Effect of Transcranial Direct Current Stimulation Augmented with Motor Imagery and Upper-Limb Functional Training for Upper-Limb Stroke Rehabilitation: A Prospective Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
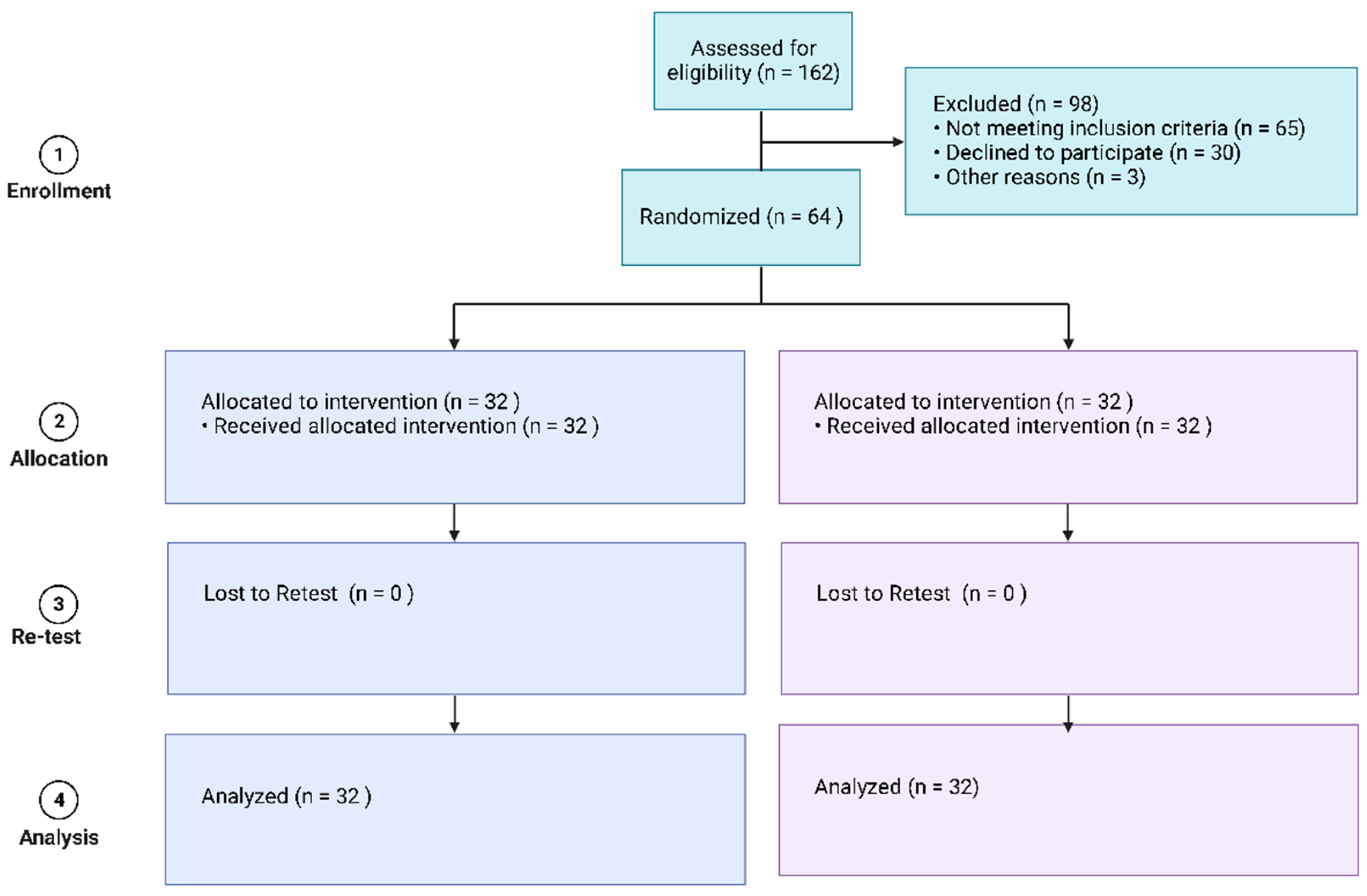
2.3. Randomization and Blinding
2.4. Sample Size Calculation
2.5. Outcome Variables
2.5.1. Fugl-Meyer Assessment of Upper-Limb Motor Recovery following Stroke
2.5.2. Action Research Arm Test (ARAT)
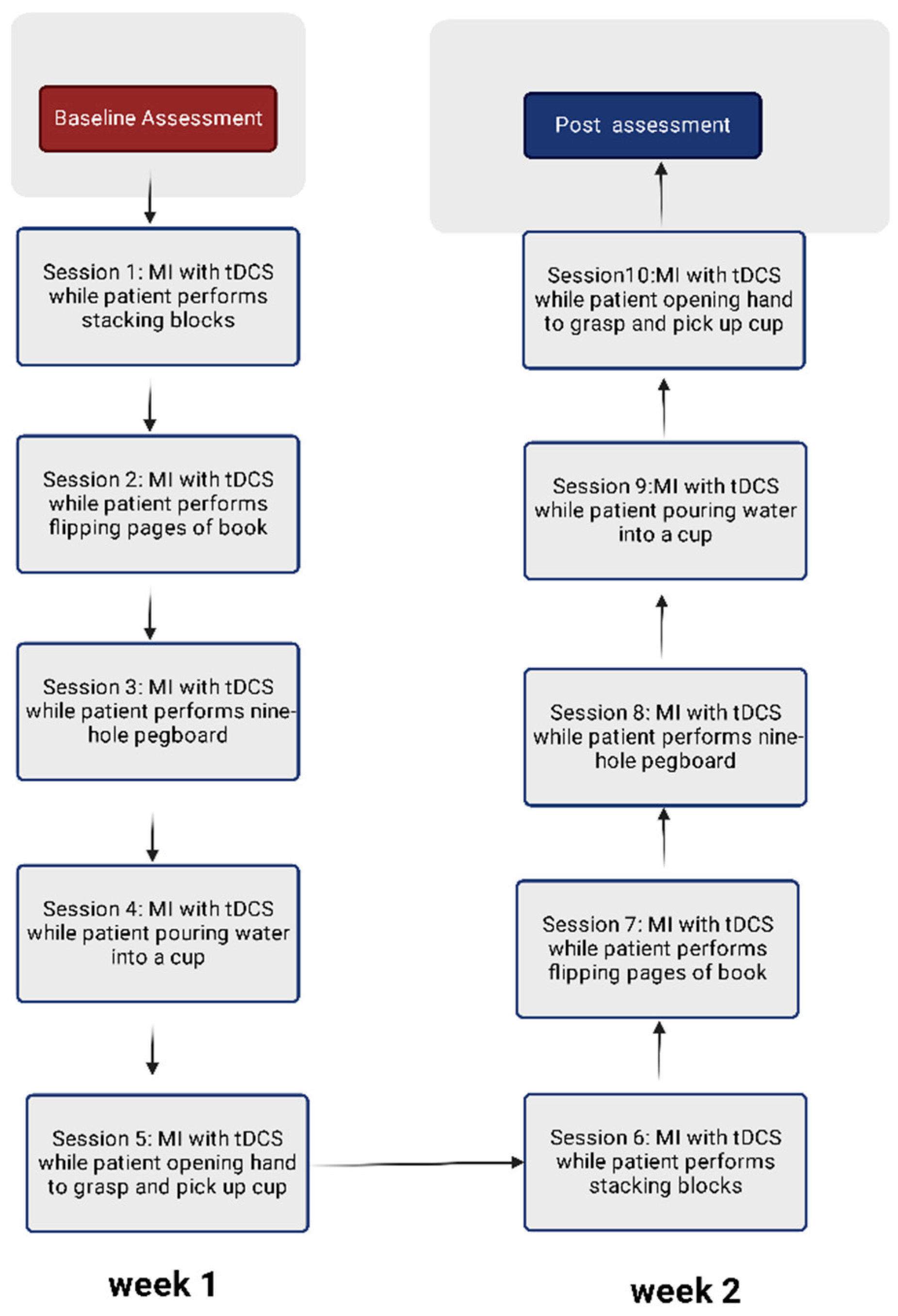
2.6. Protocol for Visual-Motor Imagery
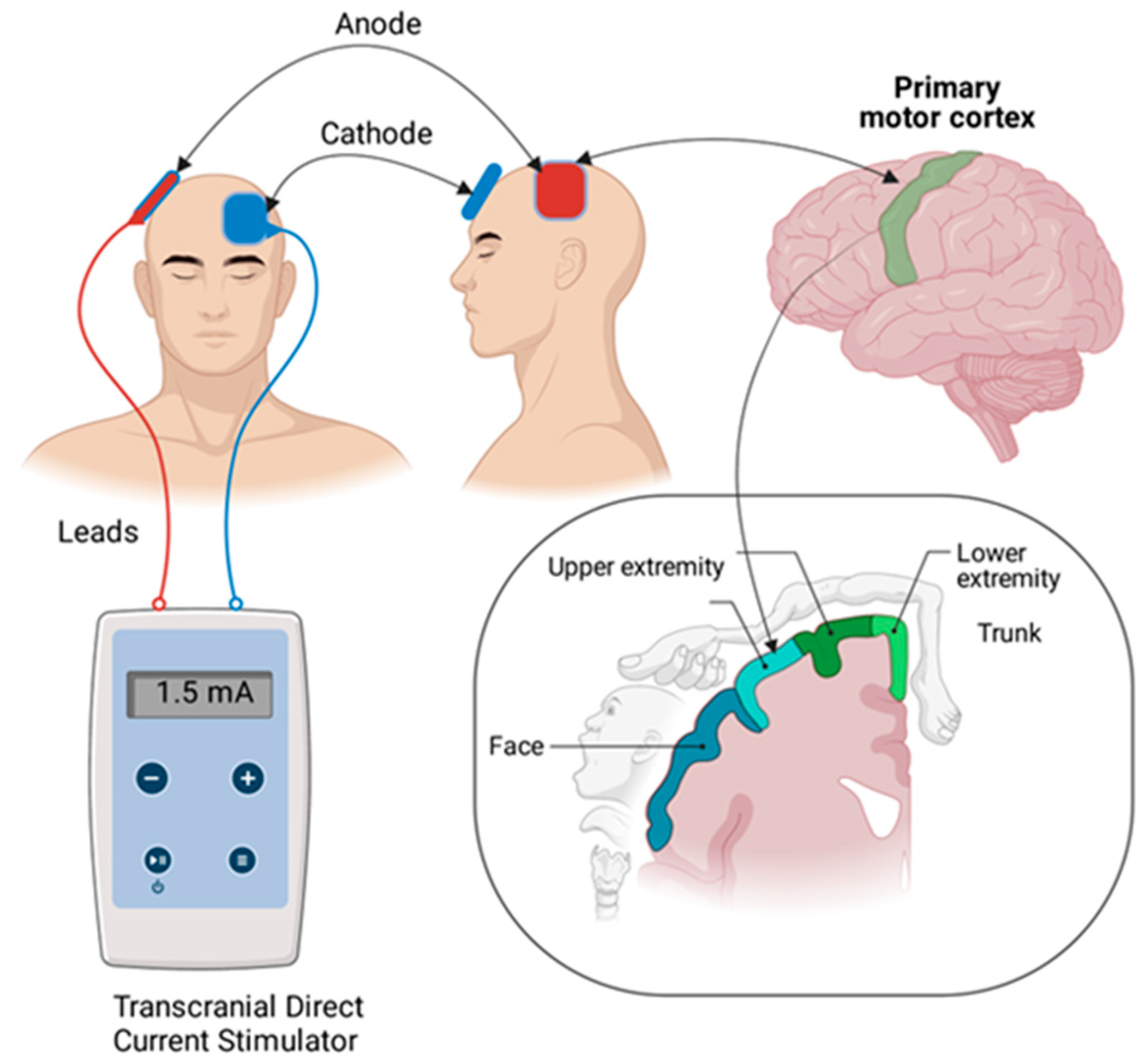
2.7. Protocol for Transcranial Direct Current Stimulation
2.8. Upper-Limb Motor Activities
2.9. Side Effects following tDCS
2.10. Statistical Analyses
3. Results
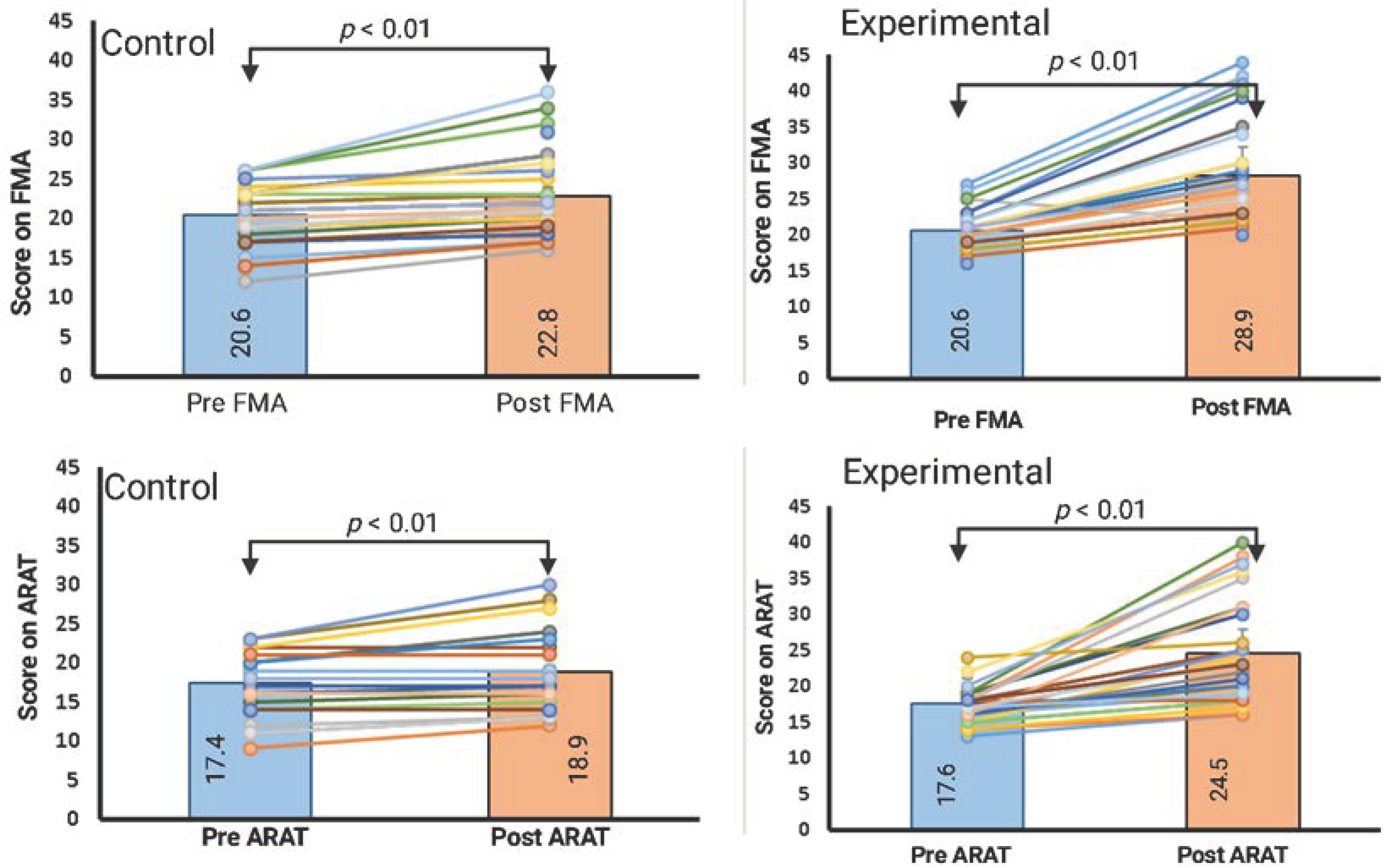
3.1. Within-Group Comparison (Paired Samples t-Test)
3.2. Between-Group Comparison (Analysis of Covariance)
3.3. Adverse Reaction following tDCS
4. Discussion
4.1. The Efficacy of Anodal tDCS in Conjunction with MI and Upper-Limb Functional Training
4.2. Protocol
4.3. Clinical Applicability
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katan, M.; Luft, A. Global burden of stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Metrot, J.; Froger, J.; Hauret, I.; Mottet, D.; van Dokkum, L.; Laffont, I. Motor recovery of the ipsilesional upper limb in subacute stroke. Arch. Phys. Med. Rehabil. 2013, 94, 2283–2290. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Stinear, C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010, 9, 1228–1232. [Google Scholar] [CrossRef]
- Shelton, F.D.N.A.P.; Volpe, B.T.; Reding, M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabil. Neural Repair 2001, 15, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.S.; Mulroy, S.; Amdur, R.L.; Requejo, P.; Prilutsky, B.I.; Dromerick, A.W. Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Top. Stroke Rehabil. 2009, 16, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Application and Refinement of Cross-Education Strength Training in Stroke. Ph.D. Thesis, University of Victoria, Oak Bay and Saanich, BC, Canada, 2019. [Google Scholar]
- Shi, Y.X.; Tian, J.H.; Yang, K.H.; Zhao, Y. Modified constraint-induced movement therapy versus traditional rehabilitation in patients with upper-extremity dysfunction after stroke: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2011, 92, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of using virtual reality–supported exercise therapy for upper extremity motor rehabilitation in patients with stroke: Systematic review and meta-analysis of randomized controlled trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst. Rev. 2017, 6, 1–21. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, H.; Zhang, J.; Yang, S.; Cai, S. Robot-assisted therapy for upper extremity motor impairment after stroke: A systematic review and meta-analysis. Phys. Ther. 2021, 101, pzab010. [Google Scholar] [CrossRef] [PubMed]
- Fuentes Calderon, M.A.; Miralles, A.N.; Pimienta, M.J.; Estella, J.M.G.; Ledesma, M.J.S. Analysis of the factors related to the effectiveness of transcranial current stimulation in upper limb motor function recovery after stroke: A systematic review. J. Med. Syst. 2019, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, S. Motor imagery on upper extremity function for persons with stroke: A systematic review and meta-analysis. Phys. Ther. Rehabil. Sci. 2019, 8, 52–59. [Google Scholar] [CrossRef]
- Sharma, N.; Pomeroy, V.M.; Baron, J.-C. Motor imagery: A backdoor to the motor system after stroke? Stroke 2006, 37, 1941–1952. [Google Scholar] [CrossRef]
- Braun, S.M.; Beurskens, A.J.; Borm, P.J.; Schack, T.; Wade, D.T. The effects of mental practice in stroke rehabilitation: A systematic review. Arch. Phys. Med. Rehabil. 2006, 87, 842–852. [Google Scholar] [CrossRef]
- Park, S.-W.; Kim, J.-H.; Yang, Y.-J. Mental practice for upper limb rehabilitation after stroke: A systematic review and meta-analysis. Int. J. Rehabil. Res. 2018, 41, 197–203. [Google Scholar] [CrossRef]
- de Vries, S.; Mulder, T. Motor imagery and stroke rehabilitation: A critical discussion. Acta Derm. Venereol. 2007, 39, 5–13. [Google Scholar] [CrossRef]
- Bastani, A.; Jaberzadeh, S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin. Neurophysiol. 2012, 123, 644–657. [Google Scholar] [CrossRef]
- Dong, K.; Meng, S.; Guo, Z.; Zhang, R.; Xu, P.; Yuan, E.; Lian, T. The effects of transcranial direct current stimulation on balance and gait in stroke patients: A systematic review and meta-analysis. Front. Neurol. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Van Hoornweder, S.; Vanderzande, L.; Bloemers, E.; Verstraelen, S.; Depestele, S.; Cuypers, K.; van Dun, K.; Strouwen, C.; Meesen, R. The effects of transcranial direct current stimulation on upper-limb function post-stroke: A meta-analysis of multiple-session studies. Clin. Neurophysiol. 2021, 132, 1897–1918. [Google Scholar] [CrossRef]
- Hummel, F.; Cohen, L.G. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil. Neural Repair 2005, 19, 14–19. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, V.; Paolazzi, C.L.; Miceli, G. tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex 2015, 63, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Schlaug, G.; Renga, V.; Nair, D. Transcranial direct current stimulation in stroke recovery. Arch. Neurol. 2008, 65, 1571–1576. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L.; Jackson, P.; LaFleur, M.F.; Durand, A.; Doyon, J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: A reliability and construct validity study. J. Neurol. Phys. Ther. 2007, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Fregni, F.; Martinuzzi, C.; Pavarelli, C.; Salvioli, S.; Basaglia, N. tDCS and robotics on upper limb stroke rehabilitation: Effect modification by stroke duration and type of stroke. BioMed Res. Int. 2016, 2016, 5068127. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Takahashi, K.; Moriwaki, M.; Sakamoto, T.; Domen, K. Improvement of upper extremity deficit after constraint-induced movement therapy combined with and without preconditioning stimulation using dual-hemisphere transcranial direct current stimulation and peripheral neuromuscular stimulation in chronic stroke patients: A pilot randomized controlled trial. Front. Neurol. 2017, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Tauchi, Y.; Kyougoku, M.; Okita, Y.; Takebayashi, T. Structural validity and internal consistency of a hypothesized factor structure of the Fugl-Meyer Assessment of the upper extremity. Top. Stroke Rehabil. 2022, 1–11. [Google Scholar] [CrossRef]
- Hellstrom, K.; Lindmark, B.; Wahlberg, B.; Fugl-Meyer, A.R. Self-efficacy in relation to impairments and activities of daily living disability in elderly patients with stroke: A prospective investigation. J. Rehabil. Med. 2003, 35, 202–207. [Google Scholar]
- Lin, J.-H.; Hsueh, I.-P.; Sheu, C.-F.; Hsieh, C.-L. Psychometric properties of the sensory scale of the Fugl-Meyer Assessment in stroke patients. Clin. Rehabil. 2004, 18, 391–397. [Google Scholar] [CrossRef]
- Amano, S.; Umeji, A.; Uchita, A.; Hashimoto, Y.; Takebayashi, T.; Takahashi, K.; Uchiyama, Y.; Domen, K. Clinimetric properties of the Fugl-Meyer assessment with adapted guidelines for the assessment of arm function in hemiparetic patients after stroke. Top. Stroke Rehabil. 2018, 25, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; Di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Hiragami, S.; Inoue, Y.; Harada, K. Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J. Phys. Ther. Sci. 2019, 31, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Rech, K.D.; Salazar, A.P.; Marchese, R.; Schifino, G.; Cimolin, V.; Pagnussat, A.S. Fugl-Meyer assessment scores are related with kinematic measures in people with chronic hemiparesis after stroke. J. Stroke Cerebrovasc. Dis. 2019, 29, 104463. [Google Scholar] [CrossRef]
- Hernández, E.; Galeano, C.; Barbosa, N.; Forero, S.; Sunnerhagen, K.; Murphy, M. Intra- and inter-rater reliability of Fugl-Meyer assessment of upper extremity in stroke. J. Rehabil. Med. 2019, 51, 652–659. [Google Scholar] [CrossRef]
- Van der Lee, J.H.; de Groot, V.; Beckerman, H.; Wagenaar, R.C.; Lankhorst, G.J.; Bouter, L.M. The intra-and interrater reliability of the action research arm test: A practical test of upper extremity function in patients with stroke. Arch. Phys. Med. Rehabil. 2001, 82, 14–19. [Google Scholar] [CrossRef]
- Crajé, C.; van der Graaf, C.; Lem, F.C.; Geurts, A.C.; Steenbergen, B. Determining specificity of motor imagery training for upper limb improvement in chronic stroke patients: A training protocol and pilot results. Int. J. Rehabil. Res. 2010, 33, 359–362. [Google Scholar] [CrossRef]
- Figlewski, K.; Blicher, J.U.; Mortensen, J.; Severinsen, K.E.; Nielsen, J.F.; Andersen, H. Transcranial direct current stimulation potentiates improvements in functional ability in patients with chronic stroke receiving constraint-induced movement therapy. Stroke 2017, 48, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Carneiro, M.I.S.; Bolognini, N.; Fregni, F. Safety review of transcranial direct current stimulation in stroke. Neuromodul. Technol. Neural Interface 2017, 20, 215–222. [Google Scholar] [CrossRef]
- Silva, E.; Rocha, S.; Foerster, Á.; Wiesiolek, C.; Chagas, A.P.; Machado, G.; Baltar, A.; Monte-Silva, K. The impact of transcranial direct current stimulation (tDCS) combined with modified constraint-induced movement therapy (mCIMT) on upper limb function in chronic stroke: A double-blind randomized controlled trial. Disabil. Rehabil. 2016, 38, 653–660. [Google Scholar] [CrossRef]
- Goodwill, A.M.; Teo, W.-P.; Morgan, P.; Daly, R.M.; Kidgell, D.J. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: A pilot study. Front. Hum. Neurosci. 2016, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]
- Lee, D.-G.; Lee, D.-Y. Effects of adjustment of transcranial direct current stimulation on motor function of the upper extremity in stroke patients. J. Phys. Sci. 2015, 27, 3511–3513. [Google Scholar] [CrossRef]
- Gong, Y.; Long, X.-M.; Xu, Y.; Cai, X.-Y.; Ye, M. Effects of repetitive transcranial magnetic stimulation combined with transcranial direct current stimulation on motor function and cortex excitability in subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2020, 35, 718–727. [Google Scholar] [CrossRef]
- Chang, M.C.; Kim, D.Y.; Park, D.H. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. 2015, 8, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Prathum, T.; Piriyaprasarth, P.; Aneksan, B.; Hiengkaew, V.; Pankhaew, T.; Vachalathiti, R.; Klomjai, W. Effects of home-based dual-hemispheric transcranial direct current stimulation combined with exercise on upper and lower limb motor performance in patients with chronic stroke. Disabil. Rehabil. 2021, 44, 3868–3879. [Google Scholar] [CrossRef]
- Beaulieu, L.-D.; Blanchette, A.K.; Mercier, C.; Bernard-Larocque, V.; Milot, M.-H. Efficacy, safety, and tolerability of bilateral transcranial direct current stimulation combined to a resistance training program in chronic stroke survivors: A double-blind, randomized, placebo-controlled pilot study. Restor. Neurol. Neurosci. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Vestito, L.; Rosellini, S.; Mantero, M.; Bandini, F. Long-term effects of transcranial direct-current stimulation in chronic post-stroke aphasia: A pilot study. Front. Hum. Neurosci. 2014, 8, 785. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Z.; Bai, Z.; Fong, K.N. Timing-dependent interaction effects of tDCS with mirror therapy on upper extremity motor recovery in patients with chronic stroke: A randomized controlled pilot study. J. Neurol. Sci. 2019, 405, 116436. [Google Scholar] [CrossRef]
- Budhota, A.; Chua, K.S.G.; Hussain, A.; Kager, S.; Cherpin, A.; Contu, S.; Vishwanath, D.; Kuah, C.W.K.; Ng, C.Y.; Yam, L.H.L.; et al. Robotic assisted upper limb training post stroke: A randomized control trial using combinatory approach toward reducing workforce demands. Front. Neurol. 2021, 12, 622014. [Google Scholar] [CrossRef]
- Cho, K.H.; Hong, M.-R.; Song, W.-K. Upper limb robotic rehabilitation for chronic stroke survivors: A single-group preliminary study. J. Phys. Ther. Sci. 2018, 30, 580–583. [Google Scholar] [CrossRef][Green Version]
- Simonetti, D.; Zollo, L.; Milighetti, S.; Miccinilli, S.; Bravi, M.; Ranieri, F.; Magrone, G.; Guglielmelli, E.; Di Lazzaro, V.; Sterzi, S. Literature review on the effects of tDCS coupled with robotic therapy in post stroke upper limb rehabilitation. Front. Hum. Neurosci. 2017, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Vallar, G.; Casati, C.; Latif, L.A.; El-Nazer, R.; Williams, J.; Banco, E.; Macea, D.D.; Tesio, L.; Chessa, C.; et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabilit. Neural Repair 2011, 25, 819–829. [Google Scholar] [CrossRef]
- Rizzo, V.; Terranova, C.; Crupi, D.; Sant’Angelo, A.; Girlanda, P.; Quartarone, A. Increased transcranial direct current stimulation after effects during concurrent peripheral electrical nerve stimulation. Brain Stimul. 2014, 7, 113–121. [Google Scholar] [CrossRef]
- von Rein, E.; Hoff, M.; Kaminski, E.; Sehm, B.; Steele, C.J.; Villringer, A.; Ragert, P. Improving motor performance without training: The effect of combining mirror visual feedback with transcranial direct current stimulation. J. Neurophysiol. 2015, 113, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Groppa, S.; Bergmann, T.; Siems, C.; Mölle, M.; Marshall, L.; Siebner, H. Slow-oscillatory transcranial direct current stimulation can induce bidirectional shifts in motor cortical excitability in awake humans. Neuroscience 2010, 166, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Foerster, Á.; Dutta, A.; Kuo, M.; Paulus, W.; Nitsche, M.A. Effects of anodal transcranial direct current stimulation over lower limb primary motor cortex on motor learning in healthy individuals. Eur. J. Neurosci. 2018, 47, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guerrero, G. Transcranial Direct Current Stimulation Effects on Cortical Excitability and Learning during a Dorsiflexion Motor Task. Ph.D. Thesis, University of Jyväskylä, Jyväskylä, Finland, 2019. [Google Scholar]
- Alharbi, R.A.; Aloyuni, S.A.; Kashoo, F.; Waly, M.I.; Singh, H.; Ahmad, M. Does transcranial direct current stimulation affect selective visual attention in children with left-sided infantile hemiplegia? A randomized, controlled pilot study. Brain Impair. 2020, 22, 152–164. [Google Scholar] [CrossRef]
| Adverse Effects | Number of Patients |
|---|---|
| Tingling sensation | 8 |
| Itching under electrodes | 5 |
| Fatigue following stimulation | 0 |
| Difficulties in concentrating | 0 |
| Nausea or vomiting | 0 |
| Insomnia | 0 |
| Burning sensation | 0 |
| Pain | 0 |
| Headache | 0 |
| Variables | Control (n = 32) | Experimental (n = 32) | p |
|---|---|---|---|
| Age (years) | 59.9 ± 5.6 | 58.7 ± 5.7 | 0.161 * |
| Gender (male/female) | 24/8 | 25/7 | 0.500 *** |
| Mini-mental state examination | 27.9 (0.89) | 28.0 (0.8) | 0.459 * |
| Onset (months) | 7.4 ± 1.2 | 7.8 ± 1.3 | 0.145 * |
| Type of stroke | |||
| Ischemic | 30 (93.8) | 28 (87.5) | 0.306 *** |
| Hemorrhagic | 2 (6.3) | 4 (12.5) | |
| Side affected | |||
| Right | 12 (53.1) | 18 (56.3) | |
| Left | 15 (46.9) | 14 (43.8) | |
| Associated risk factors | |||
| Hypertension | 21 | 18 | 0.069 ** |
| Diabetes mellitus | 4 | 4 | |
| High cholesterol | 0 | 5 | |
| Cardiac disorders | 5 | 2 | |
| Others | 2 | 3 | |
| Region of stroke | |||
| Cortical | 1 | 3 | 0.613 *** |
| Subcortical | 31 | 29 |
| Outcome | Experimental Group (n = 32) | Control Group (n = 32) | Between-Group Difference p-Value (η2 Value) |
|---|---|---|---|
| FMA score (upper limb) | |||
| Baseline | 20.6 (2.6) | 20.4 (3.7) | p = 0.76 a |
| Post-intervention | 28.3 (6.9) | 22.8 (5.0) | p < 0.001 (0.37) b |
| Improvement | 7.6 | 2.4 | |
| 95% confidence interval of the difference | 5.9–9.4 | 1.6–3.3 | |
| Standard error of mean | 0.86 | 0.87 | |
| Within-group difference p-value | p < 0.01 c | p < 0.01 c | |
| ARAT score | |||
| Baseline | 17.6 (2.6) | 17.4 (3.7) | p = 0.78 a |
| Post-intervention | 24.5 (6.9) | 18.9 (5.1) | p < 0.001 (0.38) b |
| Improvement | 6.8 | 1.5 | |
| 95% confidence interval of the difference | 5.0–8.6 | 0.6–2.3 | |
| Standard error of mean | 0.42 | 0.41 | |
| Within-group difference p-value | p < 0.01 c | p < 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashoo, F.Z.; Al-Baradie, R.S.; Alzahrani, M.; Alanazi, A.; Manzar, M.D.; Gugnani, A.; Sidiq, M.; Shaphe, M.A.; Sirajudeen, M.S.; Ahmad, M.; et al. Effect of Transcranial Direct Current Stimulation Augmented with Motor Imagery and Upper-Limb Functional Training for Upper-Limb Stroke Rehabilitation: A Prospective Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 15199. https://doi.org/10.3390/ijerph192215199
Kashoo FZ, Al-Baradie RS, Alzahrani M, Alanazi A, Manzar MD, Gugnani A, Sidiq M, Shaphe MA, Sirajudeen MS, Ahmad M, et al. Effect of Transcranial Direct Current Stimulation Augmented with Motor Imagery and Upper-Limb Functional Training for Upper-Limb Stroke Rehabilitation: A Prospective Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(22):15199. https://doi.org/10.3390/ijerph192215199
Chicago/Turabian StyleKashoo, Faizan Zaffar, Raid Saleem Al-Baradie, Msaad Alzahrani, Ahmad Alanazi, Md Dilshad Manzar, Anchit Gugnani, Mohammad Sidiq, Mohammad Abu Shaphe, Mohamed Sherif Sirajudeen, Mehrunnisha Ahmad, and et al. 2022. "Effect of Transcranial Direct Current Stimulation Augmented with Motor Imagery and Upper-Limb Functional Training for Upper-Limb Stroke Rehabilitation: A Prospective Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 22: 15199. https://doi.org/10.3390/ijerph192215199
APA StyleKashoo, F. Z., Al-Baradie, R. S., Alzahrani, M., Alanazi, A., Manzar, M. D., Gugnani, A., Sidiq, M., Shaphe, M. A., Sirajudeen, M. S., Ahmad, M., Althumayri, B., Aljandal, A., Almansour, A., Alshewaier, S. A., & Chahal, A. (2022). Effect of Transcranial Direct Current Stimulation Augmented with Motor Imagery and Upper-Limb Functional Training for Upper-Limb Stroke Rehabilitation: A Prospective Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(22), 15199. https://doi.org/10.3390/ijerph192215199








