Hygiene of Medical Devices and Minimum Inhibitory Concentrations for Alcohol-Based and QAC Disinfectants among Isolates from Physical Therapy Departments
Abstract
1. Introduction
2. Materials and Methods
- (i)
- Incidin Liquid (Ecolab, Maribor, Slovenia): 35 g propan-2-ol and 25 g propan-1-ol in 100 g solution;
- (ii)
- Sani-Cloth 70% (PDI): 70 mL isopropyl alcohol in 100 mL solution;
- (iii)
- Descosept Sensitive Wipes (Dr. Schumacher): 45 g ethanol in 100 g solution;
- (iv)
- Mikrozid Sensitive Liquid (Schülke & Mayr, Norderstedt, Germany): 0.25 g alkyl dimethylbenzyl ammonium chloride, 0.25 g didecyldimethylammonium chloride, and 0.25 g alkyl ethylbenzylammonium chloride in 100 g solution;
- (v)
- L + R Surface Disinfect Universal (Lohmann & Rauscher, Rengsdorf, Germany): 0.4 g alkyl dimethylbenzyl ammonium chloride and 0.4 g didecyldimethyl ammonium chloride in 100 g solution.
3. Results
3.1. Hygiene of Devices in the Physiotherapy Department
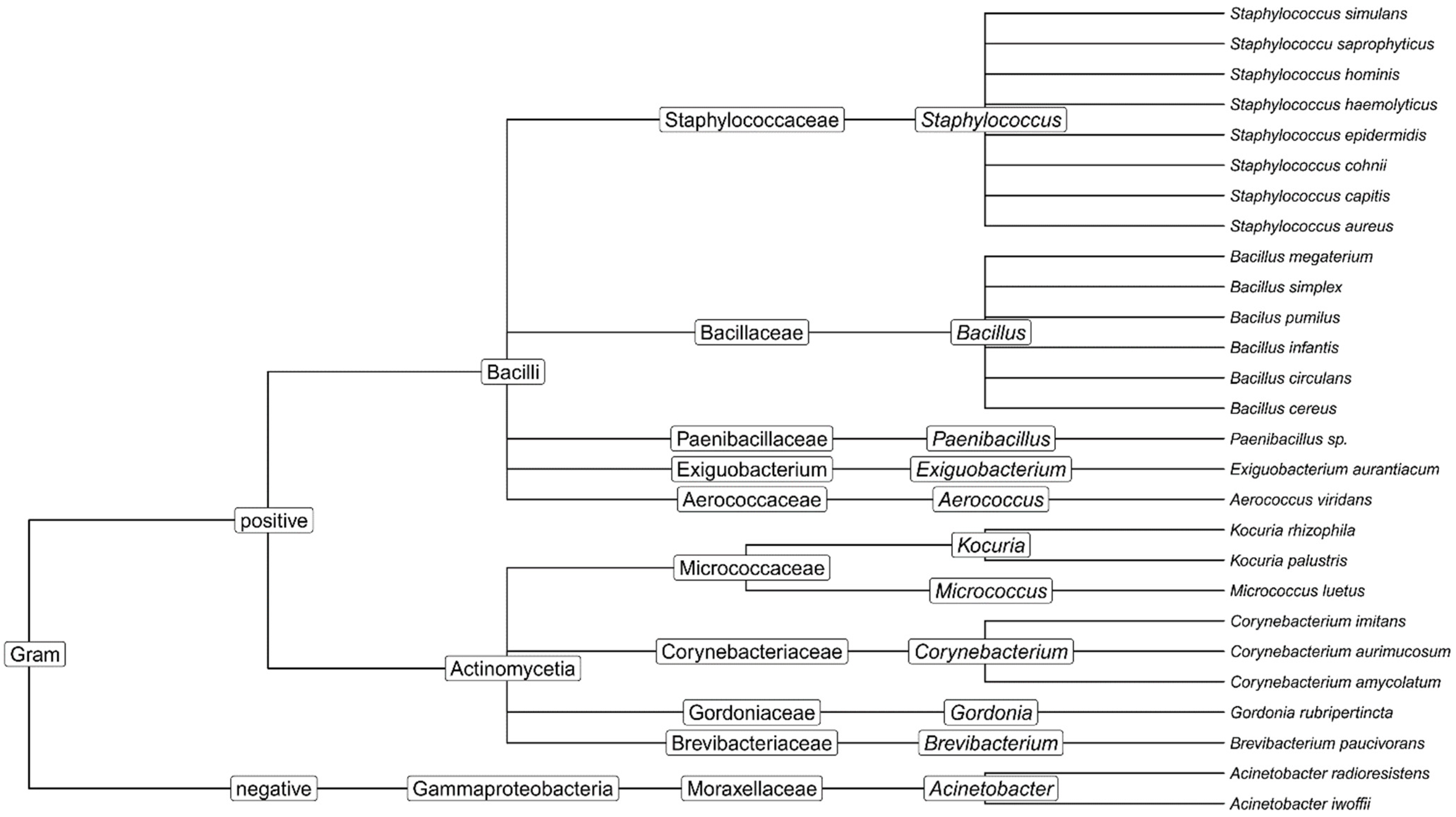
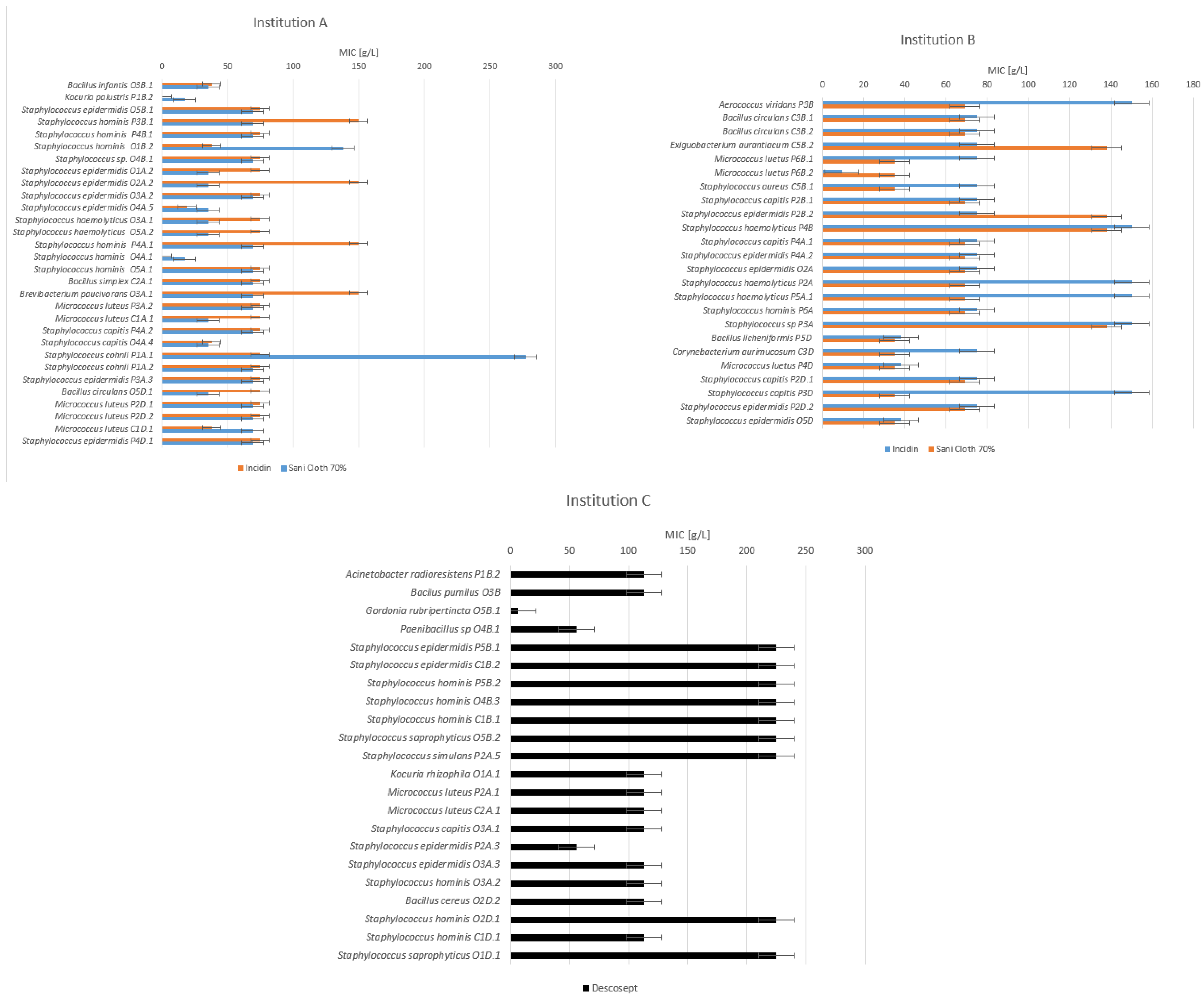
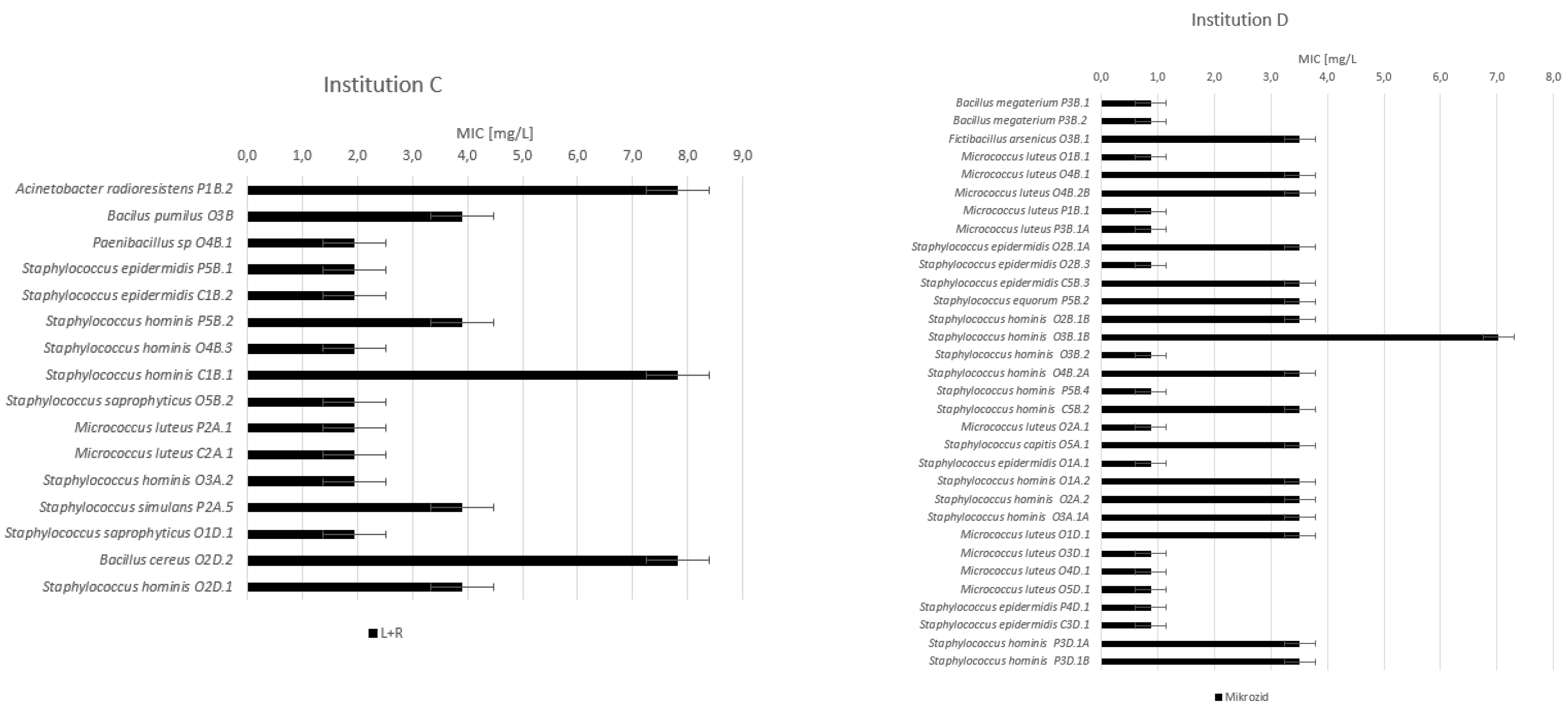
3.2. Minimum Inhibitory Concentrations (MIC)
- -
- Acinetobacter radioresistens in institution C at the physiotherapy department on the therapeutic pillow before use;
- -
- Bacillus cereus in institution C at the occupational therapy department on an LED monitor after disinfection;
- -
- Staphylococcus hominis in institution C from the common area on the keyboard before use.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljadi, S.H.; Al-Shemmari, M.; Al-Ramzi, J.; Al-Abdullatif, S.; Hajeyah, Z.; Jamal, L.; Al-Bahar, S. Bacterial contamination in physical therapy departments in the State of Kuwait. J. Phys. Ther. Sci. 2017, 29, 1014–1018. [Google Scholar] [CrossRef][Green Version]
- Lambert, I.; Tebbs, S.; Hill, D.; Moss, H.; Davies, A.; Elliott, T. Interferential therapy machines as possible vehicles for cross-infection. J. Hosp. Infect. 2000, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Koibuchi, H.; Kotani, K.; Taniguchi, N. Ultrasound probes as a possible vector of bacterial transmission. Med. Ultrason. 2013, 15, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. Disposable vs reusable electrocardiography leads in development of and cross-contamination by resistant bacteria. Crit. Care Nurse 2011, 31, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Biocide use and antibiotic resistance: The relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 2003, 3, 794–803. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9880479 (accessed on 27 January 2017). [CrossRef] [PubMed]
- MarketsandMarkets, Biocides Market by Type (Oxidizing Biocides, Non-Oxidizing Biocides), Application (Water treatment, Personal care, Wood preservation, Paints & coating), and Region(APAC, North America, Europe, MEA, and South America)-Global Trends and Forecasts to 2026. Biocides Market. 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/plant-based-protein-market-14715651.html (accessed on 8 July 2021).
- Forman, M.E.; Fletcher, M.H.; Jennings, M.C.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Structure-Resistance Relationships: Interrogating Antiseptic Resistance in Bacteria with Multicationic Quaternary Ammonium Dyes. ChemMedChem. 2016, 11, 958–962. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. Use of Germicides in the Home and the Healthcare Setting Is There a Relationship Between Germicide Use and Antibiotic Resistance? Infect. Control Hosp. Epidemiol. 2006, 27, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y.; Bloomfield, S.; Coelho, J.R.; Collier, P.; Cookson, B.; Fanning, S.; Hill, A.; Hartemann, P.; Mcbain, A.J.; Oggioni, M.; et al. Does microbicide use in consumer products promote antimicrobial resistance? A critical review and recommendations for a cohesive approach to risk assessment. Microb. Drug Resist. 2013, 19, 344–354. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Guidance on the Biocidal Products Regulation, Finland. 2018. Available online: https://echa.europa.eu/documents/10162/23036412/bpr_guidance_vol_i_parts_abc_en.pdf/31b245e5-52c2-f0c7-04db-8988683cbc4b (accessed on 30 June 2022).
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward chlorhexidine in oral bacteria-is there cause for concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. -Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Latimer, J.; Bazaid, A.; McBain, A.J. Altered competitive fitness, antimicrobial susceptibility, and cellular morphology in a triclosan-induced small-colony variant of Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 4809–4816. [Google Scholar] [CrossRef] [PubMed]
- Wieland, N.; Boss, J.; Lettmann, S.; Fritz, B.; Schwaiger, K.; Bauer, J.; Hölzel, C. Susceptibility to disinfectants in antimicrobial-resistant and -susceptible isolates of Escherichia coli, Enterococcus faecalis and Enterococcus faecium from poultry-ESBL/AmpC-phenotype of E. coli is not associated with resistance to a quaternary ammonium compound, DDAC. J. Appl. Microbiol. 2017, 122, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, S.P.; Scheie, A.A.; Høiby, E.A.; Lunestad, B.-T.; Heir, E.; Fotland, T.; Naterstad, K.; Kruse, H. Triclosan and Antimicrobial Resistance in Bacteria: An Overview. Microb. Drug Resist. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Navidinia, M. Overview Perspective of Bacterial Strategies of Resistance to Biocides and Antibiotics. Arch. Clin. Infect. Dis. 2019, 14, 65744. [Google Scholar] [CrossRef]
- Romero, J.L.; Burgos, M.J.G.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Resistance to Antibiotics, Biocides, Preservatives and Metals in Bacteria Isolated from Seafoods: Co-Selection of Strains Resistant or Tolerant to Different Classes of Compounds. Front. Microbiol. 2017, 8, 1650. [Google Scholar] [CrossRef]
- Maillard, J.Y. Bacterial target sites for biocide action. Symp. Ser. Soc. Appl. Microbiol. 2002, 16S–27S. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12481825 (accessed on 1 June 2022). [CrossRef]
- EUCAST, Eucast: Disk Diffusion Methodology. Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method. 2017. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 7 October 2022).
- Schug, A.R.; Bartel, A.; Scholtzek, A.D.; Meurer, M.; Brombach, J.; Hensel, V.; Fanning, S.; Schwarz, S.; Feßler, A.T. Biocide susceptibility testing of bacteria: Development of a broth microdilution method. Vet. Microbiol. 2020, 248, 108791. [Google Scholar] [CrossRef] [PubMed]
- Humayoun, S.B.; Hiott, L.M.; Gupta, S.; Barrett, J.B.; Woodley, T.A.; Johnston, J.J.; Jackson, C.R.; Frye, J.G. An assay for determining the susceptibility of Salmonella isolates to commercial and household biocides. PLoS ONE 2018, 13, e0209072. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.T.; Rodloff, A.C.; Labahn, M.; Reinhardt, M.; Truyen, U.; Speck, S. Evaluation of disinfectant efficacy against multidrug-resistant bacteria: A comprehensive analysis of different methods. Am. J. Infect. Control 2019, 47, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.L.; Morton, H.E. Antiseptics. In APIC Infection Control & Applied Epidemiology: Principles & Practices; Olmstad, R.N., Ed.; Mosby-Year Book. Inc.: St. Louis, MO, USA, 1996; pp. 19-1–19-7. [Google Scholar]
- Rutala, W.A. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. Association for Professionals in Infection Control and Epidemiology, Inc. Am. J. Infect. Control 1996, 24, 313–342. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8870916 (accessed on 3 October 2018). [CrossRef]
- European Chemicals Agency. Guidance on the Biocidal Products Regulation Volume II Efficacy-Assessment and Evaluation (Parts B+C); European Chemicals Agency: Helsinki, Finland, 2018. [Google Scholar] [CrossRef]
- Anderson, R.E.; Young, V.; Stewart, M.; Robertson, C.; Dancer, S.J. Cleanliness audit of clinical surfaces and equipment: Who cleans what? J. Hosp. Infect. 2011, 78, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Afle, F.C.D.; Agbankpe, A.J.; Johnson, R.C.; Houngbégnon, O.; Houssou, S.C.; Bankole, H.S. Healthcare-associated infections: Bacteriological characterization of the hospital surfaces in the University Hospital of Abomey-Calavi/so-ava in South Benin (West Africa). BMC Infect. Dis. 2019, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Vossebein, L.; Zille, A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrob. Resist. Infect. Control 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Dumigan, D.G.; Boyce, J.M.; Havill, N.L.; Golebiewski, M.; Balogun, O.; Rizvani, R. Who is really caring for your environment of care? Developing standardized cleaning procedures and effective monitoring techniques. Am. J. Infect. Control 2010, 38, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Jiang, S.; Zeng, J.; Zhou, X.; Li, Y. Drug Resistance and Gene Transfer Mechanisms in Respiratory/Oral Bacteria. J. Dent. Res. 2018, 97, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 55S–64S. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.Y. Bacterial resistance to biocides in the healthcare environment: Should it be of genuine concern? J. Hosp. Infect. 2007, 65, 60–72. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. J. Hosp. Infect. 2003, 55, 98–107. [Google Scholar] [CrossRef]
- Gilbert, P.; Allison, D.G.; McBain, A.J. Biofilms in vitro and in vivo: Do singular mechanisms imply cross-resistance? Symp. Ser. Soc. Appl. Microbiol. 2002, 98S–110S. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12481835 (accessed on 4 February 2021). [CrossRef]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018, 10, eaar6115. [Google Scholar] [CrossRef]
- Borana, R.; Bari, S. Ethanol tolerance in Escherichia coli DH5-Alpha developed by serial exposure to sublethal doses is conferred to wild strains by horizontal gene transfer. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gravel, J.; Paradis-Bleau, C.; Schmitzer, A.R. Adaptation of a bacterial membrane permeabilization assay for quantitative evaluation of benzalkonium chloride as a membrane-disrupting agent. Medchemcomm 2017, 8, 1408–1413. [Google Scholar] [CrossRef]
- Tandukar, M.; Oh, S.; Tezel, U.; Konstantinidis, K.T.; Pavlostathis, S.G. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ. Sci. Technol. 2013, 47, 9730–9738. [Google Scholar] [CrossRef]
- Morrissey, I.; Oggioni, M.R.; Knight, D.R.; Curiao, T.; Coque, T.; Kalkanci, A.; Martinez, J.L. Evaluation of Epidemiological Cut-Off Values Indicates that Biocide Resistant Subpopulations Are Uncommon in Natural Isolates of Clinically-Relevant Microorganisms. PLoS ONE 2014, 9, e86669. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. EUCAST Definitions (and Breakpoint Table, MIC and Zone DistributionWebsite Conventions), ECCMID 2011, Milan. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_Presentations/2011/EW1_Brown_Definitionsf2.pdf (accessed on 1 June 2022).
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cowley, N.L.; Forbes, S.; Amézquita, A.; McClure, P.; Humphreys, G.J.; McBain, A.J. Effects of Formulation on Microbicide Potency and Mitigation of the Development of Bacterial Insusceptibility. Appl. Environ. Microbiol. 2015, 81, 7330–7338. [Google Scholar] [CrossRef]
- McDonald, L.C.; Chen, F.-J.; Lo, H.-J.; Yin, H.-C.; Lu, P.-L.; Huang, C.-H.; Chen, P.; Lauderdale, T.-L.; Ho, M. Emergence of Reduced Susceptibility and Resistance to Fluoroquinolones in Escherichia coli in Taiwan and Contributions of Distinct Selective Pressures. Antimicrob. Agents Chemother. 2001, 45, 3084–3091. [Google Scholar] [CrossRef]
- Rodloff, A.; Bauer, T.; Ewig, S.; Kujath, P.; Müller, E. Susceptible, Intermediate, and Resistant—The Intensity of Antibiotic Action. Dtsch. Arztebl. Int. 2008, 105, 657–662. [Google Scholar] [CrossRef]
- Conrad, S.; Oethinger, M.; Kaifel, K.; Klotz, G.; Marre, R.; Kern, W.V. gyrA mutations in high-level fluoroquinolone-resistant clinical isolates of Escherichia coli. J. Antimicrob. Chemother. 1996, 38, 443–456. [Google Scholar] [CrossRef]
- Gales, A.C.; Gordon, K.A.; Wilke, W.W.; Pfaller, M.A.; Jones, R.N. Occurrence of single-point gyrA mutations among ciprofloxacin-susceptible Escherichia coli isolates causing urinary tract infections in Latin America. Diagn. Microbiol. Infect. Dis. 2000, 36, 61–64. [Google Scholar] [CrossRef]
- Ozeki, S.; Deguchi, T.; Yasuda, M.; Nakano, M.; Kawamura, T.; Nishino, Y.; Kawada, Y. Development of a rapid assay for detecting gyrA mutations in Escherichia coli and determination of incidence of gyrA mutations in clinical strains isolated from patients with complicated urinary tract infections. J. Clin. Microbiol. 1997, 35, 2315–2319. [Google Scholar] [CrossRef]
- Manaye, G.; Muleta, D.; Henok, A.; Asres, A.; Mamo, Y.; Feyissa, D.; Ejeta, F.; Niguse, W. Evaluation of the Efficacy of Alcohol-Based Hand Sanitizers Sold in Southwest Ethiopia. Infect. Drug Resist. 2021, 14, 547–554. [Google Scholar] [CrossRef]
- Mazzola, P.G.; Jozala, A.F.; Novaes, L.C.D.L.; Moriel, P.; Penna, T.C.V. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009, 45, 241–248. [Google Scholar] [CrossRef]
- Kotb, S.; Sayed, M. Sensitivity of Methicillin-Resistance and Methicillin-Susceptible Staphylococcus aureus Strains to Some Different Disinfectants. Int. J. Livest. Res. 2015, 5, 45. [Google Scholar] [CrossRef]
- Kampf, G.; Kramer, A. Epidemiologic Background of Hand Hygiene and Evaluation of the Most Important Agents for Scrubs and Rubs. Clin. Microbiol. Rev. 2004, 17, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health 2018, 11, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Maertens, H.; de Reu, K.; Meyer, E.; van Coillie, E.; Dewulf, J. Limited association between disinfectant use and either antibiotic or disinfectant susceptibility of Escherichia coli in both poultry and pig husbandry. BMC Vet. Res. 2019, 15, 310. [Google Scholar] [CrossRef]
- Ramzi, A.; Oumokhtar, B.; Zoubi, Y.E.; Mouatassem, T.F.; Benboubker, M.; Lalami, A.e. Evaluation of Antibacterial Activity of Three Quaternary Ammonium Disinfectants on Different Germs Isolated from the Hospital Environment. Biomed. Res. Int. 2020, 2020, 6509740. [Google Scholar] [CrossRef]
- World Health Organization. Combating Waterborne Disease at the Household Level. 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/43621/9789241595223_eng.pdf (accessed on 17 March 2022).
- McBain, A.; Rickard, A.H.; Gilbert, P. Possible Implications of Biocide Accumulation in the Environment on the Prevalence of Bacterial Antibiotic Resistance; Springer: Berlin/Heidelberg, Germany, 2002; Volume 29, pp. 326–330. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations/the Review on Antimicrobial Resistance Chaired by Jim O’Neill|Wellcome Collection, United Kingdom. 2016. Available online: https://wellcomecollection.org/works/thvwsuba (accessed on 4 February 2021).
- Hardy, K.; Sunnucks, K.; Gil, H.; Shabir, S.; Trampari, E.; Hawkey, P.; Webber, M. Increased usage of antiseptics is associated with reduced susceptibility in clinical isolates of Staphylococcus aureus. mBio 2018, 9, e00894-18. [Google Scholar] [CrossRef]
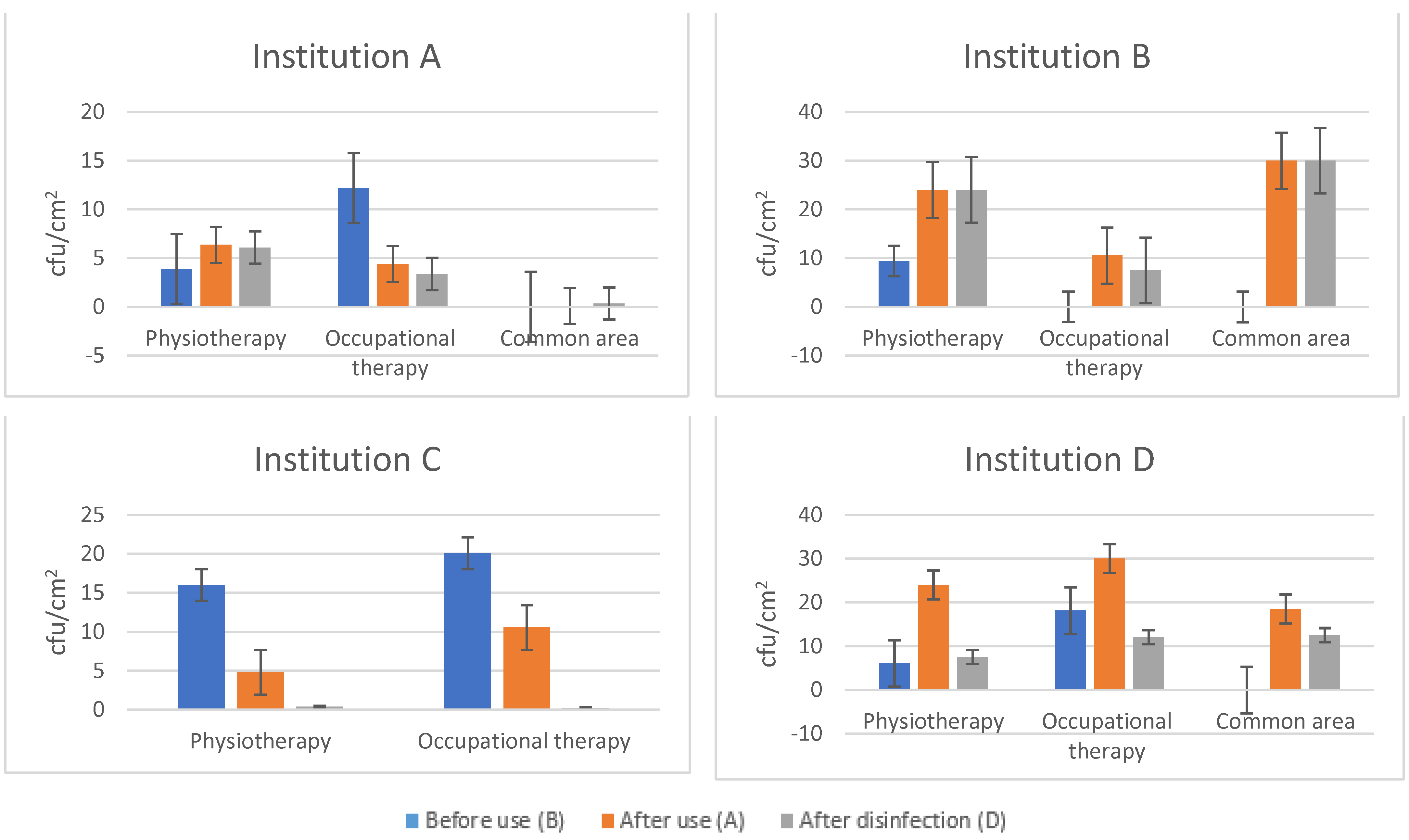
| Before Use (B) | After Use (A) | After Disinfection (D) | Log Reduction (A/D) | |
|---|---|---|---|---|
| Institution A | 8.04 ± 9.38 | 4.50 ± 6.54 | 4.00 ± 6.64 | 0.051 (11.11%) |
| Institution B | 5.16 ± 9.62 | 19.21 ± 14.35 | 18.00 ± 15.49 | 0.028 (6.30%) |
| Institution C | 18.05 ± 14.18 | 9.10 ±14.08 | 0.28 ± 0.19 | 1.512 (96.92%) |
| Institution D | 12.11 ± 14.16 | 25.18 ± 9.56 | 10.52 ± 14.52 | 0.379 (58.22%) |
| Incidin [g/L] | Sani-Cloth 70% [g/L] | Descosept [g/L] | L + R [mg/L] | Mikrozid [mg/L] | |
|---|---|---|---|---|---|
| Institution A | |||||
| Before use | 75.20 ± 45.72 | 66.17 ± 41.41 | |||
| After use | 82.33 ± 34.65 | 64.68 ± 54.62 | |||
| After disinfection | 67.60 ± 16.55 | 62.20 ± 15.21 | |||
| Institution B | |||||
| Before use | 83.44 ± 40.61 | 79.50 ± 43.01 | |||
| After use | 107.14 ± 40.09 | 78.86 ± 26.08 | |||
| After disinfection | 69.86 ± 39.89 | 44.71 ± 16.59 | |||
| Institution C | |||||
| Before use | 163.90 ± 84.22 | 3.69 ± 2.48 | |||
| After use | 119.88 ± 46.93 | 2.44 ± 0.98 | |||
| After disinfection | 169.00 ± 64.66 | 4.55 ± 2.98 | |||
| Institution D | |||||
| Before use | 2.54 ± 1.73 | ||||
| After use | 2.63 ± 1.36 | ||||
| After disinfection | 1.87 ± 1.36 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozman, U.; Duh, D.; Cimerman, M.; Turk, S.Š. Hygiene of Medical Devices and Minimum Inhibitory Concentrations for Alcohol-Based and QAC Disinfectants among Isolates from Physical Therapy Departments. Int. J. Environ. Res. Public Health 2022, 19, 14690. https://doi.org/10.3390/ijerph192214690
Rozman U, Duh D, Cimerman M, Turk SŠ. Hygiene of Medical Devices and Minimum Inhibitory Concentrations for Alcohol-Based and QAC Disinfectants among Isolates from Physical Therapy Departments. International Journal of Environmental Research and Public Health. 2022; 19(22):14690. https://doi.org/10.3390/ijerph192214690
Chicago/Turabian StyleRozman, Urška, Darja Duh, Mojca Cimerman, and Sonja Šostar Turk. 2022. "Hygiene of Medical Devices and Minimum Inhibitory Concentrations for Alcohol-Based and QAC Disinfectants among Isolates from Physical Therapy Departments" International Journal of Environmental Research and Public Health 19, no. 22: 14690. https://doi.org/10.3390/ijerph192214690
APA StyleRozman, U., Duh, D., Cimerman, M., & Turk, S. Š. (2022). Hygiene of Medical Devices and Minimum Inhibitory Concentrations for Alcohol-Based and QAC Disinfectants among Isolates from Physical Therapy Departments. International Journal of Environmental Research and Public Health, 19(22), 14690. https://doi.org/10.3390/ijerph192214690






