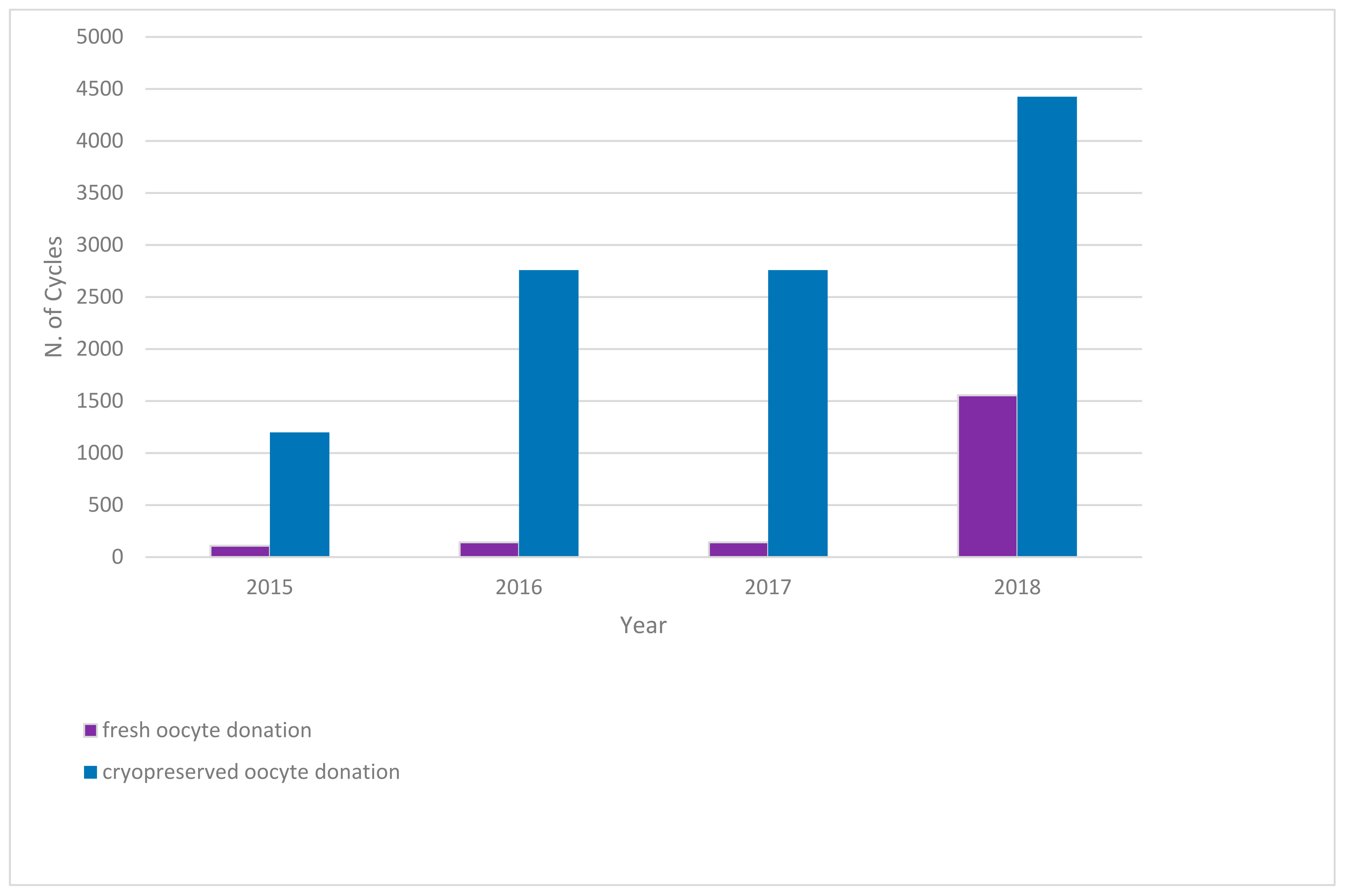
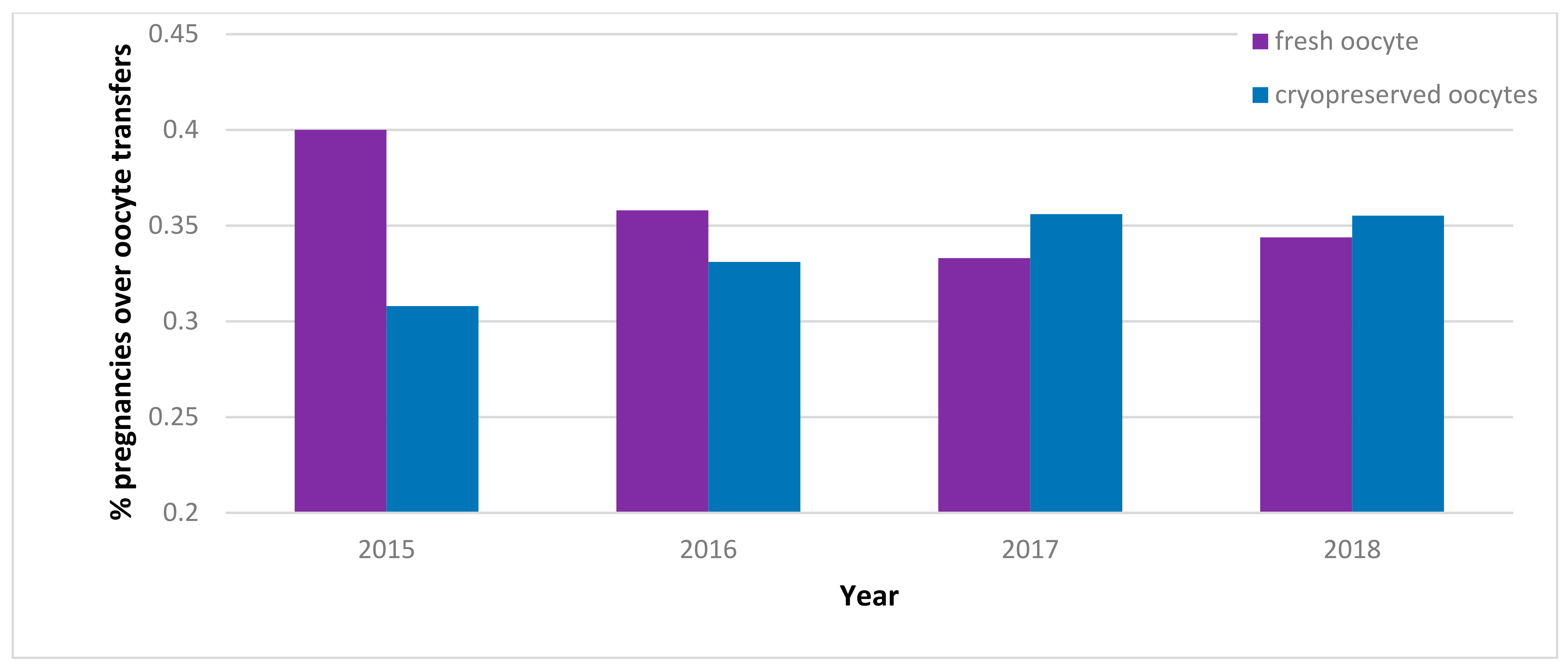
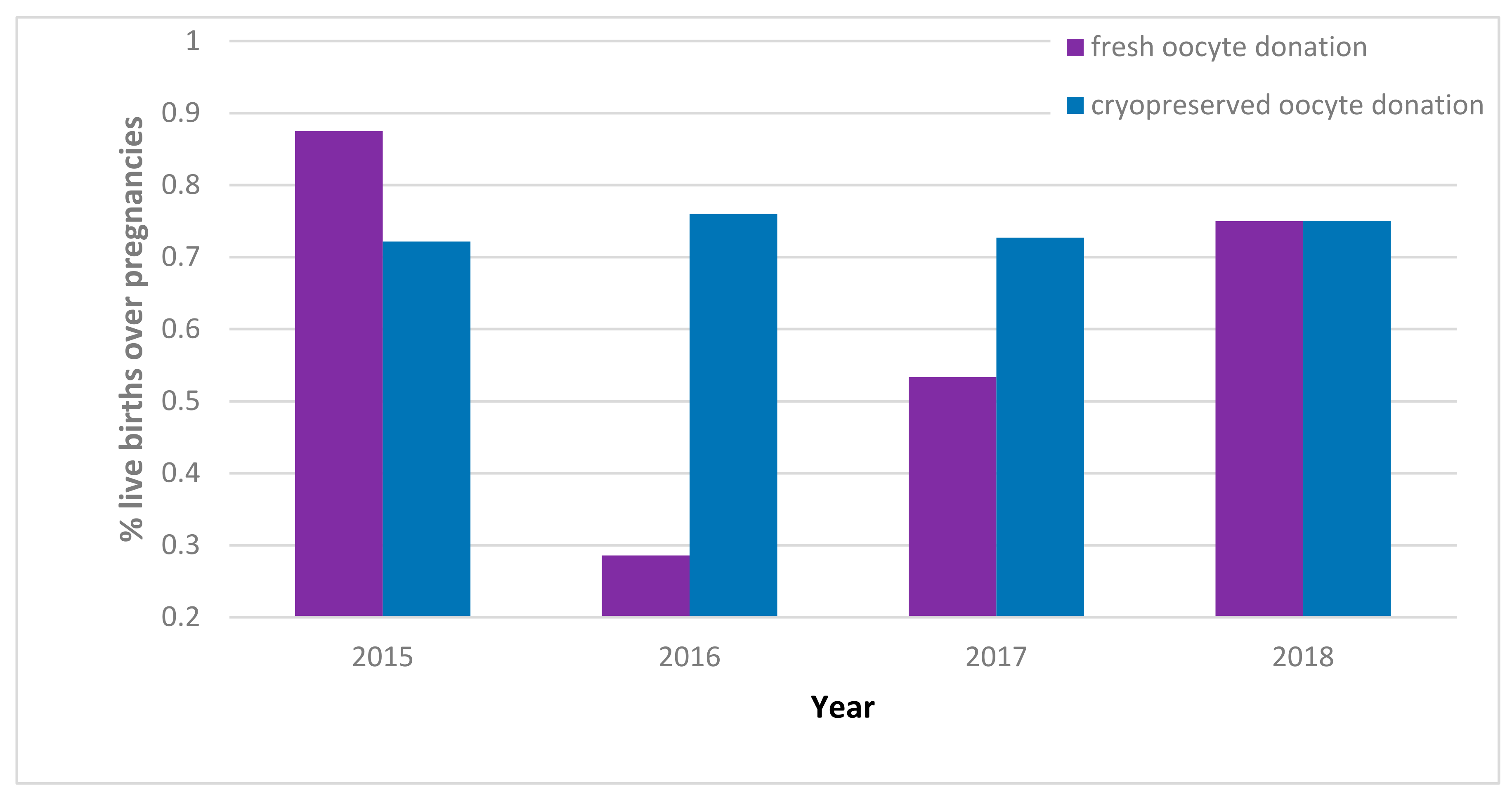
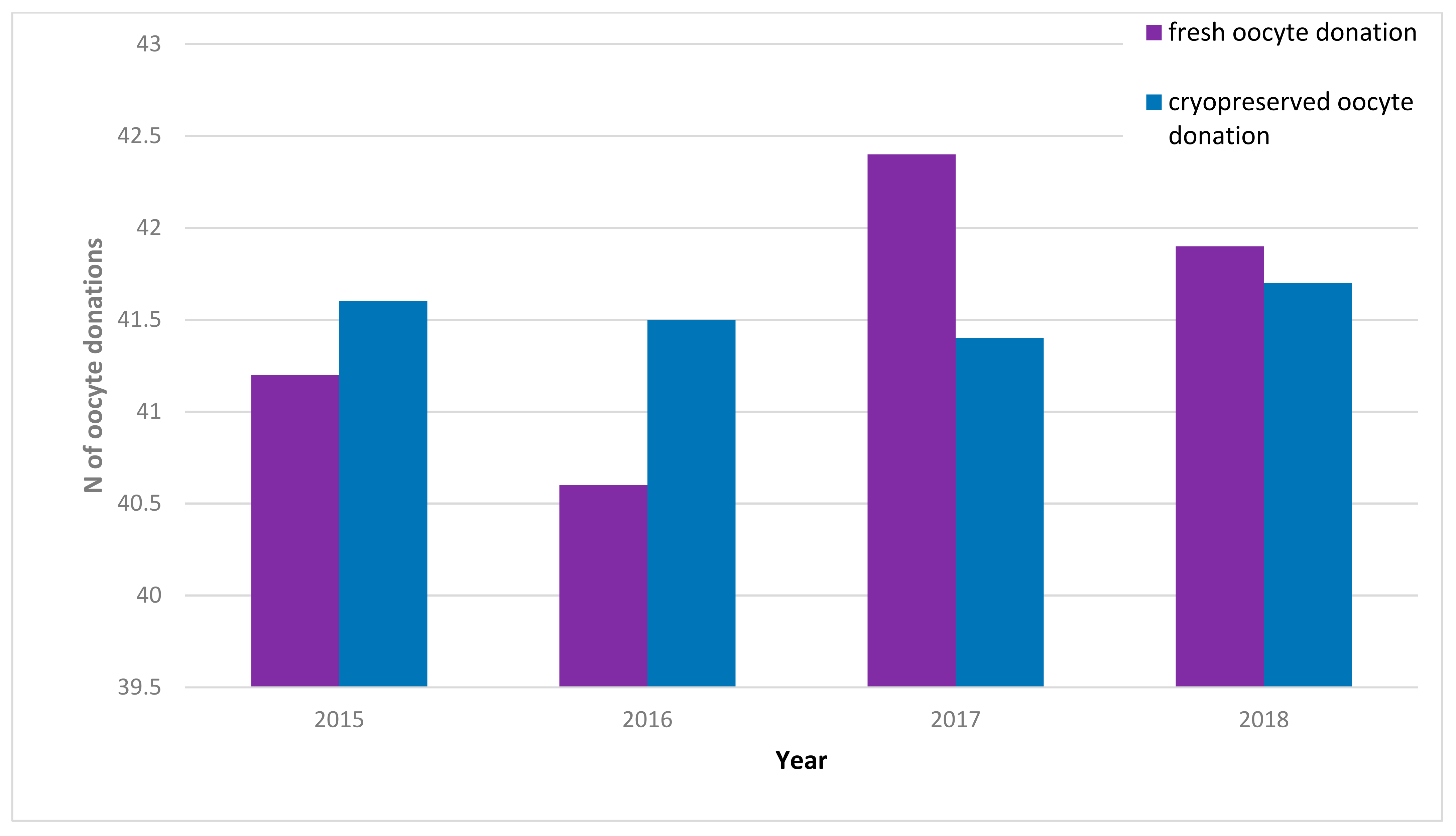
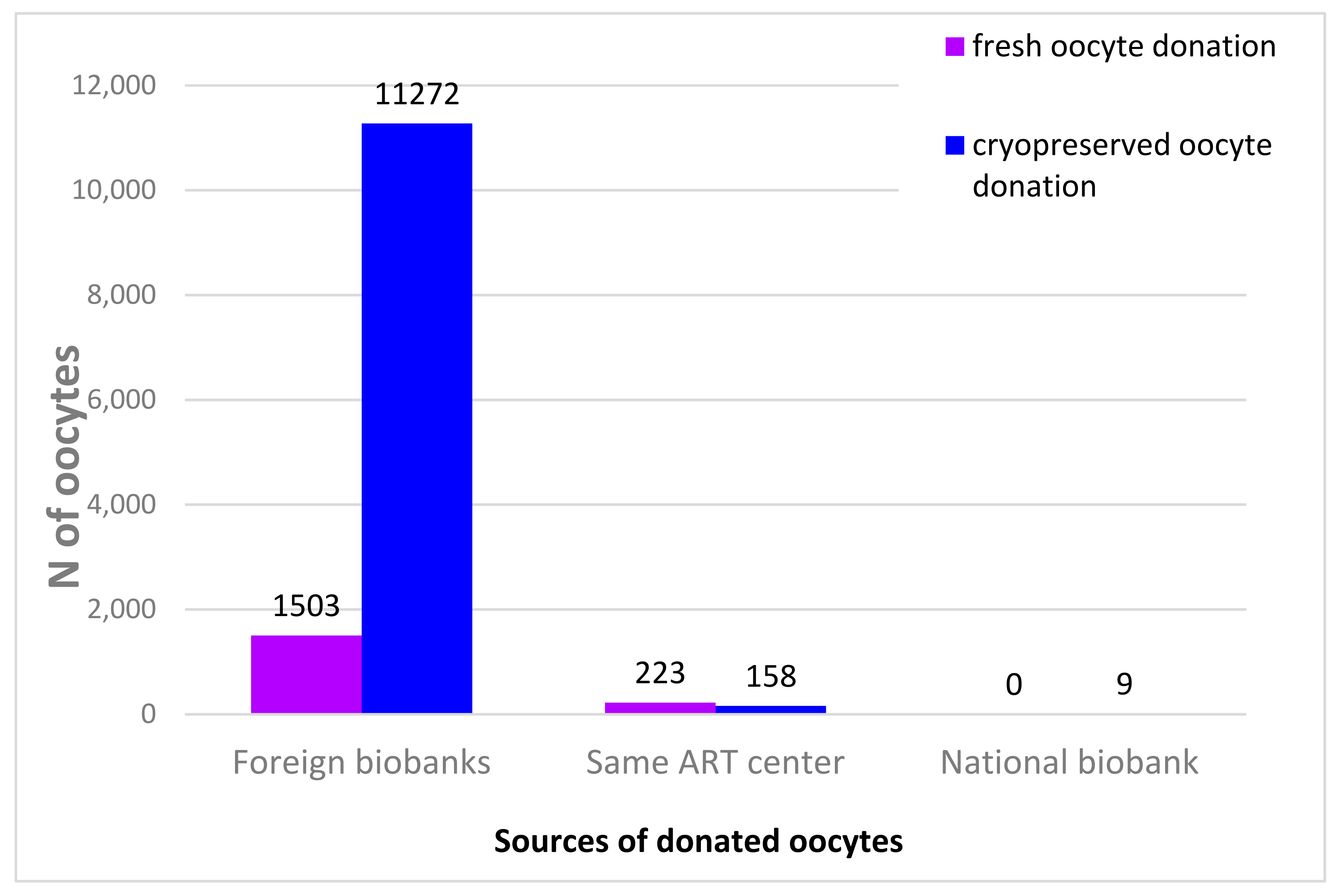
| 2015 | 2016 | 2017 | 2018 | |||||
|---|---|---|---|---|---|---|---|---|
| FOD | COD | FOD | COD | FOD | COD | FOD | COD | |
| No. of pregnancies | 40 | 341 | 49 | 833 | 15 | 1018 | 524 | 1430 |
| No. of live births | 35 | 246 | 14 | 633 | 8 | 740 | 393 | 1073 |
| No. of stillborn | 0 | 3 | 0 | 5 | 0 | 2 | 2 | 9 |
| No. of neonatal deaths | n.a. | n.a. | n.a. | n.a. | 0 | 6 | 4 | 4 |
| No. of malformed births | 0 | 3 | 0 | 5 | 0 | 4 | 3 | 6 |
| 2014 | 2015 | 2016 | 2017 | 2018 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % PFT | % POT | % PFT | % POT | % PFT | % POT | % PFT | % POT | % PFT | % POT | |
| ≤34 years old | 19.3 | n.a. | 19.9 | n.a. | 17.5 | 21.8 | 19.5 | 23.1 | 21.9 | 26.8 |
| 35–39 years old | 16.4 | n.a. | 14.4 | n.a. | 16.5 | 19.9 | 17.6 | 21.7 | 16.3 | 20.1 |
| 40–42 years old | 12.6 | n.a. | 13.6 | n.a. | 14.1 | 18 | 12.3 | 15.3 | 11.3 | 15.8 |
| ≥43 years old | 7.5 | n.a. | 16.3 | n.a. | 12.2 | 13.6 | 5.6 | 7 | 5.1 | 7.9 |
| Total | 16.7 | n.a. | 16.6 | n.a. | 16.3 | 20.1 | 16.9 | 20.5 | 16.9 | 21.5 |
| 2015 | 2016 | 2017 | 2018 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % POSC | % POW | % POT | % POSC | % POW | % POT | % POSC | % POW | % POT | % POSC | % POW | % POT | |
| ≤34 years old | 25.5 | 27.3 | n.a. | 23.6 | 25.3 | 34.7 | 23.5 | 25.2 | 36.1 | 21.7 | 23.1 | 36.6 |
| 35–39 years old | 19.6 | 21.2 | n.a. | 19.8 | 21.5 | 28.7 | 19.4 | 21.1 | 29.8 | 17.9 | 19.4 | 29.6 |
| 40–42 years old | 11.1 | 12.5 | n.a. | 10.6 | 12.1 | 16.6 | 11.4 | 12.8 | 18.4 | 10.3 | 11.7 | 18 |
| ≥43 years old | 6.2 | 7.3 | n.a. | 4.4 | 5.3 | 8 | 5.6 | 6.8 | 10.8 | 4.9 | 5.9 | 9.8 |
| Total | 17.8 | 19.6 | n.a. | 16.9 | 18.7 | 25.6 | 17.2 | 19 | 27.4 | 15.8 | 17.4 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremonese, J.; Marcon, M.; Oppi, L.; Paletti, G.; Romolo, V.; Tozzo, P.; Caenazzo, L. An Update on Planned Oocyte Cryopreservation (POC) in Italy: Medical, Epidemiological and Legal Consideration. Int. J. Environ. Res. Public Health 2022, 19, 2371. https://doi.org/10.3390/ijerph19042371
Cremonese J, Marcon M, Oppi L, Paletti G, Romolo V, Tozzo P, Caenazzo L. An Update on Planned Oocyte Cryopreservation (POC) in Italy: Medical, Epidemiological and Legal Consideration. International Journal of Environmental Research and Public Health. 2022; 19(4):2371. https://doi.org/10.3390/ijerph19042371
Chicago/Turabian StyleCremonese, Jessica, Marianna Marcon, Laura Oppi, Giulia Paletti, Vincenzo Romolo, Pamela Tozzo, and Luciana Caenazzo. 2022. "An Update on Planned Oocyte Cryopreservation (POC) in Italy: Medical, Epidemiological and Legal Consideration" International Journal of Environmental Research and Public Health 19, no. 4: 2371. https://doi.org/10.3390/ijerph19042371
APA StyleCremonese, J., Marcon, M., Oppi, L., Paletti, G., Romolo, V., Tozzo, P., & Caenazzo, L. (2022). An Update on Planned Oocyte Cryopreservation (POC) in Italy: Medical, Epidemiological and Legal Consideration. International Journal of Environmental Research and Public Health, 19(4), 2371. https://doi.org/10.3390/ijerph19042371







