Response of Macrophyte Diversity in Coastal Lakes to Watershed Land Use and Salinity Gradient
Abstract
1. Introduction
2. Materials and Methods
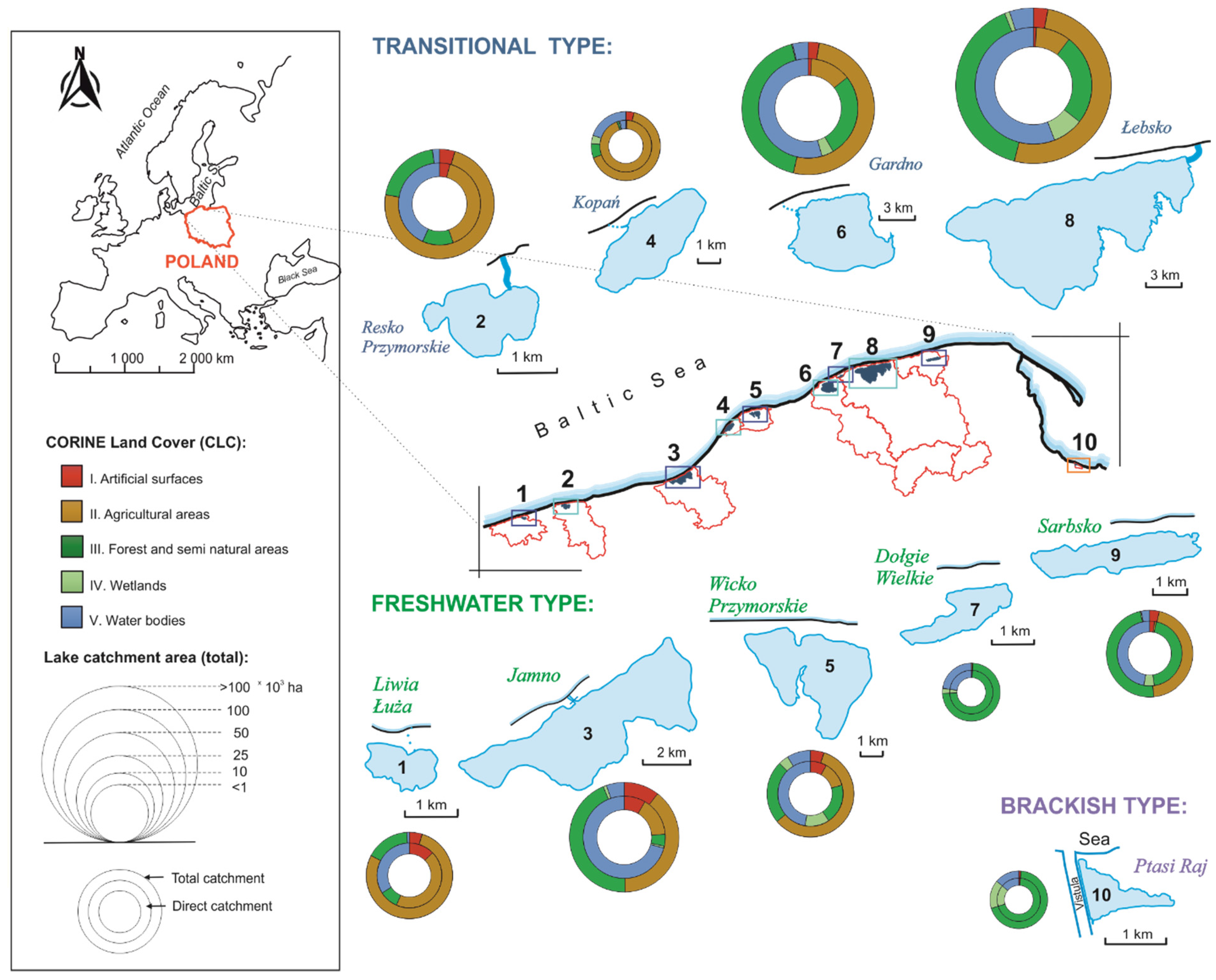
2.1. Study Sites
2.2. Phytocenotic Studies and Evaluation
2.3. Water-Quality Parameters
2.4. Statistical Analyses
3. Results
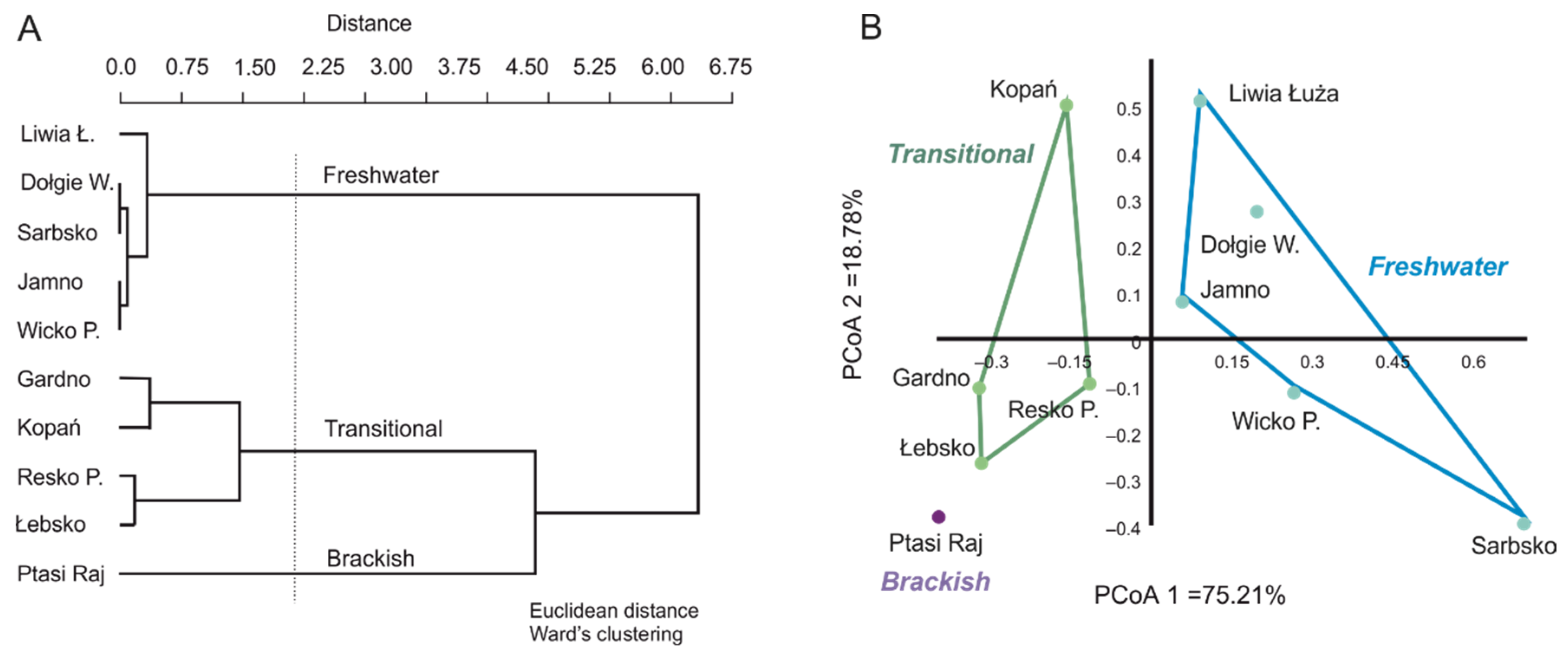
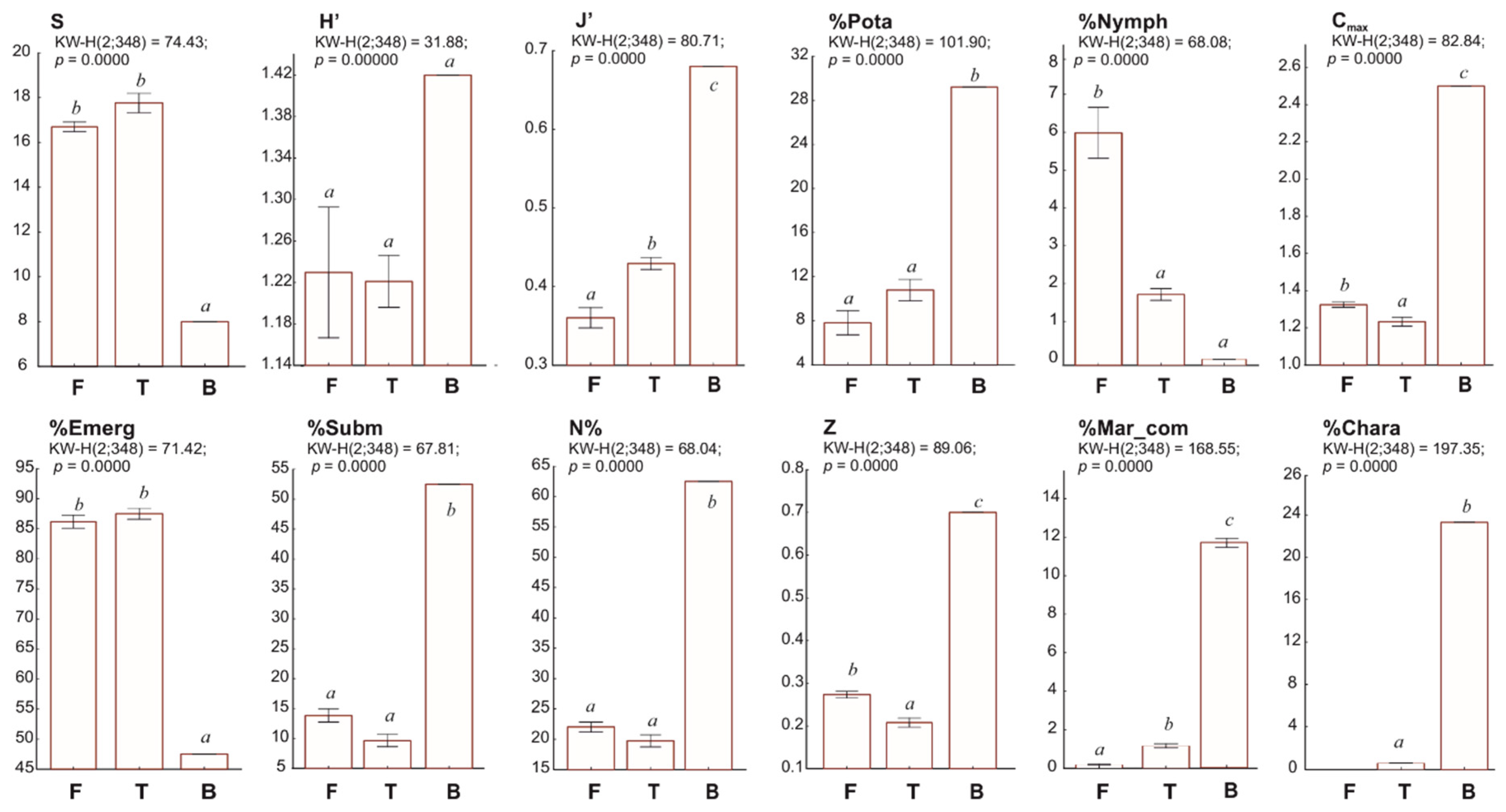
3.1. Spatial and Qualitative Variation of Macrophyte Communities in Coastal Lakes
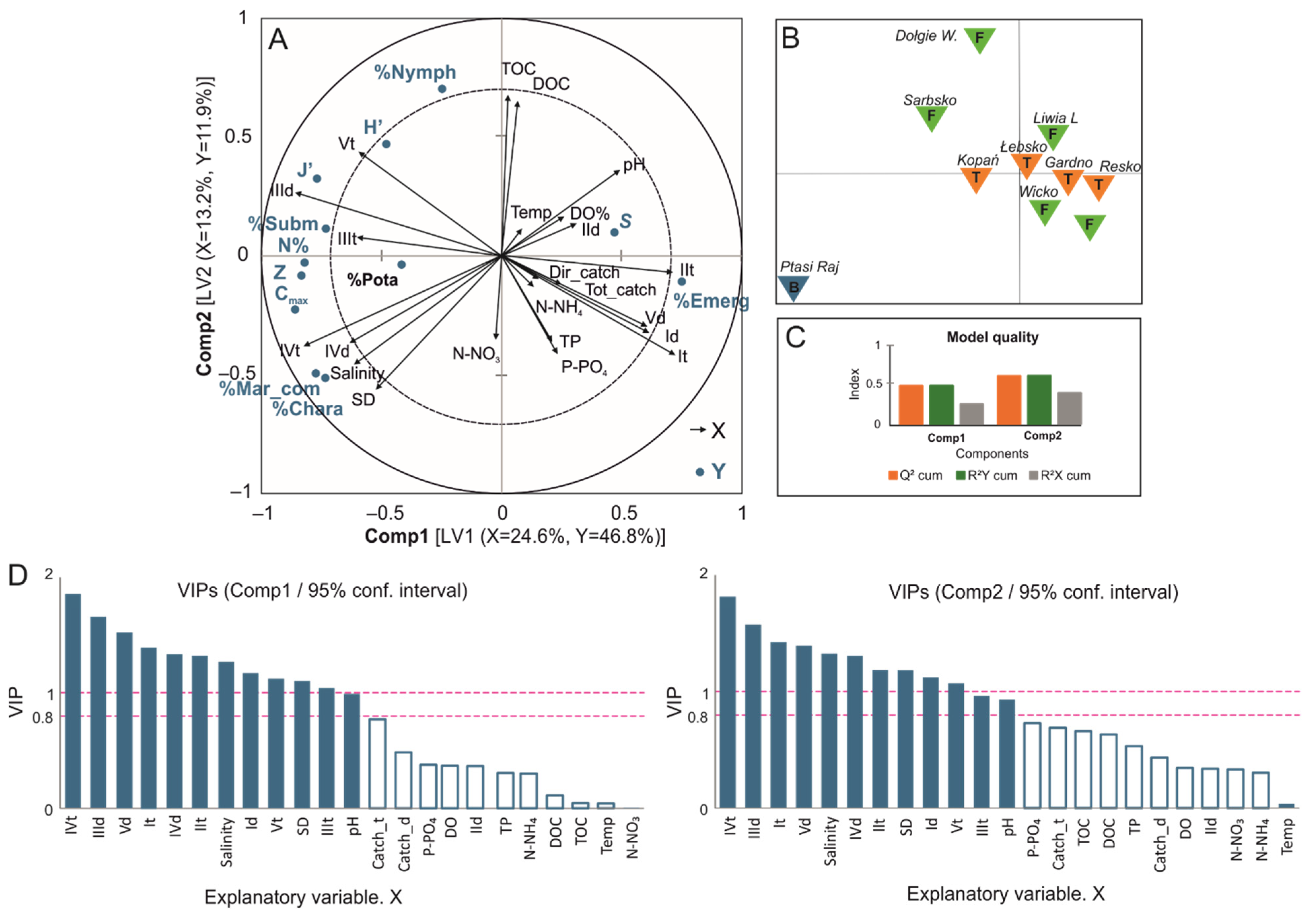
3.2. Factors Influencing the Diversity of Macrophytes in Coastal Lakes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: A Framework for Assessment; Island Press: Washington, DC, USA, 2003; p. 212. [Google Scholar]
- Karus, K.; Feldman, T. Factors influencing macrophyte metrics in Estonian coastal lakes in the light of ecological status assessment. Hydrobiologia 2013, 704, 153–163. [Google Scholar] [CrossRef]
- Rajasree, B.R.; Deo, M.C. Assessment of coastal vulnerability considering the future climate: A case study along the Central West Coast of India. J. Waterw. Port Coast. Ocean. Eng. 2020, 146, 05019005. [Google Scholar] [CrossRef]
- European Commission. Managing Natura 2000 Sites—The Provisions of Article 6 of the ‘Habitats’ Directive 92/43/EEC.; European Commission: Brussels, Belgium, 2000; p. 73.
- European Commission. Directive 2000/60/EC of the European Parliament and the Council of 23rd October 2000 Establishing a Framework for Community Action in the Field of Water Policy (OJ L 327, 22/12/2000, 1–72). 2000. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:327:0001:0072:EN:PDF (accessed on 31 August 2022).
- Obolewski, K.; Glińska-Lewczuk, K. Connectivity and complexity of coastal lakes as determinants for their restoration—A case study of the southern Baltic Sea. Ecol. Eng. 2020, 155, 105948. [Google Scholar] [CrossRef]
- Coccossis, H. Integrated coastal management and river basin management. Water Air Soil Pollut. 2004, 4, 411–419. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, S.; Zhang, Z. Dilution or enrichment: The effects of flood on pollutants in urban rivers. Environ. Sci. Eur. 2022, 34, 61. [Google Scholar] [CrossRef]
- European Commission. Biodiversity Strategy for 2030. 2021. Available online: https://environment.ec.europa.eu/strategy/biodiversity-strategy-2030pl (accessed on 31 August 2022).
- Cieśliński, R.; Olszewska, A. New insight into defining the lakes of the southern Baltic coastal zone. Environ. Monit. Assess. 2018, 190, 102. [Google Scholar] [CrossRef]
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Borics, G.; Borja, A.; Feld, C.K.; Ferreira, M.T.; et al. Protecting and restoring Europe’s waters: An analysis of the future development needs of the Water Framework Directive. Sci. Total Environ. 2019, 658, 1228–1238. [Google Scholar] [CrossRef]
- Schneider, S.C.; Biberdžić, V.; Braho, V.; Budzakoska-Gjoreska, B.; Cara, M.; Dana, Z.; Đurašković, P.; Eriksen, T.E.; Hjermann, D.; Imeri, A.; et al. Littoral eutrophication indicators are more closely related to nearshore land use than to water nutrient concentrations: A critical evaluation of stressor-response relationships. Sci. Total Environ. 2020, 748, 141193. [Google Scholar] [CrossRef]
- Obolewski, K. Using macrozoobenthos to assess the ecological condition of the estuary lake Jamno. Ochr. Srodowiska 2009, 31, 17–24. [Google Scholar]
- Melzer, A. Aquatic Macrophytes as Tools for Lake Management. Developments in Hydrobiology. In The Ecological Bases for Lake and Reservoir Management; Harper, D.M., Brierley, B., Ferguson, A.J.D., Phillips, G., Eds.; Springer: Dordrecht, The Netherlands, 1999; p. 136. [Google Scholar] [CrossRef]
- Nurminen, L. Macrophyte Species Composition Reflecting Water Quality Changes in Adjacent Water Bodies of Lake Hiidenvesi, SW Finland. Ann. Zool. Fenn. 2003, 40, 199–208. Available online: http://www.jstor.org/stable/23726718 (accessed on 20 August 2022).
- Søndergaard, M.; Johansson, L.S.; Lauridsen, T.L.; Jørgensen, T.B.; Liboriussen, L.; Jeppesen, E. Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 2010, 55, 893–908. [Google Scholar] [CrossRef]
- Duigan, C.; Kovach, W.; Palmer, M. Vegetation communities of British lakes: A revised classification scheme for conservation. Aquat. Conserv. 2007, 17, 147–173. [Google Scholar] [CrossRef]
- Schneider, S. Macrophyte trophic indicator values from a European perspective. Limnologica 2007, 37, 281–289. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. An insight to the ecological evaluation index (EEI). Ecol. Indic. 2003, 3, 27–33. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Greve, T.M.; Nielsen, K. Eelgrass as a Bioindicator Under the European Water Framework Directive. Water Resour. Manag. 2005, 19, 63–75. [Google Scholar] [CrossRef]
- Luther, H. Verbreitung und Ökologie der höheren Wasserpflanzen im Brackwasser der Ekenäs-Gegend in Siidfinnland. I. Algemeiner Tell. Act. Bot. Fenn. 1951, 49, 1–231. [Google Scholar]
- Luther, H. Verbreitung und Ökologie der höheren Wasserpflanzen im Brackwasser der Ekenäs-Gegend in Siidfinnland. II. Spezieller Teil. Act. Bot. Fenn. 1951, 50, 1–370. [Google Scholar]
- Copernicus Land Monitoring Service. 2018. Available online: https://land.copernicus.eu/ (accessed on 5 July 2022).
- Obolewski, K.; Glińska-Lewczuk, K.; Szymańska, M.; Mrozińska, N.; Bąkowska, M.; Astel, A.; Lew, S.; Paturej, E. Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS ONE 2018, 13, e0207825. [Google Scholar] [CrossRef]
- Szarek-Iwaniuk, P.A. A Comparative analysis of spatial data and land use/land cover classification in urbanized areas and areas subjected to anthropogenic pressure for the example of Poland. Sustainability 2021, 13, 3070. [Google Scholar] [CrossRef]
- Jensén, S. An objective method for sampling the macrophyte vegetation in lakes. Vegetatio 1977, 33, 107–118. [Google Scholar] [CrossRef]
- Ciecierska, H. Dissertations and Monographs. In Macrophyte Based Indices of the Ecological State of Lakes; University of Warmia and Mazury in Olsztyn: Olsztyn, Poland, 2008; p. 139, (In Polish with English Summary). [Google Scholar]
- Ciecierska, H.; Kolada, A. ESMI: A macrophyte index for assessing the ecological status of lakes. Environ. Monitor. Assess. 2014, 186, 5501–5517. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K.; Bąkowska, M.; Szymańska, M.; Mrozińska, N. Patterns of phytoplankton composition in coastal lakes differed by connectivity with the Baltic Sea. Sci. Total Environ. 2018, 631–632, 951–961. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for Examination of Water and Wastewater, 23rd ed.; American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Washington, DC, USA, 2017; p. 1545. ISBN 9780875532875. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Henningsson, M.; Sundbom, E.; Armelius, B.; Erdberg, P. PLS model building: A multivariate approach to personality test data. Scand. J. Psychol. 2001, 42, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Shawul, A.A.; Chakma, S.; Melesse, A.M. The response of water balance components to land cover change based on hydrologic modeling and partial least squares regression (PLSR) analysis in the Upper Awash Basin. J. Hydrol. Reg. Stud. 2019, 26, 100640. [Google Scholar] [CrossRef]
- Poikane, S.; Herrero, F.S.; Kelly, M.; Borja, A.; Birk, S.; van de Bund, W. European aquatic ecological assessment methods: A critical review of their sensitivity to key pressures. Sci. Total Environ. 2020, 740, 140075. [Google Scholar] [CrossRef]
- Segurado, P.; Caiola, N.; Pont, D.; Oliveira, J.M.; Delaigue, O.; Ferreira, M.T. Comparability of fish-based ecological quality assessments for geographically distinct Iberian regions. Sci. Total Environ. 2014, 476, 785–794. [Google Scholar] [CrossRef]
- Kuhar, U.; Germ, M.; Gaberščik, A.; Urbanič, G. Development of a river Macrophyte Index (RMI) for assessing river ecological status. Limnologica 2011, 41, 235–243. [Google Scholar] [CrossRef]
- Pinedo, S.; Jordana, E.; Ballesteros, E. A critical analysis on the response of macroinvertebrate communities along disturbance gradients: Description of MEDOCC (MEDiterranean OCCidental) index. Mar. Ecol. 2015, 36, 141–154. [Google Scholar] [CrossRef]
- Europaean Commision, Report—Fitness Check of the Water Framework Directive and the Floods Directive. 2020. Available online: https://ec.europa.eu/info/publications/fitness-check-water-framework-directive-and-floods-directive_en (accessed on 1 July 2022).
- Schinegger, R.; Trautwein, C.; Melcher, A.; Schmutz, S. Multiple human pressures and their spatial patterns in European running waters. Water Environ. J. 2012, 26, 261–273. [Google Scholar] [CrossRef]
- Camp, J.; Flo, E.; Vila, M.; Arin, L.; Reñé, A.; Sampedro, N.; Manzanera, M.; Garcés, E. Pros and Cons of Biological Quality Element Phytoplankton as a Water-Quality Indicator in the NW Mediterranean Sea. In Experiences from Ground, Coastal and Transitional Water Quality Monitoring; Munné, A., Ginebreda, A., Prat, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 135–160. [Google Scholar]
- Torras, X.; Pinedo, S.; García, M.; Weitzmann, B.; Ballesteros, E. Environmental Quality of Catalan Coastal Waters Based on Macroalgae: The Interannual Variability of CARLIT Index and Its Ability to Detect Changes in Anthropogenic Pressures over Time. In Experiences from Ground, Coastal and Transitional Water Quality Monitoring. The Handbook of Environmental Chemistry; Munné, A., Ginebreda, A., Prat, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 43, pp. 183–200. [Google Scholar]
- Baattrup-Pedersen, A.; Larsen, S.E.; Rasmussen, J.J.; Riis, T. The future of European water management: Demonstration of a new WFD compliant framework to support sustainable management under multiple stress. Sci. Total Environ. 2019, 654, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Poikane, S.; Portielje, R.; Denys, L.; Elferts, D.; Kelly, M.; Kolada, A.; Mäemets, H.; Phillips, G.; Søndergaard, M.; Willby, N.; et al. Macrophyte assessment in European lakes: Diverse approaches but convergent views of ‘good’ ecological status. Ecol. Indic. 2018, 94, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.G.; Birk, S.; Willby, N.J.; Denys, L.; Drakare, S.; Kahlert, M.; Karjalainen, S.M.; Marchetto, A.; Pitt, J.A.; Urbanič, G.; et al. Redundancy in the ecological assessment of lakes: Are phytoplankton, macrophytes and phytobenthos all necessary? Sci. Total Environ. 2016, 568, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.B.G.; Hilt, S.; Kosten, S.; De Klein, J.J.M.; Paerl, H.W.; Van De Waal, D.B. Shifting states, shifting services: Linking regime shifts to changes in ecosystem services of shallow lakes. Freshw. Biol. 2021, 66, 1–12. [Google Scholar] [CrossRef]
- Scheffer, M.; Jeppesen, E. Regime shifts in shallow lakes. Ecosystems 2007, 10, 1–3. [Google Scholar] [CrossRef]
- Zeng, L.; He, F.; Dai, Z.; Xu, D.; Liu, B.; Zhou, Q.; Wu, Z. Effect of submerged macrophyte restoration on improving aquatic ecosystem in a subtropical, shallow lake. Ecol. Eng. 2017, 106, 578–587. [Google Scholar] [CrossRef]
- Njagi, D.M.; Routh, J.; Odhiambo, M.; Luo, C.; Basapuram, L.G.; Olago, D.; Klump, V.; Stager, C. A century of human-induced environmental changes and the combined roles of nutrients and land use in Lake Victoria catchment on eutrophication. Sci. Total Environ. 2022, 835, 155425. [Google Scholar] [CrossRef]
- Yan, D.; Li, J.; Xie, S.; Liu, Y.; Sheng, Y.; Luan, Z. Examining the expansion of Spartina alterniflora in coastal wetlands using an MCE-CA-Markov model. Front. Mar. Sci. 2022, 9, 964172. [Google Scholar] [CrossRef]
- Akasaka, M.; Takamura, N.; Mitsuhashi, H.; Kadono, Y. Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshw. Biol. 2010, 55, 909–922. [Google Scholar] [CrossRef]
- Jones, R.I.; King, L.; Dent, M.M.; Maberly, S.C.; Gibson, C.E. Nitrogen stable isotope ratios in surface sediments, epilithon and macrophytes from upland lakes with differing nutrient status. Freshw. Biol. 2004, 49, 382–391. [Google Scholar] [CrossRef]
- Matias, N.G.; Boavida, M.J. Effects of catchment development on the trophic status of a deep and shallow reservoir in Portugal. Lake Reserv. Manag. 2005, 21, 350–360. [Google Scholar] [CrossRef]
- Nakano, T.; Tayasu, I.; Yamada, Y.; Hosono, T.; Igeta, A.; Hyodo, F.; Ando, A.; Saitoh, Y.; Tanaka, T.; Wada, E.; et al. Effect of agriculture on water quality of Lake Biwa, Japan. Sci. Total Environ. 2008, 389, 132–148. [Google Scholar] [CrossRef] [PubMed]
- McColl, R.H.S. Chemistry and trophic status of seven New Zealand lakes. N. Z. J. Mar. Freshw. Res. 1972, 6, 399–447. [Google Scholar] [CrossRef]
- Novikmec, M.; Hamerlík, L.; Kočický, D.; Hrivnák, R.; Kochjarová, J.; Oťaheľová, H.; Paľove-Balang, P.; Svitok, M. Ponds and their catchments: Size relationships and influence of land use across multiple spatial scales. Hydrobiologia 2016, 774, 155–166. [Google Scholar] [CrossRef]
- Mozgawa, J. Photointerpretation analysis of landscape structure in lake watersheds of Suwałki Landscape. Park. Ekol. Pol. 1993, 41, 53–74. [Google Scholar]
- Mioduszewski, W. Small water reservoirs on agricultural areas. Inst. Melior. Użytków Ziel. Falenty 2008, 10, 32–34. (In Polish) [Google Scholar]
- Wilk-Woźniak, E.; Walusiak, E.; Burchardt, L.; Cerbin, S.; Chmura, D.; Gąbka, M.; Glińska-Lewczuk, K.; Gołdyn, R.; Grabowska, M.; Karpowicz, M.; et al. Effects of the environs of waterbodies on aquatic plants in oxbow lakes (habitat 3150). Ecol. Indic. 2018, 98, 736–742. [Google Scholar] [CrossRef]
- Dudzińska, A.; Szpakowska, B.; Pajchrowska, M. Influence of land development on the ecological status of small water bodies. Oceanol. Hydrobiol. Stud. 2020, 49, 345–353. [Google Scholar] [CrossRef]
- Harris, L.R.; Bessinger, M.; Dayaram, A.; Holness, S.; Kirkman, S.; Livingstone, T.-C.; Lombard, A.T.; Lück-Vogel, M.; Pfaff, M.; Sink, K.J.; et al. Advancing land-sea integration for ecologically meaningful coastal conservation and management. Biol. Conserv. 2019, 237, 81–89. [Google Scholar] [CrossRef]
- Kotta, J.; Tiit Kutser, T.; Teeveer, K.; Vahtmäe, E.; Pärnoja, M. Predicting species cover of marine macrophyte and invertebrate species combining hyperspectral remote sensing, machine learning and regression techniques. PLoS ONE 2013, 8, e63946. [Google Scholar] [CrossRef]
- Lew, S.; Glińska-Lewczuk, K.; Burandt, P.; Kulesza, K.; Kobus, S.; Obolewski, K. Salinity as a Determinant Structuring Microbial Communities in Coastal Lakes. Int. J. Environ. Res. Public Health 2022, 19, 4592. [Google Scholar] [CrossRef] [PubMed]
- Raśkiewicz, J.; Cieśliński, R. Hydrological and Morphometric Conditions of Lakes Ptasi Raj and Karaś (Northern Poland). In Anthropogenic and Natural Transformations of Lakes; Kubiak, J., Bajkiewicz-Grabowska, E., Eds.; Agricultural University of Szczecin, Polish Limnological Society: Szczecin, Poland, 2007; pp. 104–107. [Google Scholar]
- Blindow, I.; Dietrich, J.; Möllmann, N.; Schubert, H. Growth, photosynthesis and fertility of Chara aspera under different light and salinity conditions. Aquat. Bot. 2003, 76, 213–234. [Google Scholar] [CrossRef]
- Pitkänen, H.; Peuraniemi, M.; Westerbom, M.; Kilpi, M.; Numers, M.V. Long-term changes in distribution and frequency of aquatic vascular plants and charophytes in an estuary in the Baltic Sea. Ann. Bot. Fenn. 2013, 50, 1–54. [Google Scholar] [CrossRef]
- Pajusalu, L.; Martin, G.; Põllumäe, A.; Paalme, T. Direct effects of increased CO2 concentrations in seawater on the net primary production of charophytes in a shallow, coastal, brackishwater ecosystem. Boreal Environ. Res. 2015, 20, 413–422. [Google Scholar]
- Rodrigo, M.A.; Rojo, C.; Segura, M.; Alonso-Guillén, J.L.; Martín, M.; Vera, P. The role of charophytes in a Mediterranean pond created for restoration purposes. Aquat. Bot. 2015, 120, 101–111. [Google Scholar] [CrossRef]
- Wells, E.; Wilkinson, M.; Wood, P.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruzafa, A.; Hegazi, M.I.; Pérez-Ruzafa, I.M.; Marcos, C. Differences in spatial and seasonal patterns of macrophyte assemblages between a coastal lagoon and the open sea. Mar. Environ. Res. 2008, 65, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.T.; Wright, J.F.; Furse, M.T. RIVPACS models for predicting the expected macroinvertebrate fauna and assessing the ecological quality of rivers. Ecol. Model. 2003, 160, 219–233. [Google Scholar] [CrossRef]
- Santos, J.S.; Pontes, M.S.; Arruda, G.J.; Caires, A.R.L.; Lima, S.M.; Andrade, L.H.C.; Bueno, M.L.; da Silva, V.F.B.; Grillo, R.; Santiago, E.F. Ecological Aspects of Aquatic Macrophytes for Environmental Pollution Control: An Eco-Remedial Approach. In Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water; Elsevier: Amsterdam, The Netherlands, 2022; pp. 497–523. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.-Y.; Guo, X.; Hazelton, E.L.G.; Kettenring, K.M.; et al. Cosmopolitan species as models for ecophysiological responses to global change: The common reed Phragmites australis. Front. Plant Sci. 2017, 8, 1833. [Google Scholar] [CrossRef]
- Yang, Z.-D.; Yuan, S.-B.; Liu, X.-Q.; Wang, H.-Z. Water level fluctuation requirements of emergent macrophyte Typha angustifolia L. Water 2020, 12, 127. [Google Scholar] [CrossRef]
- Global Invasive Species Database Species Profile: Glyceria Maxima. 2022. Available online: http://www.iucngisd.org/gisd/species.php?sc=891 (accessed on 10 August 2022).
- Costa, J.C.; Capelo, J.; Espírito Santo, M.D.; Lousã, M.; Monteiro, A.; Mesquita, S.; Vasconcelos, M.T.; Moreira, I. Plant communities of the lagoons of the Portuguese Coastal Superdistrict—A multivariate approach. Hydrobiologia 1999, 415, 67–75. [Google Scholar] [CrossRef]
- Daigneault, A.; Strong, A.L.; Meyer, S.R. Benefits, costs, and feasibility of scaling up land conservation for maintaining ecosystem services in the Sebago Lake watershed, Maine, USA. Ecosyst. Serv. 2021, 48, 101238. [Google Scholar] [CrossRef]
- Fenemor, A.; Phillips, C.; Allen, W.; Young, R.G.; Harmsworth, G.; Bowden, B. Integrated catchment management—Interweaving social process and science knowledge. N. Z. J. Mar. Freshwater Res. 2011, 45, 313–331. [Google Scholar] [CrossRef]

| S | H’ | J’ | N% | Cmax | %Emerg | %Subm | Z | %Mar_comm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLC 1 | VID | CLC | VID | CLC | VID | CLC | VID | CLC | VID | CLC | VID | CLC | VID | CLC | VID | CLC | VID |
| Freshwater lake type | |||||||||||||||||
| 242t | 0.91 | 313d | 0.95 | 313d | 0.99 | 313d | 0.93 | 313d | 0.99 | 313d | −0.92 | 313d | 0.92 | 313d | 0.98 | 112t | 0.92 |
| 313t | 0.88 | 324t | 0.93 | 324t | 0.94 | 324t | 0.91 | 324t | 0.95 | 324t | −0.91 | 324t | 0.91 | 324t | 0.94 | 112d | 0.87 |
| 312d | 0.87 | 312d | 0.86 | 312d | 0.80 | 313d | −0.98 | ||||||||||
| 331d | 0.83 | 112d | −0.87 | 324t | −0.92 | ||||||||||||
| 324d | 0.82 | 112t | −0.86 | 312d | −0.88 | ||||||||||||
| 112t | −0.90 | ||||||||||||||||
| 112d | −0.87 | ||||||||||||||||
| Transitional lake type | |||||||||||||||||
| 512d | 0.99 | 121t | 0.93 | 411d | 0.99 | 313d | 0.96 | 313d | 0.99 | 324t | 0.99 | 242t | 0.95 | 313d | 0.98 | 313t | 0.99 |
| 243t | 0.88 | 331t | 0.93 | 313d | 0.98 | 411d | 0.81 | 411d | 0.96 | 243t | 0.97 | 411t | 0.94 | 411d | 0.87 | 121t | 0.98 |
| 313t | 0.88 | 412t | 0.93 | 411t | 0.90 | 324t | −0.85 | 411t | −0.99 | 112d | 0.91 | 411t | 0.85 | 331d | 0.98 | ||
| 331d | 0.87 | 411d | 0.89 | 512t | 0.86 | 242t | −0.97 | 231d | 0.89 | 331t | 0.98 | ||||||
| 324t | 0.86 | 141t | 0.88 | 242t | 0.85 | 512t | −0.97 | 243t | −0.98 | 412t | 0.98 | ||||||
| 311t | 0.85 | 331d | 0.88 | 324t | −0.98 | 141t | 0.87 | ||||||||||
| 231d | −0.98 | 313t | 0.88 | 512d | −0.92 | 211t | −0.99 | ||||||||||
| 112d | −0.99 | 211t | −0.91 | 211d | −0.94 | ||||||||||||
| 112t | −0.92 | 211d | −0.86 | 112t | −0.85 | ||||||||||||
| 211t | −0.85 | 112d | −0.85 | ||||||||||||||
| 231d | −0.85 | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzybowski, M.; Burandt, P.; Glińska-Lewczuk, K.; Lew, S.; Obolewski, K. Response of Macrophyte Diversity in Coastal Lakes to Watershed Land Use and Salinity Gradient. Int. J. Environ. Res. Public Health 2022, 19, 16620. https://doi.org/10.3390/ijerph192416620
Grzybowski M, Burandt P, Glińska-Lewczuk K, Lew S, Obolewski K. Response of Macrophyte Diversity in Coastal Lakes to Watershed Land Use and Salinity Gradient. International Journal of Environmental Research and Public Health. 2022; 19(24):16620. https://doi.org/10.3390/ijerph192416620
Chicago/Turabian StyleGrzybowski, Mirosław, Paweł Burandt, Katarzyna Glińska-Lewczuk, Sylwia Lew, and Krystian Obolewski. 2022. "Response of Macrophyte Diversity in Coastal Lakes to Watershed Land Use and Salinity Gradient" International Journal of Environmental Research and Public Health 19, no. 24: 16620. https://doi.org/10.3390/ijerph192416620
APA StyleGrzybowski, M., Burandt, P., Glińska-Lewczuk, K., Lew, S., & Obolewski, K. (2022). Response of Macrophyte Diversity in Coastal Lakes to Watershed Land Use and Salinity Gradient. International Journal of Environmental Research and Public Health, 19(24), 16620. https://doi.org/10.3390/ijerph192416620









