Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
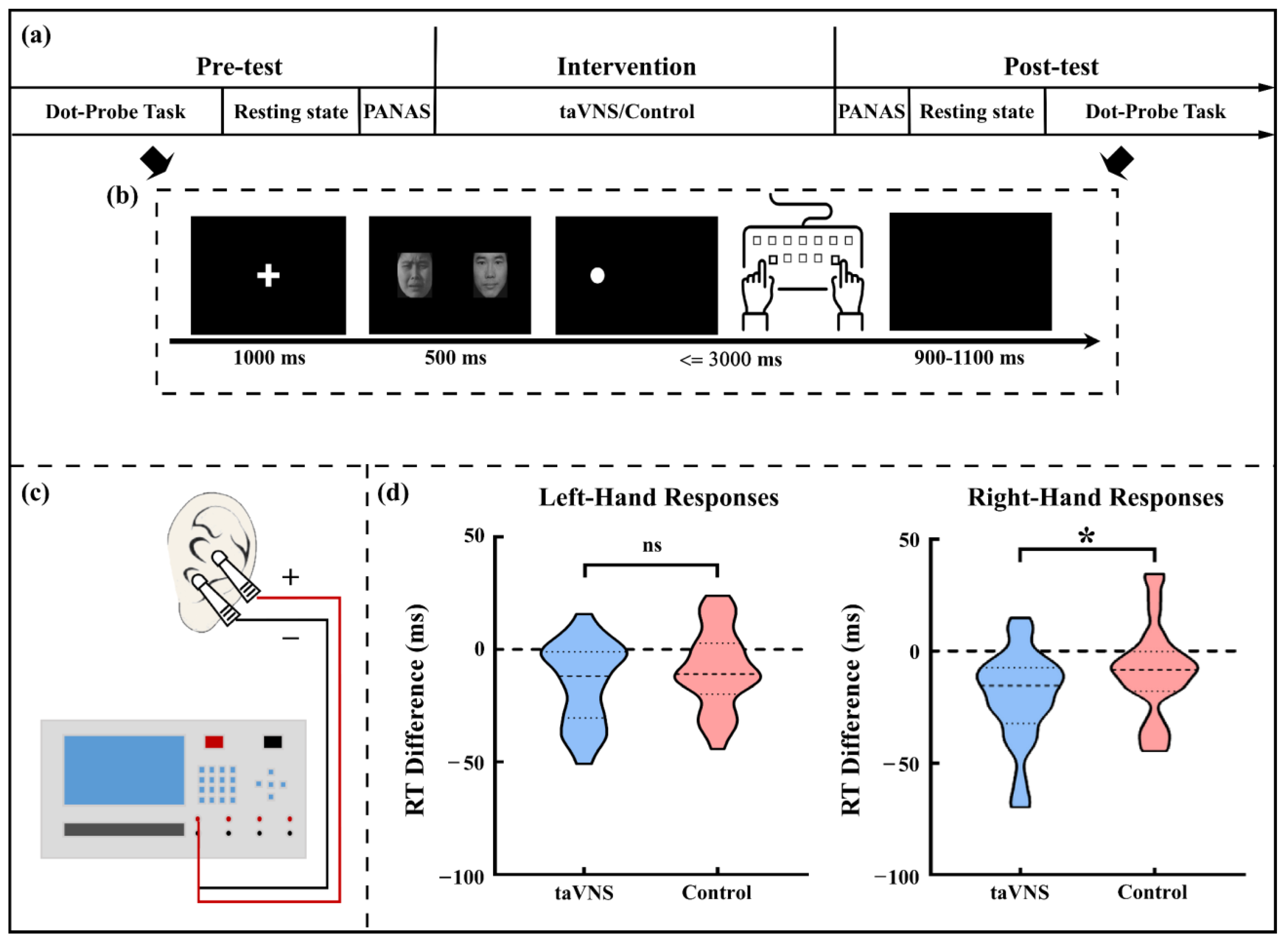
2.2. The Cue-Target Pattern Task
2.3. taVNS and Control Intervention
2.4. Experimental Procedure
2.5. EEG Recording
2.6. EEG Data Pre-Processing and Analyses
2.6.1. Resting State EEG Power Spectrum Analysis
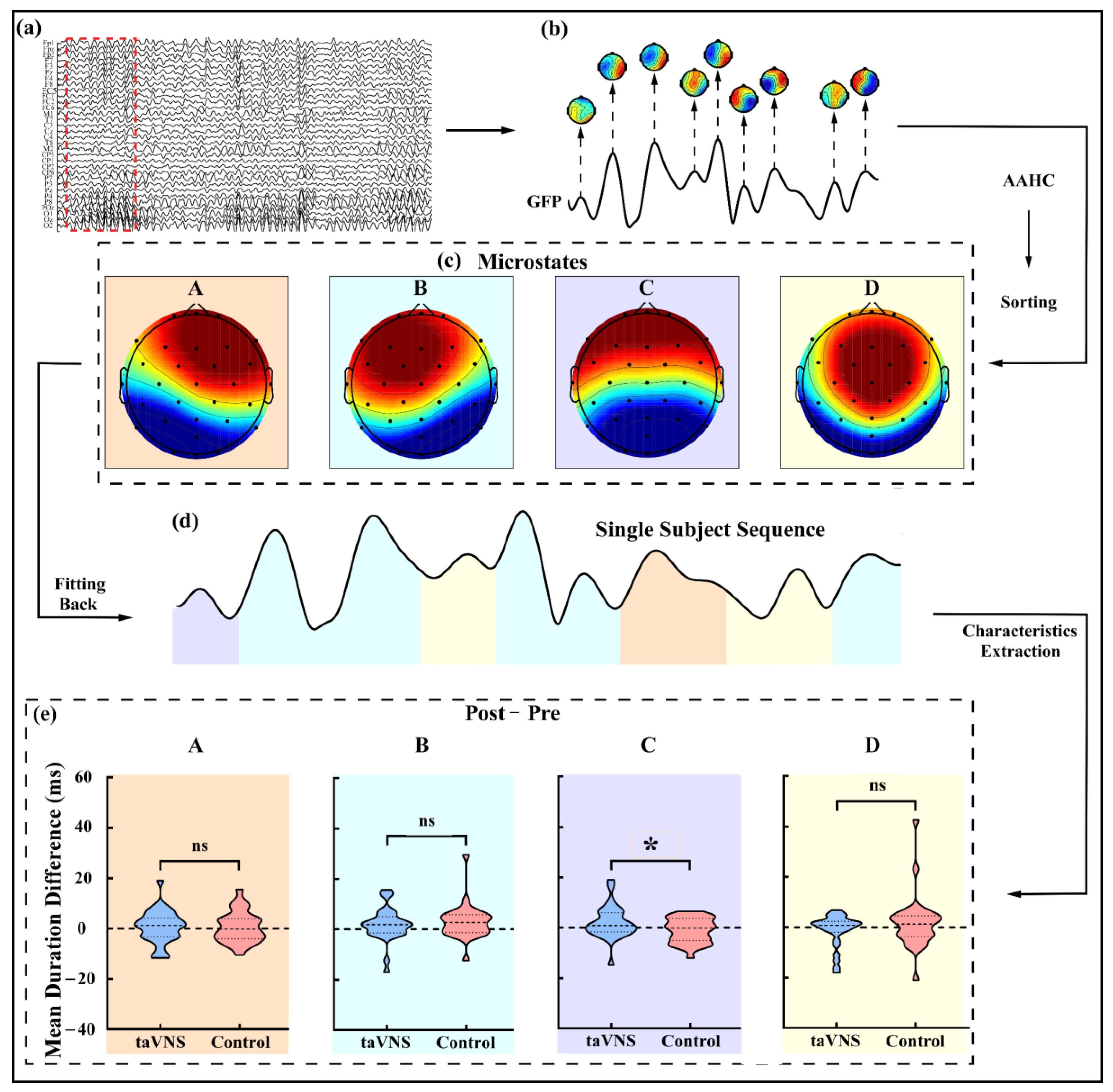
2.6.2. Microstate Analysis
2.7. Statistic Analyses
3. Results
3.1. taVNS Effects on the Affective Score
3.2. taVNS Effects on the RT
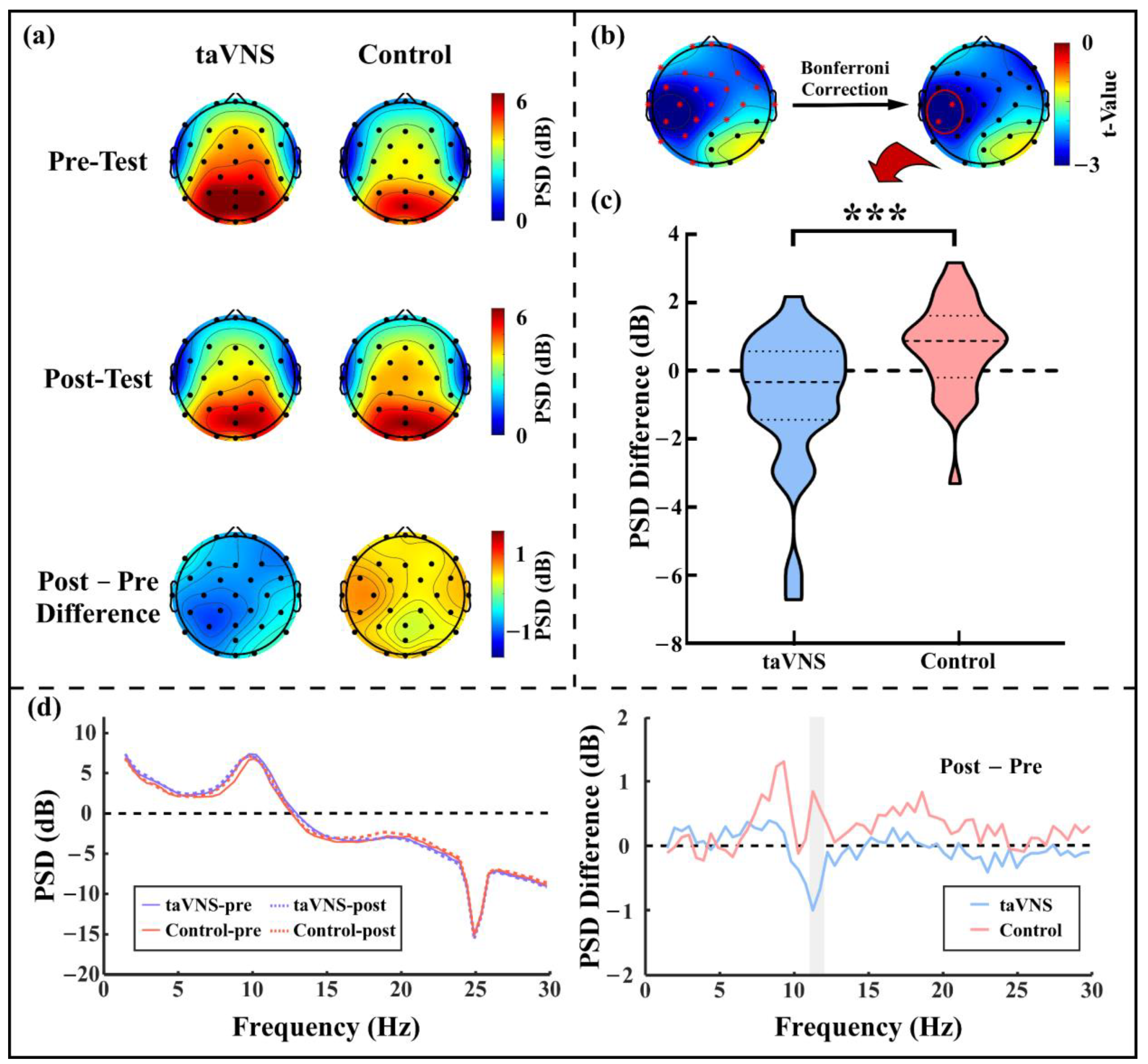
3.3. taVNS Effects on the Resting-State α Oscillations
3.4. taVNS Effects on the Microstates
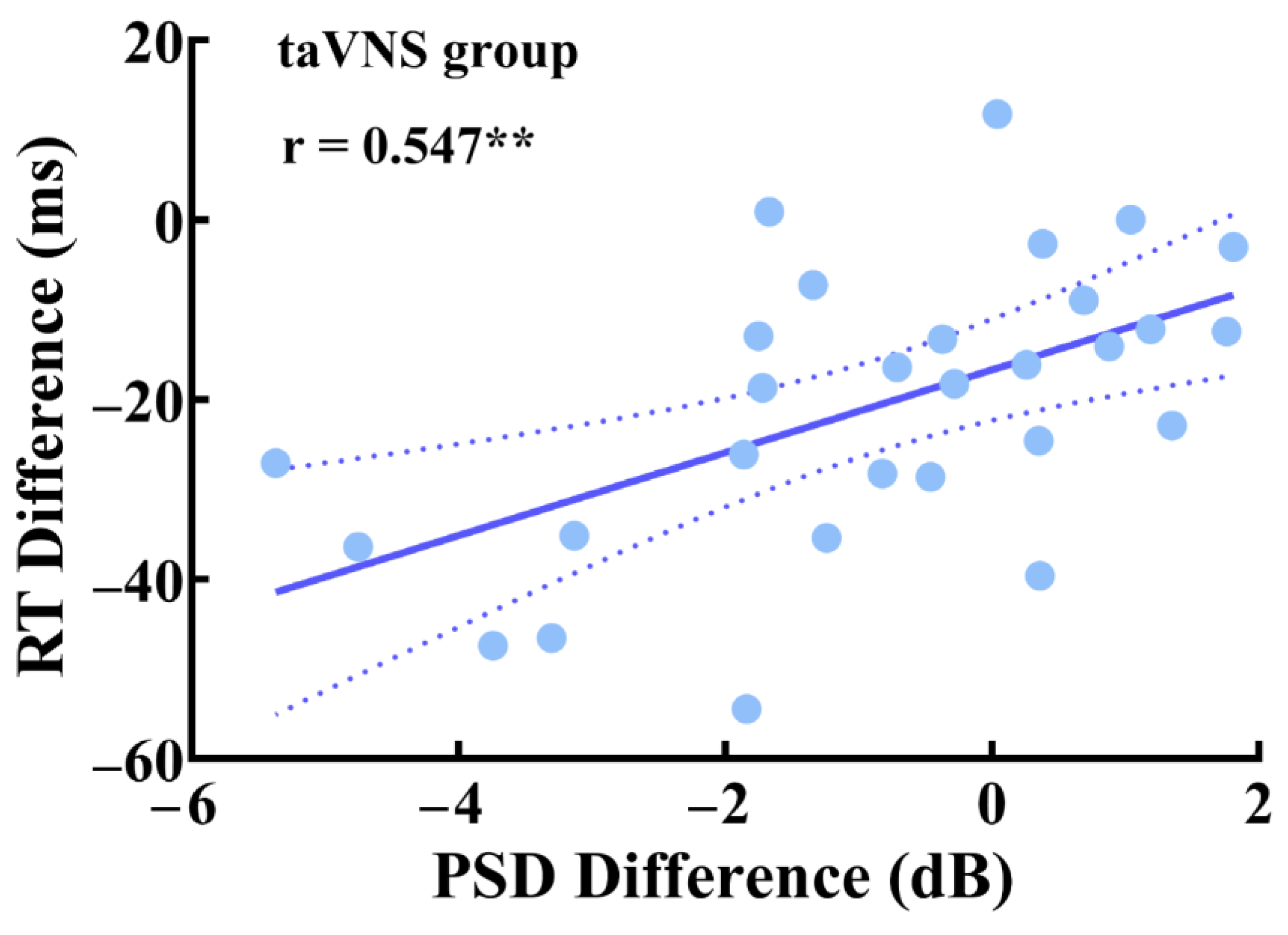
3.5. Parietal Correlations between Behavioral Performance and EEG Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oken, B.S.; Salinsky, M.C.; Elsas, S.M. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clin. Neurophysiol. 2006, 117, 1885–1901. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Dan, Y. Neuromodulation of brain states. Neuron 2012, 76, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.F.; Albusoda, A.; Farmer, A.D.; Aziz, Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 2020, 236, 588–611. [Google Scholar] [CrossRef] [PubMed]
- Sturm, W.; Willmes, K. On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage 2001, 14, S76–S84. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef]
- Yu, Y.T.; Yang, Y.; Wang, L.B.; Fang, J.L.; Chen, Y.Y.; He, J.H.; Rong, P.J. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI: The first case report. Brain Stimul. 2017, 10, 328–330. [Google Scholar] [CrossRef]
- Noe, E.; Ferri, J.; Colomer, C.; Moliner, B.; O’Valle, M.; Ugart, P.; Rodriguez, C.; Llorens, R. Feasibility, safety and efficacy of transauricular vagus nerve stimulation in a cohort of patients with disorders of consciousness. Brain Stimul. 2020, 13, 427–429. [Google Scholar] [CrossRef]
- Zaehle, T.; Krauel, K. Transcutaneous vagus nerve stimulation in patients with attention-deficit/hyperactivity disorder: A viable option? In Non-Invasive Brain Stimulation; Kadosh, R.C., Zaehle, T., Krauel, K., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2021; Volume 264, pp. 171–190. [Google Scholar]
- Kuo, T.B.; Chen, C.Y.; Hsu, Y.C.; Yang, C.C. EEG beta power and heart rate variability describe the association between cortical and autonomic arousals across sleep. Auton. Neurosci. Basic Clin. 2016, 194, 32–37. [Google Scholar] [CrossRef]
- McGinley, M.J.; Vinck, M.; Reimer, J.; Batista-Brito, R.; Zagha, E.; Cadwell, C.R.; Tolias, A.S.; Cardin, J.A.; McCormick, D.A. Waking state: Rapid variations modulate neural and behavioral responses. Neuron 2015, 87, 1143–1161. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous vagus nerve stimulation in humans induces pupil dilation and attenuates alpha oscillations. J. Neurosci. 2021, 41, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Lewine, J.D.; Paulson, K.; Bangera, N.; Simon, B.J. Exploration of the impact of brief noninvasive vagal nerve stimulation on EEG and event-related potentials. Neuromodulation 2019, 22, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Halasz, P.; Terzano, M.; Parrino, L.; Bodizs, R. The nature of arousal in sleep. J. Sleep Res. 2004, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Leopold, D.A.; Scholvinck, M.L.; Mandelkow, H.; Picchioni, D.; Liu, X.; Ye, F.Q.; Turchi, J.N.; Duyn, J.H. Tracking brain arousal fluctuations with fMRI. Proc. Natl. Acad. Sci. USA 2016, 113, 4518–4523. [Google Scholar] [CrossRef]
- Liu, X.; de Zwart, J.A.; Scholvinck, M.L.; Chang, C.T.; Ye, F.Q.; Leopold, D.A.; Duyn, J.H. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 2018, 9, 10. [Google Scholar] [CrossRef]
- Lerman, I.; Klaming, R.; Spadoni, A.; Baker, D.G.; Simmons, A.N. Non-invasive cervical vagus nerve stimulation effects on reaction time and valence image anticipation response. Brain Stimul. 2022, 15, 946–956. [Google Scholar] [CrossRef]
- Kreuzer, P.M.; Landgrebe, M.; Resch, M.; Husser, O.; Schecklmann, M.; Geisreiter, F.; Poeppl, T.B.; Prasser, S.J.; Hajak, G.; Rupprecht, R.; et al. Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: An open pilot study. Brain Stimul. 2014, 7, 740–747. [Google Scholar] [CrossRef]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical review of transcutaneous vagus nerve stimulation: Challenges for translation to clinical practice. Front. Neurosci. 2020, 14, 24. [Google Scholar] [CrossRef]
- Rong, P.; Liu, A.; Zhang, J.; Wang, Y.; He, W.; Yang, A.; Li, L.; Ben, H.; Li, L.; Liu, H.; et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: A randomized controlled trial. Clin. Sci. 2014. [Google Scholar] [CrossRef]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 2018, 180, 577–593. [Google Scholar] [CrossRef]
- Liu, Z.A.; Si, L.C.X.; Xu, W.W.; Zhang, K.X.; Wang, Q.; Chen, B.D.; Wang, G. Characteristics of EEG microstate sequences during propofol-induced alterations of brain consciousness states. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Britz, J.; Van De Ville, D.; Michel, C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage 2010, 52, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Assenza, G.; Di Pino, G.; Musumeci, G.; Ranieri, F.; Florio, L.; Barbato, C.; Di Lazzaro, V. The effect of transcutaneous vagus nerve stimulation on cortical excitability. J. Neural Transm. 2015, 122, 679–685. [Google Scholar] [CrossRef]
- Keute, M.; Ruhnau, P.; Heinze, H.J.; Zaehle, T. Behavioral and electrophysiological evidence for GABAergic modulation through transcutaneous vagus nerve stimulation. Clin. Neurophysiol. 2018, 129, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Maraver, M.J.; Steenbergen, L.; Hossein, R.; Actis-Grosso, R.; Ricciardelli, P.; Hommel, B.; Colzato, L.S. Transcutaneous vagus nerve stimulation modulates attentional resource deployment towards social cues. Neuropsychologia 2020, 143, 10. [Google Scholar] [CrossRef] [PubMed]
- Peuker, E.T.; Filler, T.J. The nerve supply of the human auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef]
- Hilz, M.J. Transcutaneous vagus nerve stimulation—A brief introduction and overview. Auton. Neurosci. Basic Clin. 2022, 243, 103038. [Google Scholar] [CrossRef]
- Fechner, G.T. Elementeder Psychophysik, 2nd ed.; Breitkopfand Härtel: Leipzig, Germany, 1889. [Google Scholar]
- Zhen, W.; ChengMei, Y.; Jia, H.; ZeZhi, L.I.; Jue, C.; HaiYin, Z.; YiRu, F.; ZePing, X. Reliability and validity of the Chinese version of Beck Depression Inventory-I among depression patients. Chin. Ment. Health J. 2011, 25, 476–479. [Google Scholar]
- Wenli, L.; Mingyi, Q. Revision of the State-Trait Anxiety Inventory with sample of Chinese college students. Acta Sci. Nat. Univ. Pekin. 1995, 31, 108–114. [Google Scholar] [CrossRef]
- Huang, L.; Yang, T.; Li, Z. Applicability of the positive and negative affect scale in Chinese. Chin. Ment. Health J. 2003, 17, 54–56. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Peng, W.W.; Valentini, E.; Zhang, Z.G.; Hu, Y. Functional features of nociceptive-induced suppression of alpha band electroencephalographic oscillations. J. Pain 2013, 14, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Tenke, C.E.; Deldin, P.J.; Trivedi, M.H.; Weissman, M.M.; Auerbach, R.P.; Bruder, G.E.; Pizzagalli, D.A.; Kayser, J. Frontal theta and posterior alpha in resting EEG: A critical examination of convergent and discriminant validity. Psychophysiology 2020, 57, 20. [Google Scholar] [CrossRef]
- Murray, M.M.; Brunet, D.; Michel, C.M. Topographic ERP analyses: A step-by-step tutorial review. Brain Topogr. 2008, 20, 249–264. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kavakbasi, E.; Gross, J.; Wollbrink, A.; Fellmeth, R.; Baune, B.T. Acute effect of vagus nerve stimulation (VNS) on brain function. J. Psychiatr. Res. 2021, 141, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Bodin, C.; Aubert, S.; Daquin, G.; Carron, R.; Scavarda, D.; McGonigal, A.; Bartolomei, F. Responders to vagus nerve stimulation (VNS) in refractory epilepsy have reduced interictal cortical synchronicity on scalp EEG. Epilepsy Res. 2015, 113, 98–103. [Google Scholar] [CrossRef]
- Castillo, G.; Gaitero, L.; Fonfara, S.; Czura, C.J.; Monteith, G.; James, F. Transcutaneous cervical vagus nerve stimulation induces changes in the electroencephalogram and heart rate variability of healthy dogs, a pilot study. Front. Vet. Sci. 2022, 9, 16. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A., Jr.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Haegens, S.; Nácher, V.; Luna, R.; Romo, R.; Jensen, O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl. Acad. Sci. USA 2011, 108, 19377–19382. [Google Scholar] [CrossRef]
- Sadaghiani, S.; Kleinschmidt, A. Brain networks and α-oscillations: Structural and functional foundations of cognitive control. Trends Cogn. Sci. 2016, 20, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, K.E.; Lleras, A.; Beck, D.M.; Fabiani, M.; Ro, T.; Gratton, G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front. Psychol. 2011, 2, 99. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Gerloff, C.; Hummel, F.C. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 2009, 47, 284–288. [Google Scholar] [CrossRef]
- Kraus, T.; Kiess, O.; Hösl, K.; Terekhin, P.; Kornhuber, J.; Forster, C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal—A pilot study. Brain Stimul. 2013, 6, 798–804. [Google Scholar] [CrossRef]
- Dietrich, S.; Smith, J.; Scherzinger, C.; Hofmann-Preiss, K.; Freitag, T.; Eisenkolb, A.; Ringler, R. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed. Tech. Biomed. Eng. 2008, 53, 104–111. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lu, X.J.; Hu, L. The modulatory effect of transcutaneous auricular vagus nerve stimulation (taVNS) on attentional processes. Hum. Brain Mapp. 2022. under review. [Google Scholar]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Croce, P.; Lanzone, J.; Boscarino, M.; Zappasodi, F.; Tombini, M.; Di Lazzaro, V.; Assenza, G. Transcutaneous vagus nerve stimulation modulates EEG microstates and delta activity in healthy subjects. Brain Sci. 2020, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Luo, F.; Zhang, L.; Han, R.; Wang, B. Variation of the default mode network with altered alertness levels induced by propofol. Neuropsychiatr. Dis. Treat. 2015, 11, 2573–2581. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Zhong, M.; Huang, H.; Weng, Y.; Niu, M.; Zhao, L.; Huang, R. Dynamic properties of human default mode network in eyes-closed and eyes-open. Brain Topogr. 2020, 33, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Coste, C.P.; Kleinschmidt, A. Cingulo-opercular network activity maintains alertness. NeuroImage 2016, 128, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Krylova, M.; Alizadeh, S.; Izyurov, I.; Teckentrup, V.; Chang, C.; van der Meer, J.; Erb, M.; Kroemer, N.; Koenig, T.; Walter, M.; et al. Evidence for modulation of EEG microstate sequence by vigilance level. NeuroImage 2021, 224, 117393. [Google Scholar] [CrossRef]
- Tomescu, M.I.; Rihs, T.A.; Rochas, V.; Hardmeier, M.; Britz, J.; Allali, G.; Fuhr, P.; Eliez, S.; Michel, C.M. From swing to cane: Sex differences of EEG resting-state temporal patterns during maturation and aging. Dev. Cogn. Neurosci. 2018, 31, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Milz, P.; Pascual-Marqui, R.D.; Achermann, P.; Kochi, K.; Faber, P.L. The EEG microstate topography is predominantly determined by intracortical sources in the alpha band. NeuroImage 2017, 162, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Kochi, K.; Kinoshita, T. On the relation between EEG microstates and cross-spectra. arXiv 2022, arXiv:2208.02540. [Google Scholar] [CrossRef]
- Croce, P.; Quercia, A.; Costa, S.; Zappasodi, F. EEG microstates associated with intra- and inter-subject alpha variability. Sci. Rep. 2020, 10, 2469. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.G.; Cannon, E.N.; Fox, N.A. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin. Neurophysiol. 2016, 127, 254–269. [Google Scholar] [CrossRef]
- Manshanden, I.; De Munck, J.C.; Simon, N.R.; da Silva, F.H.L. Source localization of MEG sleep spindles and the relation to sources of alpha band rhythms. Clin. Neurophysiol. 2002, 113, 1937–1947. [Google Scholar] [CrossRef]
- Briand, M.M.; Gosseries, O.; Staumont, B.; Laureys, S.; Thibaut, A. Transcutaneous auricular vagal nerve stimulation and disorders of consciousness: A hypothesis for mechanisms of action. Front. Neurol. 2020, 11, 933. [Google Scholar] [CrossRef]
- Manta, S.; El Mansari, M.; Debonnel, G.; Blier, P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int. J. Neuropsychopharmacol. 2013, 16, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, F.; Yue, L.; Zhang, H.; Peng, W.; Hu, L. Cross-species investigation on resting state electroencephalogram. Brain Topogr. 2019, 32, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.W.; Tang, Z.Y.; Zhang, F.R.; Li, H.; Kong, Y.Z.; Iannetti, G.D.; Hu, L. Neurobiological mechanisms of TENS-induced analgesia. NeuroImage 2019, 195, 396–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lu, X.; Hu, L. Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness. Int. J. Environ. Res. Public Health 2023, 20, 1402. https://doi.org/10.3390/ijerph20021402
Chen Y, Lu X, Hu L. Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness. International Journal of Environmental Research and Public Health. 2023; 20(2):1402. https://doi.org/10.3390/ijerph20021402
Chicago/Turabian StyleChen, Yuxin, Xuejing Lu, and Li Hu. 2023. "Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness" International Journal of Environmental Research and Public Health 20, no. 2: 1402. https://doi.org/10.3390/ijerph20021402
APA StyleChen, Y., Lu, X., & Hu, L. (2023). Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness. International Journal of Environmental Research and Public Health, 20(2), 1402. https://doi.org/10.3390/ijerph20021402





