Chronic Community Exposure to Environmental Metal Mixtures Is Associated with Selected Cytokines in the Navajo Birth Cohort Study (NBCS)
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Survey Information
2.3. Biological Sample Collection
2.4. Metal Biomonitoring
2.5. Serum Cytokines
2.6. Statistical Analysis
3. Results
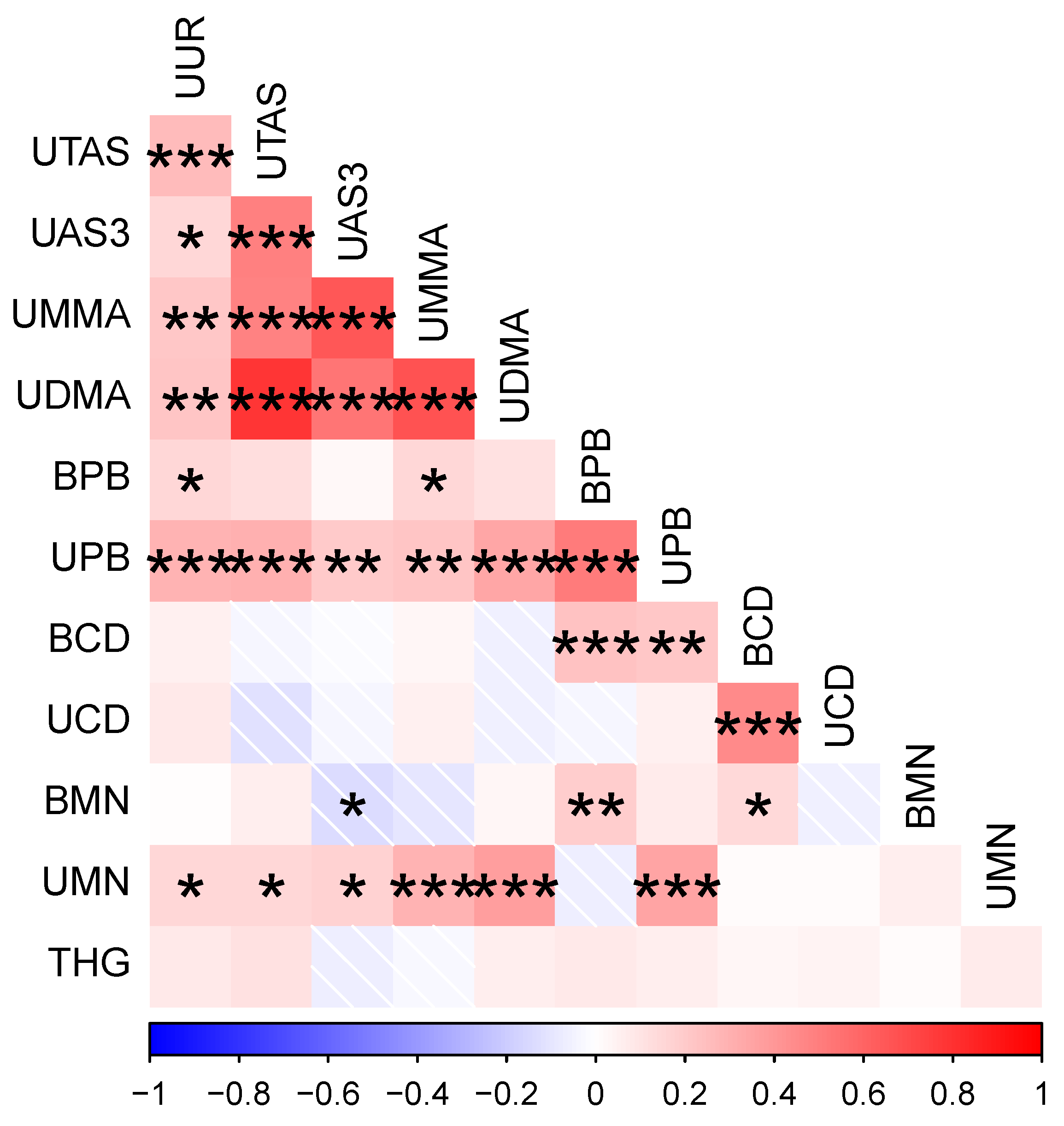
3.1. Participant Metal Concentrations and Their Correlations
3.2. Participant Cytokine Levels and Correlations with Metal Exposures
3.3. Multivariable Linear Regression Models
4. Discussion
4.1. Immunomodulatory Effects of Metals and Comparisons with Previous Literature
4.2. Implications for Health Outcomes
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, J.; Hoover, J.; MacKenzie, D. Mining and Environmental Health Disparities in Native American Communities. Curr. Environ. Health Rep. 2017, 4, 130–141. [Google Scholar] [CrossRef] [PubMed]
- NNDWR. Safe Drinking Water Hauling Feasibility Study and Pilot Project. In Proceedings of the Uranium Contamination Stakeholders Workshop, Window Rock, AZ, USA, 1 January 2020. [Google Scholar]
- Hoover, J.H.; Coker, E.; Barney, Y.; Shuey, C.; Lewis, J. Spatial Clustering of Metal and Metalloid Mixtures in Unregulated Water Sources on the Navajo Nation—Arizona, New Mexico, and Utah, USA. Sci. Total Environ. 2018, 633, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Marques, C.R.; Ferreira, M.J.S.; Neves, M.F.J.V.; Caetano, A.L.; Antunes, S.C.; Mendo, S.; Gonçalves, F. Phytotoxicity and Genotoxicity of Soils from an Abandoned Uranium Mine Area. Appl. Soil Ecol. 2009, 42, 209–220. [Google Scholar] [CrossRef]
- National Research Council. Potential Environmental Effects of Uranium Mining, Processing, and Reclamation. In Uranium Mining in Virginia: Scientific, Technical, Environmental, Human Health and Safety, and Regulatory Aspects of Uranium Mining and Processing in Virginia; National Academies Press: Washington, DC, USA, 19 December 2011. [Google Scholar]
- Samuel-Nakamura, C. Uranium and Other Heavy Metals in the Plant-Animal-Human Food Chain near Abandoned Mining Sites and Structures in an American Indian Community in Northwestern New Mexico. Ph.D. Thesis, UCLA, Los Angeles, CA, USA, 2013. [Google Scholar]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of Lead and Cadmium on the Immune System and Cancer Progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.L.; Hopkins, W.A.; Congdon, J.D. Ecotoxicological Implications of Aquatic Disposal of Coal Combustion Residues In the United States: A Review. Environ. Monit. Assess. 2002, 80, 207–276. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.; Gonzales, M.; Shuey, C.; Barney, Y.; Lewis, J. Elevated Arsenic and Uranium Concentrations in Unregulated Water Sources on the Navajo Nation, USA. Expo. Health 2017, 9, 113–124. [Google Scholar] [CrossRef]
- Cowan, E. Inspections Show Navajo Utility Had Years of Violations. Arizona Daily Sun, 9 December 2016. [Google Scholar]
- Kurttio, P.; Komulainen, H.; Leino, A.; Salonen, L.; Auvinen, A.; Saha, H. Bone as a Possible Target of Chemical Toxicity of Natural Uranium in Drinking Water. Environ. Health Perspect. 2005, 113, 68–72. [Google Scholar] [CrossRef]
- Erdei, E.; Shuey, C.; Pacheco, B.; Cajero, M.; Lewis, J.; Rubin, R.L. Elevated Autoimmunity in Residents Living near Abandoned Uranium Mine Sites on the Navajo Nation. J. Autoimmun. 2019, 99, 15–23. [Google Scholar] [CrossRef]
- Hund, L.; Bedrick, E.J.; Miller, C.; Huerta, G.; Nez, T.; Ramone, S.; Shuey, C.; Cajero, M.; Lewis, J. A Bayesian Framework for Estimating Disease Risk Due to Exposure to Uranium Mine and Mill Waste on the Navajo Nation. J. R. Stat. Soc. Ser. A Stat. Soc. 2015, 178, 1069–1091. [Google Scholar] [CrossRef]
- Prasad, P.; Sinha, D. Low-Level Arsenic Causes Chronic Inflammation and Suppresses Expression of Phagocytic Receptors. Environ. Sci. Pollut. Res. Int. 2017, 24, 11708–11721. [Google Scholar] [CrossRef]
- Corlin, L.; Rock, T.; Cordova, J.; Woodin, M.; Durant, J.L.; Gute, D.M.; Ingram, J.; Brugge, D. Health Effects and Environmental Justice Concerns of Exposure to Uranium in Drinking Water. Curr. Environ. Health Rep. 2016, 3, 434–442. [Google Scholar] [CrossRef] [PubMed]
- James, K.A.; Byers, T.; Hokanson, J.E.; Meliker, J.R.; Zerbe, G.O.; Marshall, J.A. Association between Lifetime Exposure to Inorganic Arsenic in Drinking Water and Coronary Heart Disease in Colorado Residents. Environ. Health Perspect. 2015, 123, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.L.; Tracy, B.L.; Zielinski, J.M.; Meyerhof, D.P.; Moss, M.A. Chronic Ingestion of Uranium in Drinking Water: A Study of Kidney Bioeffects in Humans. Toxicol. Sci. 1998, 43, 68–77. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Di Salvatore, V.; Falzone, L.; Libra, M. Immunological Effects of Occupational Exposure to Lead (Review). Mol. Med. Rep. 2017, 15, 3355–3360. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Padula, A.M.; Ma, C.; Huang, H.; Morello-Frosch, R.; Woodruff, T.J.; Carmichael, S.L. Drinking Water Contaminants in California and Hypertensive Disorders in Pregnancy. Environ. Epidemiol. 2021, 5, e149. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Noble, B.N.; Joya, S.A.; Ibn Hasan, M.O.S.; Lin, P.-I.; Rahman, M.L.; Mostofa, G.; Quamruzzaman, Q.; Rahman, M.; Christiani, D.C.; et al. A Prospective Cohort Study Examining the Associations of Maternal Arsenic Exposure With Fetal Loss and Neonatal Mortality. Am. J. Epidemiol. 2019, 188, 347–354. [Google Scholar] [CrossRef]
- Quansah, R.; Armah, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Nketiah-Amponsah, E.; Namujju, P.B.; Obiri, S.; et al. Association of Arsenic with Adverse Pregnancy Outcomes/Infant Mortality: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 412–421. [Google Scholar] [CrossRef]
- Brugge, D.; Buchner, V. Health Effects of Uranium: New Research Findings. Rev. Environ. Health 2011, 26, 231–249. [Google Scholar] [CrossRef]
- Dangleben, N.L.; Skibola, C.F.; Smith, M.T. Arsenic Immunotoxicity: A Review. Environ. Health 2013, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, K.A.; Sillé, F.C.M. Environmental Exposures during Pregnancy: Mechanistic Effects on Immunity. Birth. Defects Res. 2019, 111, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Chapter Seven—Metalloimmunology: The Metal Ion-Controlled Immunity. In Advances in Immunology; Dong, C., Jiang, Z., Eds.; Advances in Immunology in China—Part B; Academic Press: Cambridge, MA, USA, 2020; Volume 145, pp. 187–241. [Google Scholar]
- Bigazzi, P.E. Metals and Kidney Autoimmunity. Environ. Health Perspect. 1999, 107, 753–765. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of Chromium on the Immune System. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.H.; Sarkar, S.N.; Ram, G.C.; Tripathi, H.C. Immunosuppressive Effect of Subchronic Exposure to a Mixture of Eight Heavy Metals, Found as Groundwater Contaminants in Different Areas of India, through Drinking Water in Male Rats. Arch. Environ. Contam. Toxicol. 2007, 53, 450–458. [Google Scholar] [CrossRef]
- Ferrario, D.; Gribaldo, L.; Hartung, T. Arsenic Exposure and Immunotoxicity: A Review Including the Possible Influence of Age and Sex. Curr. Environ. Health Rep. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Marth, E.; Jelovcan, S.; Kleinhappl, B.; Gutschi, A.; Barth, S. The Effect of Heavy Metals on the Immune System at Low Concentrations. Int. J. Occup. Med. Environ. Health 2001, 14, 375–386. [Google Scholar]
- Dublineau, I.; Souidi, M.; Gueguen, Y.; Lestaevel, P.; Bertho, J.M.; Manens, L.; Delissen, O.; Grison, S.; Paulard, A.; Monin, A.; et al. Unexpected Lack of Deleterious Effects of Uranium on Physiological Systems Following a Chronic Oral Intake in Adult Rat. BioMed Res. Int. 2014, 2014, 181989. [Google Scholar] [CrossRef]
- Gera, R.; Singh, V.; Mitra, S.; Sharma, A.K.; Singh, A.; Dasgupta, A.; Singh, D.; Kumar, M.; Jagdale, P.; Patnaik, S.; et al. Arsenic Exposure Impels CD4 Commitment in Thymus and Suppress T Cell Cytokine Secretion by Increasing Regulatory T Cells. Sci. Rep. 2017, 7, 7140. [Google Scholar] [CrossRef]
- Medina, S.; Lauer, F.T.; Castillo, E.F.; Bolt, A.M.; Ali, A.-M.S.; Liu, K.J.; Burchiel, S.W. Exposures to Uranium and Arsenic Alter Intraepithelial and Innate Immune Cells in the Small Intestine of Male and Female Mice. Toxicol. Appl. Pharmacol. 2020, 403, 115155. [Google Scholar] [CrossRef]
- Assad, N.; Sood, A.; Campen, M.J.; Zychowski, K.E. Metal-Induced Pulmonary Fibrosis. Curr. Environ. Health Rep. 2018, 5, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A.; McCabe, M.J.J. Immunomodulation by Metals. Int. Immunopharmacol. 2002, 2, 293–302. [Google Scholar] [CrossRef]
- Gonzales, M.; Erdei, E.; Hoover, J.; Nash, J. A Review of Environmental Epidemiology Studies in Southwestern and Mountain West Rural Minority Populations. Curr. Epidemiol. Rep. 2018, 5, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.; Erdei, E.; Nash, J.; Gonzales, M. A Review of Metal Exposure Studies Conducted in the Rural Southwestern and Mountain West Region of the United States. Curr. Epidemiol. Rep. 2019, 6, 34–49. [Google Scholar] [CrossRef]
- Guéguen, Y.; Roy, L.; Hornhardt, S.; Badie, C.; Hall, J.; Baatout, S.; Pernot, E.; Tomasek, L.; Laurent, O.; Ebrahimian, T.; et al. Biomarkers for Uranium Risk Assessment for the Development of the CURE (Concerted Uranium Research in Europe) Molecular Epidemiological Protocol. Radiat. Res. 2017, 187, 107–127. [Google Scholar] [CrossRef]
- Bauman, M.D.; Van de Water, J. Translational Opportunities in the Prenatal Immune Environment: Promises and Limitations of the Maternal Immune Activation Model. Neurobiol. Dis. 2020, 141, 104864. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A Conceptual Framework for the Developmental Origins of Health and Disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef]
- Watt, J.P.; O’Brien, K.L.; Benin, A.L.; Whitney, C.G.; Robinson, K.; Parkinson, A.J.; Reid, R.; Santosham, M. Invasive Pneumococcal Disease among Navajo Adults, 1989–1998. Clin. Infect. Dis. 2004, 38, 496–501. [Google Scholar] [CrossRef]
- Hochman, M.E.; Watt, J.P.; Reid, R.; O’Brien, K.L. The Prevalence and Incidence of End-Stage Renal Disease in Native American Adults on the Navajo Reservation. Kidney Int. 2007, 71, 931–937. [Google Scholar] [CrossRef][Green Version]
- Harmon, M.E.; Campen, M.J.; Miller, C.; Shuey, C.; Cajero, M.; Lucas, S.; Pacheco, B.; Erdei, E.; Ramone, S.; Nez, T.; et al. Associations of Circulating Oxidized LDL and Conventional Biomarkers of Cardiovascular Disease in a Cross-Sectional Study of the Navajo Population. PLoS ONE 2016, 11, e0143102. [Google Scholar] [CrossRef]
- Hutchinson, R.N.; Shin, S. Systematic Review of Health Disparities for Cardiovascular Diseases and Associated Factors among American Indian and Alaska Native Populations. PLoS ONE 2014, 9, e80973. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, C.; Ferucci, E.D.; Lanier, A.P.; Slattery, M.L.; Schraer, C.D.; Raymer, T.W.; Dillard, D.; Murtaugh, M.A.; Tom-Orme, L. Metabolic Syndrome: Prevalence among American Indian and Alaska Native People Living in the Southwestern United States and in Alaska. Metab. Syndr. Relat. Disord. 2008, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.C.M.; Lewis, J.; Peter, D.; Begay, M.-G.; Ragin-Wilson, A. The Navajo Birth Cohort Study. J. Environ. Health 2015, 78, 4. [Google Scholar]
- Hoover, J.H.; Erdei, E.; Begay, D.; Gonzales, M.; Jarrett, J.M.; Cheng, P.-Y.; Lewis, J. Exposure to Uranium and Co-Occurring Metals among Pregnant Navajo Women. Environ. Res. 2020, 190, 109943. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, J.; Jones, R.; Caldwell, K.; Verdon, C. Total Urine Arsenic Measurements Using Inductively Coupled Plasma Mass Spectrometry with a Dynamic Reaction Cell. At. Spectrosc. 2007, 28, 113–122. [Google Scholar]
- Caldwell, K.; Hartel, J.; Jarrett, J.; Jones, R. Inductively Coupled Plasma Mass Spectrometry to Measure Multiple Toxic Elements in Urine in NHANES 1999–2000. At. Spectrosc. 2005, 26, 1–7. [Google Scholar]
- CDC. Urine Multi-Element ICP-DRC-MS Laboratory Procedure Manual DLS 3018.6-02 & 3018A.4-02 2014. 11 November. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/UTAS_UTASS_UM_UMS_I_MET.PDF (accessed on 9 November 2022).
- CDC. Arsenic, Chromium, and Nickel in Urine by ICP-MS Manual DLS 3031.1-01 2018. 11 November. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/UTAS-J-UCM-J-UNI-J-MET-508.pdf (accessed on 9 November 2022).
- CDC. Blood Multi-Element Analysis for Cadmium, Lead, Manganese, Mercury, and Selenium by ICP-DRC-MS Laboratory Procedure Manual DLS 3016.8-05 2019. 11 November. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2019-2020/labmethods/PBCD-K-PBY-K-R-MET-508.pdf (accessed on 9 November 2022).
- Adams, S.V.; Newcomb, P.A. Cadmium Blood and Urine Concentrations as Measures of Exposure: NHANES 1999–2010. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 163–170. [Google Scholar] [CrossRef]
- Flora, S.J.S. Chapter 29—Metals. In Biomarkers in Toxicology; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 485–519. ISBN 978-0-12-404630-6. [Google Scholar]
- Gulson, B.L.; Cameron, M.A.; Smith, A.J.; Mizon, K.J.; Korsch, M.J.; Vimpani, G.; McMichael, A.J.; Pisaniello, D.; Jameson, C.W.; Mahaffey, K.R. Blood Lead–Urine Lead Relationships in Adults and Children. Environ. Res. 1998, 78, 152–160. [Google Scholar] [CrossRef]
- Caudill, S.P.; Schleicher, R.L.; Pirkle, J.L. Multi-Rule Quality Control for the Age-Related Eye Disease Study. Stat. Med. 2008, 27, 4094–4106. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, P.H.; Lee, W.H.; Hirani, A.A. Interleukin-4, Oxidative Stress, Vascular Inflammation and Atherosclerosis. Biomol. Ther. 2010, 18, 135–144. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.F.; Pietrani, N.T.; Bosco, A.A.; Campos, F.M.F.; Sandrim, V.C.; Gomes, K.B.; Rodrigues, K.F.; Pietrani, N.T.; Bosco, A.A.; Campos, F.M.F.; et al. IL-6, TNF-α, and IL-10 Levels/Polymorphisms and Their Association with Type 2 Diabetes Mellitus and Obesity in Brazilian Individuals. Arch. Endocrinol. Metab. 2017, 61, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Huang, A.-F.; Xu, W.-D.; Su, L.-C. Insights into IL-29: Emerging Role in Inflammatory Autoimmune Diseases. J. Cell. Mol. Med. 2019, 23, 7926–7932. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Erdei, E.; Rubin, R.L.; Miller, C.; Ducheneaux, C.; O’Leary, M.; Pacheco, B.; Mahler, M.; Henderson, P.N.; Pollard, K.M.; et al. Mercury, Autoimmunity, and Environmental Factors on Cheyenne River Sioux Tribal Lands. Autoimmune Dis. 2014, 2014, 325461. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Burchiel, S.W. Toxicity of Environmentally-Relevant Concentrations of Arsenic on Developing T Lymphocyte. Environ. Toxicol. Pharmacol. 2018, 62, 107–113. [Google Scholar] [CrossRef]
- Ezeh, P.C.; Xu, H.; Lauer, F.T.; Liu, K.J.; Hudson, L.G.; Burchiel, S.W. Monomethylarsonous Acid (MMA+3) Inhibits IL-7 Signaling in Mouse Pre-B Cells. Toxicol. Sci. 2016, 149, 289–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindberg, A.-L.; Rahman, M.; Persson, L.-Å.; Vahter, M. The Risk of Arsenic Induced Skin Lesions in Bangladeshi Men and Women Is Affected by Arsenic Metabolism and the Age at First Exposure. Toxicol. Appl. Pharmacol. 2008, 230, 9–16. [Google Scholar] [CrossRef]
- Dashner-Titus, E.J.; Schilz, J.R.; Simmons, K.A.; Duncan, T.R.; Alvarez, S.C.; Hudson, L.G. Differential Response of Human T-Lymphocytes to Arsenic and Uranium. Toxicol. Lett. 2020, 333, 269–278. [Google Scholar] [CrossRef]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef]
- Dobrakowski, M.; Boroń, M.; Czuba, Z.P.; Kasperczyk, A.; Machoń-Grecka, A.; Kasperczyk, S. Cytokines Related to Three Major Types of Cell-Mediated Immunity in Short- and Long-Term Exposures to Lead Compounds. J. Immunotoxicol. 2016, 13, 770–774. [Google Scholar] [CrossRef]
- Marth, E.; Barth, S.; Jelovcan, S. Influence of Cadmium on the Immune System. Description of Stimulating Reactions. Cent. Eur. J. Public Health 2000, 8, 40–44. [Google Scholar] [PubMed]
- Aung, M.T.; Meeker, J.D.; Boss, J.; Bakulski, K.M.; Mukherjee, B.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Manganese Is Associated with Increased Plasma Interleukin-1β during Pregnancy, within a Mixtures Analysis Framework of Urinary Trace Metals. Reprod. Toxicol. 2020, 93, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; King, B.S.; Sandoval, M.M.; Liu, K.J.; Hudson, L.G. Reduction of Arsenite-Enhanced Ultraviolet Radiation-Induced DNA Damage by Supplemental Zinc. Toxicol. Appl. Pharmacol. 2013, 269, 81–88. [Google Scholar] [CrossRef] [PubMed]
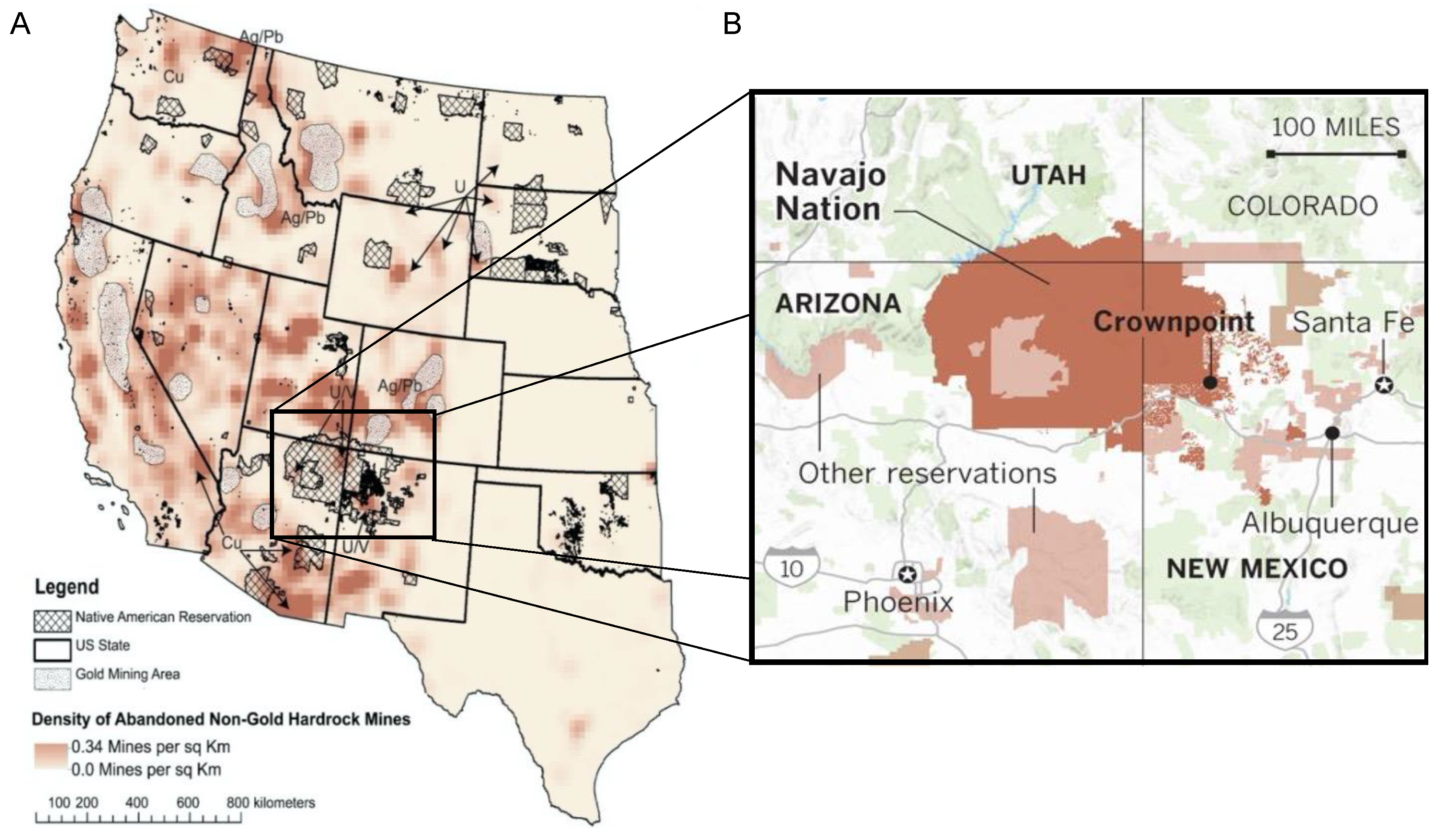

| Variable | ||
|---|---|---|
| Maternal age (years) | Mean (SD): | 27 (6.2) |
| Category | Result: n (%) * | |
| Education level | No high school diploma | 97 (57.1) |
| High school diploma | 73 (42.9) | |
| Household income | <$20,000/year | 54 (39.4) |
| >$20,000/year | 83 (60.6) | |
| Marital status | Married or living with a partner | 112 (78.9) |
| Not married or living with a partner | 30 (21.1) | |
| Pre-pregnancy BMI | Underweight or normal | 52 (26.4) |
| Overweight | 70 (35.5) | |
| Obese | 75 (38.1) | |
| Trimester at sample collection | 1st | 34 (14.7) |
| 2nd | 97 (42) | |
| 3rd | 100 (43.3) | |
| Sex of child | Female | 107 (49.3) |
| Male | 110 (50.7) |
| Abbr. | Metal/Metabolite | Matrix | Units | n | LoD | Mean (SD) | Median (IQR) | NHANES Median (95th%Tile) | Factor Navajo vs. NHANES * |
|---|---|---|---|---|---|---|---|---|---|
| UUR | Uranium | Urine | µg/dL | 216 | 0.002 | 0.03 (0.13) | 0.02 (0.01–0.03) | 0.005 (0.031) | 4× |
| UTAS | Total arsenic | Urine | µg/dL | 214 | 0.26 | 7.15 (6.12) | 5.56 (4.15–8.08) | 5.74 (49.9) | 0.97 |
| UAS3 | Arsenite (AsIII) | Urine | µg/L | 213 | 0.12 | 0.48 (0.39) | 0.38 (0.19–0.62) | 0.12 (1.11) | 3.17× |
| UMMA | Monomethylarsonic acid | Urine | µg/L | 213 | 0.2 | 0.52 (0.45) | 0.38 (0.21–0.68) | 0.28 (1.45) | 1.36× |
| UDMA | Dimethylarsinic acid | Urine | µg/L | 213 | 1.91 | 5.29 (3.61) | 4.25 (3.07–6.52) | 2.95 (12) | 1.44× |
| BPB | Lead | Blood | µg/dL | 212 | 0.07 | 0.41 (0.4) | 0.32 (0.25–0.42) | 0.88 (2.89) | 0.36 |
| UPB | Lead | Urine | µg/L | 216 | 0.03 | 0.37 (0.56) | 0.28 (0.19–0.39) | 0.32 (1.38) | 0.88 |
| BCD | Cadmium | Blood | µg/L | 212 | 0.1 | 0.34 (0.17) | 0.31 (0.24–0.4) | 0.27 (1.35) | 1.15× |
| UCD | Cadmium | Urine | µg/L | 216 | 0.036 | 0.25 (0.18) | 0.22 (0.14–0.32) | 0.179 (1.08) | 1.23× |
| BMN | Manganese | Blood | µg/L | 212 | 0.99 | 20.07 (6.82) | 19 (15–24.64) | 9.2 (16.1) | 2.07× |
| UMN | Manganese | Urine | µg/L | 215 | 0.13 | 0.37 (0.45) | 0.23 (0.15–0.41) | 0.13 (0.28) | 1.77× |
| THG | Total mercury | Blood | µg/L | 212 | 0.28 | 0.42 (0.27) | 0.34 (0.2–0.51) | 0.74 (4.66) | 0.46 |
| Cytokine | Metal | Rho | p | n |
|---|---|---|---|---|
| IL-4 | UAS3 | −0.34 | 0 | 211 |
| UMMA | −0.23 | 0.001 | 211 | |
| BMN | 0.15 | 0.033 | 209 | |
| IL-6 | UAS3 | −0.15 | 0.032 | 211 |
| BMN | 0.18 | 0.009 | 209 | |
| IL-10 | BPB | 0.15 | 0.032 | 209 |
| UCD | −0.15 | 0.028 | 214 | |
| IL-12 | UAS3 | −0.14 | 0.047 | 211 |
| BMN | 0.16 | 0.023 | 209 | |
| IL-29 | UAS3 | −0.19 | 0.006 | 203 |
| IFNα | UDMA | 0.18 | 0.011 | 203 |
| IFNγ | UCD | 0.14 | 0.035 | 214 |
| TNFα | UAS3 | −0.15 | 0.035 | 211 |
| UPB | −0.16 | 0.017 | 214 | |
| BMN | 0.16 | 0.023 | 209 | |
| CRP | UTAS | −0.18 | 0.009 | 204 |
| UAS3 | −0.16 | 0.019 | 203 | |
| UMMA | −0.17 | 0.018 | 203 | |
| UDMA | −0.16 | 0.021 | 203 |
| Cytokine | n | Predictors Retained in Multivariate Model | Estimate | Std Error | p |
|---|---|---|---|---|---|
| IL-4 | 203 | age | 0.013 | 0.008 | 0.114 |
| log(UAS3) * | −0.228 | 0.058 | 0 | ||
| log(BCD) | −0.261 | 0.113 | 0.022 | ||
| log(BMN) | 0.31 | 0.156 | 0.049 | ||
| IL-6 | 203 | log(UAS3) | −0.125 | 0.065 | 0.054 |
| log(BMN) | 0.329 | 0.172 | 0.057 | ||
| IL-7 | 203 | log(BCD) | 0.1 | 0.061 | 0.103 |
| IL-10 | 203 | age | −0.02 | 0.008 | 0.017 |
| log(UDMA) | −0.183 | 0.098 | 0.064 | ||
| log(UPB) | −0.176 | 0.12 | 0.143 | ||
| log(BPB) | 0.422 | 0.134 | 0.002 | ||
| log(UMN) | 0.139 | 0.075 | 0.066 | ||
| IL-12 | 203 | log(UTAS) | 0.17 | 0.107 | 0.112 |
| log(UAS3) | −0.113 | 0.069 | 0.103 | ||
| log(BCD) | −0.248 | 0.132 | 0.062 | ||
| log(UCD) | 0.142 | 0.088 | 0.107 | ||
| log(BMN) | 0.386 | 0.17 | 0.024 | ||
| IL-17 | 203 | log(BPB) | 0.3 | 0.128 | 0.02 |
| log(BCD) | −0.266 | 0.144 | 0.066 | ||
| IL-29 | 196 | log(UAS3) | −0.552 | 0.181 | 0.003 |
| log(UPB) | 0.545 | 0.258 | 0.036 | ||
| log(BCD) | −0.996 | 0.392 | 0.012 | ||
| log(UCD) | 0.505 | 0.253 | 0.047 | ||
| log(BMN) | 0.778 | 0.478 | 0.105 | ||
| IFNα | 196 | log(UTAS) | −0.51 | 0.305 | 0.096 |
| log(UDMA) | 0.979 | 0.316 | 0.002 | ||
| log(UMN) | −0.221 | 0.157 | 0.16 | ||
| log(THG) | 0.284 | 0.199 | 0.155 | ||
| IFNγ | 203 | trim.L | −0.248 | 0.112 | 0.028 |
| trim.Q | −0.05 | 0.094 | 0.595 | ||
| log(UUR) | −0.096 | 0.062 | 0.123 | ||
| log(BPB) | 0.255 | 0.105 | 0.017 | ||
| log(THG) | 0.173 | 0.094 | 0.066 | ||
| TNFα | 203 | age | −0.007 | 0.005 | 0.164 |
| log(UPB) | −0.183 | 0.06 | 0.003 | ||
| log(BPB) | 0.176 | 0.074 | 0.017 | ||
| log(BCD) | −0.147 | 0.071 | 0.04 | ||
| log(UCD) | 0.152 | 0.053 | 0.004 | ||
| log(BMN) | 0.186 | 0.089 | 0.037 | ||
| CRP | 171 | log(UTAS) | −0.809 | 0.24 | 0.001 |
| log(UMMA) | −0.324 | 0.162 | 0.047 | ||
| log(UDMA) | 0.667 | 0.289 | 0.022 | ||
| log(BPB) | 0.273 | 0.171 | 0.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson González, N.; Ong, J.; Luo, L.; MacKenzie, D. Chronic Community Exposure to Environmental Metal Mixtures Is Associated with Selected Cytokines in the Navajo Birth Cohort Study (NBCS). Int. J. Environ. Res. Public Health 2022, 19, 14939. https://doi.org/10.3390/ijerph192214939
Thompson González N, Ong J, Luo L, MacKenzie D. Chronic Community Exposure to Environmental Metal Mixtures Is Associated with Selected Cytokines in the Navajo Birth Cohort Study (NBCS). International Journal of Environmental Research and Public Health. 2022; 19(22):14939. https://doi.org/10.3390/ijerph192214939
Chicago/Turabian StyleThompson González, Nicole, Jennifer Ong, Li Luo, and Debra MacKenzie. 2022. "Chronic Community Exposure to Environmental Metal Mixtures Is Associated with Selected Cytokines in the Navajo Birth Cohort Study (NBCS)" International Journal of Environmental Research and Public Health 19, no. 22: 14939. https://doi.org/10.3390/ijerph192214939
APA StyleThompson González, N., Ong, J., Luo, L., & MacKenzie, D. (2022). Chronic Community Exposure to Environmental Metal Mixtures Is Associated with Selected Cytokines in the Navajo Birth Cohort Study (NBCS). International Journal of Environmental Research and Public Health, 19(22), 14939. https://doi.org/10.3390/ijerph192214939






