A Rare Case of Left Ventricular Non-Compaction with Coronary Artery Anomaly Complicated by ST-Elevation Myocardial Infarction and Subcutaneous Defibrillator Implantation
Abstract
1. Introduction
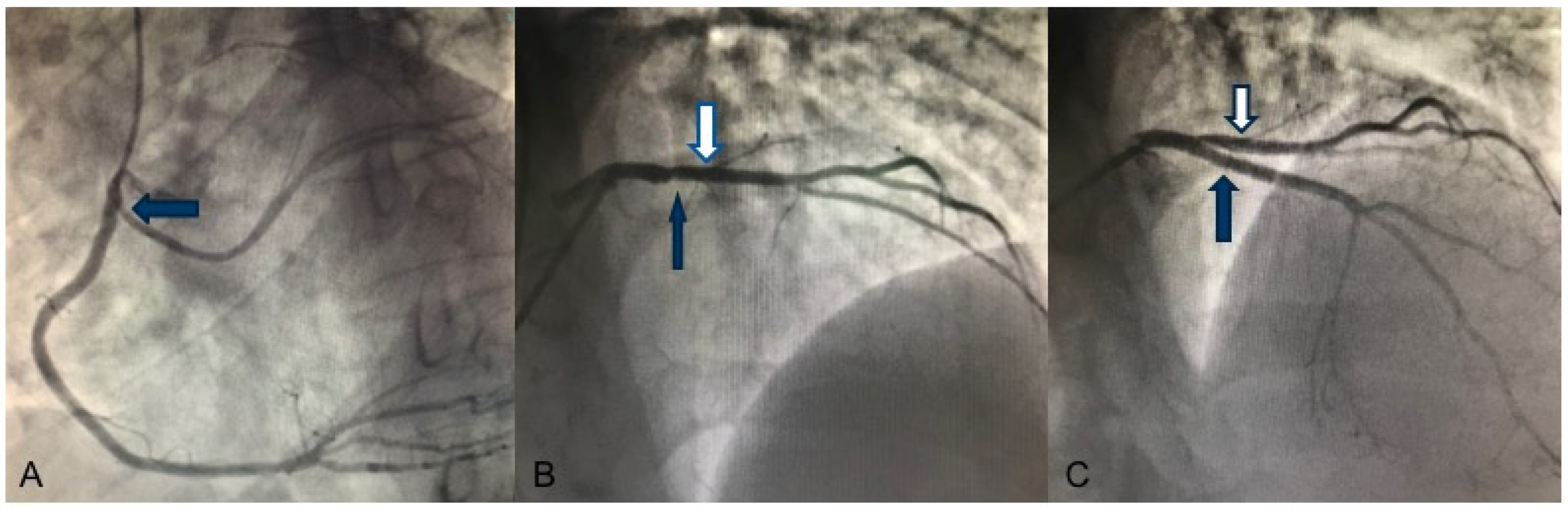
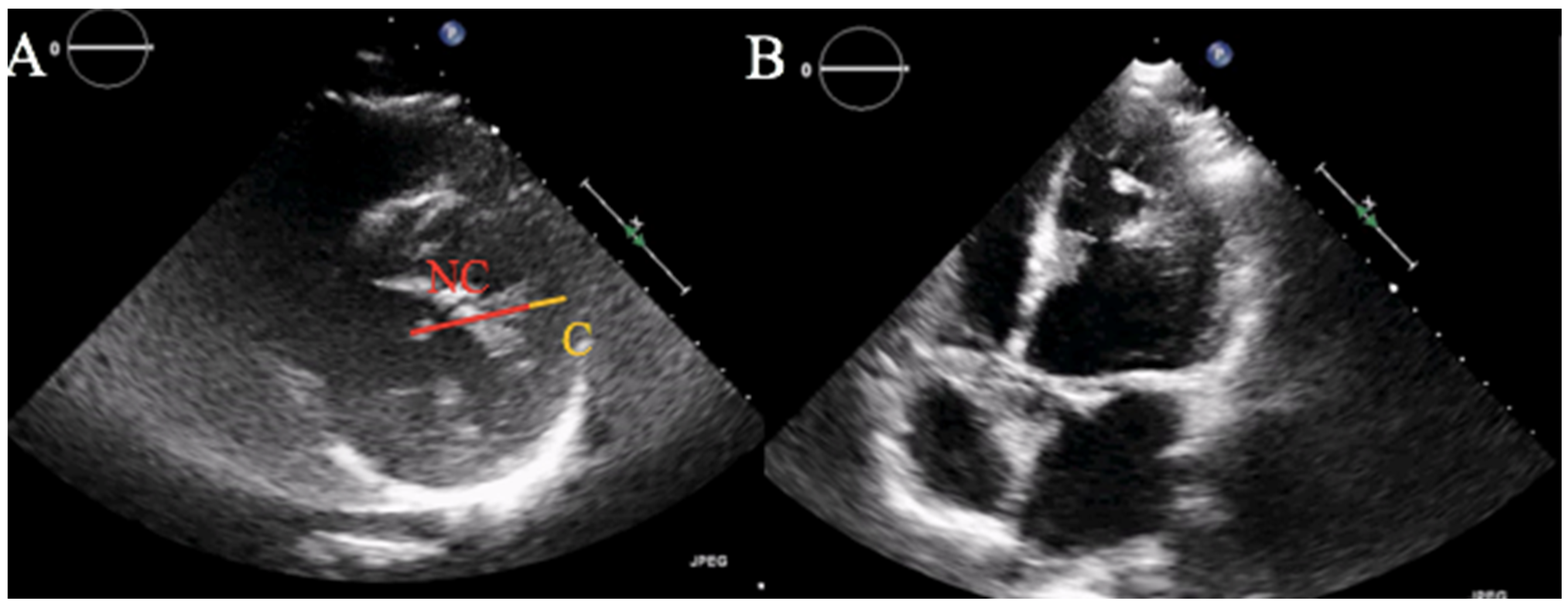
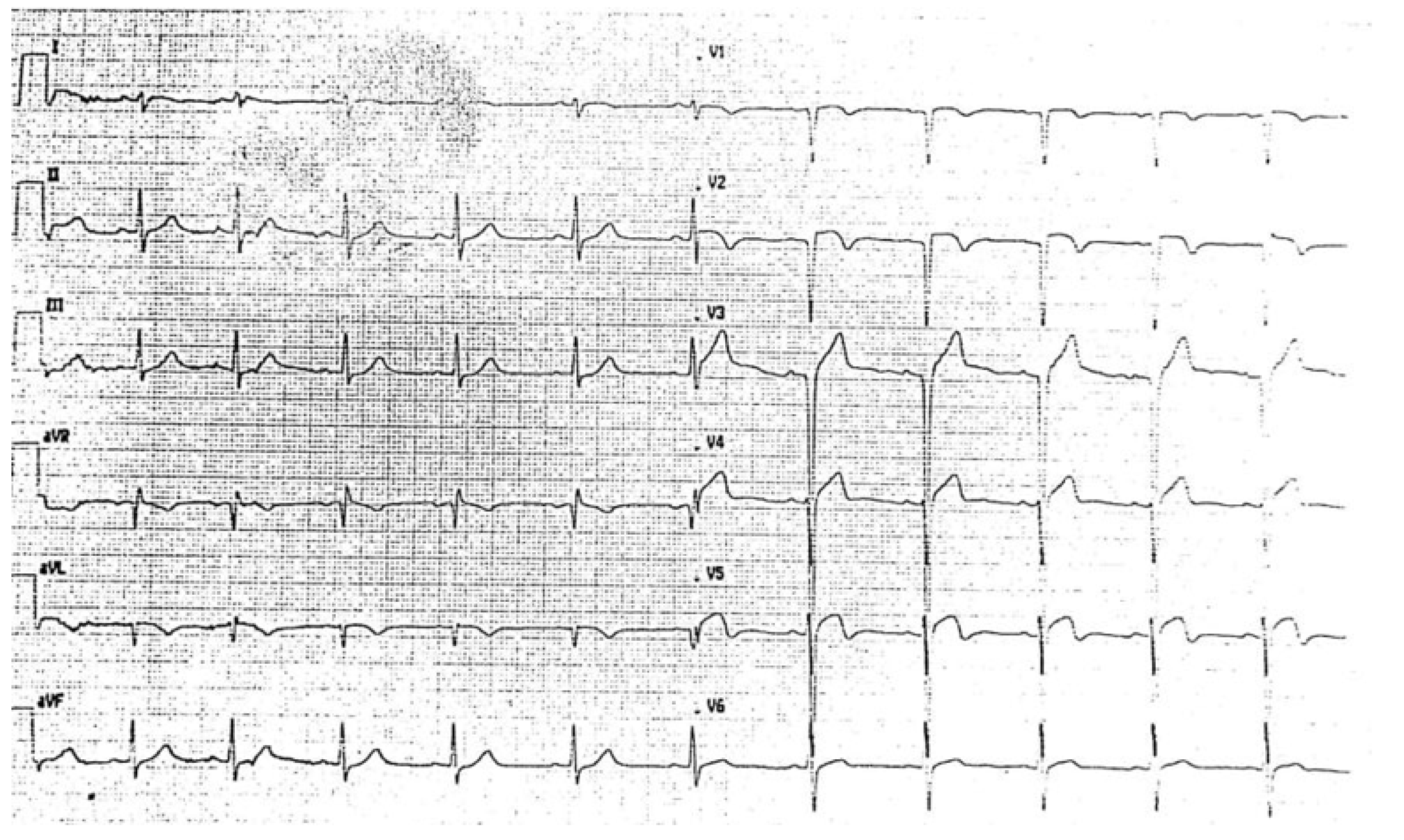
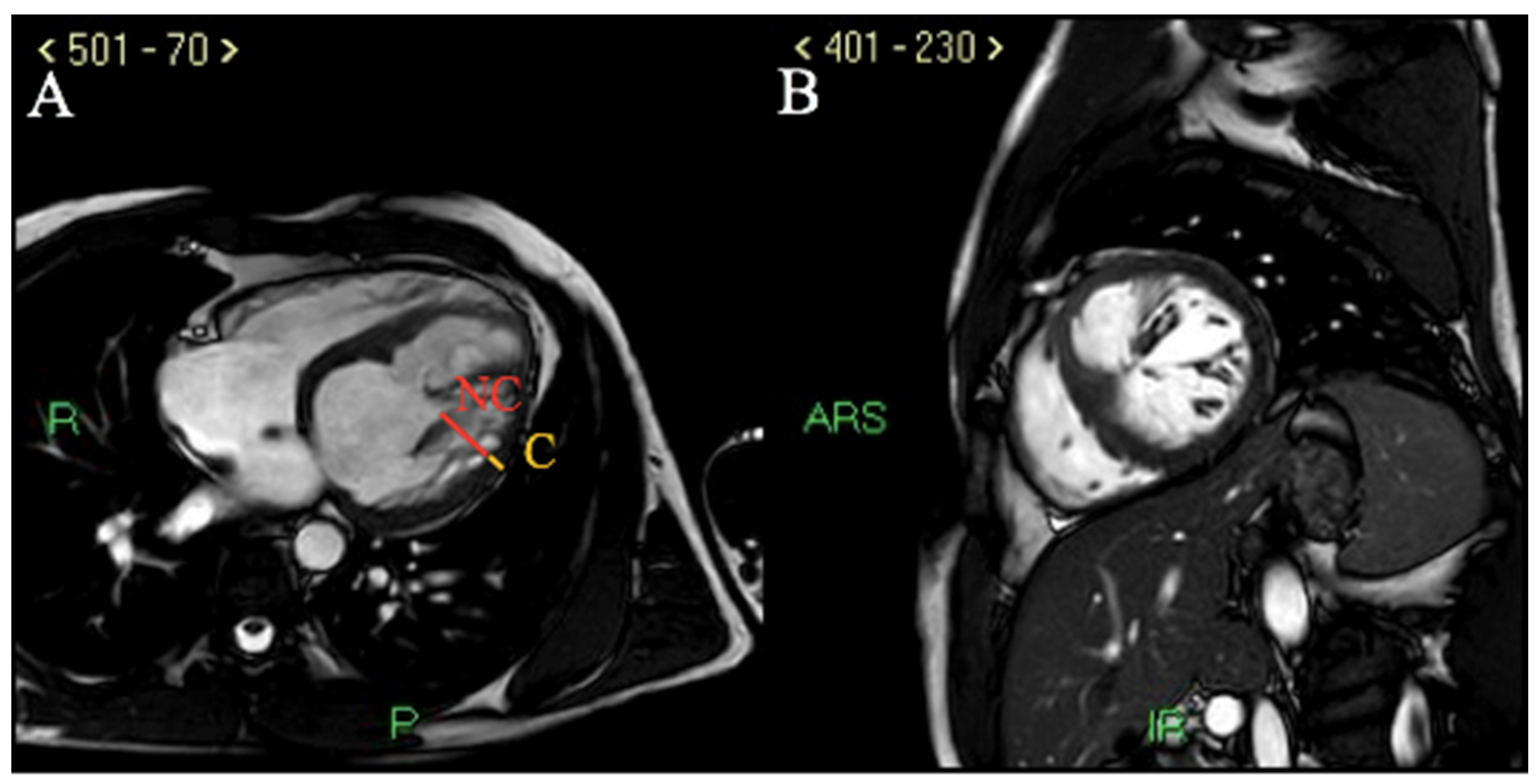
2. Case Report
3. Discussion
3.1. LVNC and Coronary Artery Anomalies
3.2. LVNC and Acute Coronary Syndrome
3.3. LVNC and Arrhythmic Risk
3.4. LVNC Diagnostic and Prognostic Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Weiford, B.C.; Subbarao, V.D.; Mulhern, K.M. Noncompaction of the Ventricular Myocardium. Circulation 2004, 109, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction. J. Am. Soc. Echocardiogr. 2004, 17, 91–100. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Towbin, J.A. Left Ventricular Noncompaction: A New Form of Heart Failure. Hear. Fail. Clin. 2010, 6, 453–469. [Google Scholar] [CrossRef]
- Stöllberger, C.; Winkler-Dworak, M.; Blazek, G.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction with and without neuromuscular disorders. Int. J. Cardiol. 2004, 97, 89–92. [Google Scholar] [CrossRef]
- Jenni, R.; Wyss, C.; Oechslin, E.N.; Kaufmann, P.A. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J. Am. Coll. Cardiol. 2002, 39, 450–454. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary Definitions and Classification of the Cardiomyopathies: An American Heart Association Scientific Statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction: Insights From Cardiovascular Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Villa, A.; Sammut, E.; Nair, A.; Rajani, R.; Bonamini, R.; Chiribiri, A. Coronary artery anomalies overview: The normal and the abnormal. World J. Radiol. 2016, 8, 537–555. [Google Scholar] [CrossRef]
- Phillips, H.M.; Hildreth, V.; Peat, J.D.; Murdoch, J.N.; Kobayashi, K.; Chaudhry, B.; Henderson, D.J. Non–Cell-Autonomous Roles for the Planar Cell Polarity Gene Vangl2 in Development of the Coronary Circulation. Circ. Res. 2008, 102, 615–623. [Google Scholar] [CrossRef]
- Mattsson, G.; Baroudi, A.; Tawfiq, H.; Magnusson, P. Left ventricular non-compaction cardiomyopathy with coronary artery anomaly complicated by ventricular tachycardia. BMC Cardiovasc. Disord. 2017, 17, 263. [Google Scholar] [CrossRef]
- Panduranga, P.; Mukhaini, M.K. Left-ventricular non-compaction with coronary artery disease. Int. J. Cardiol. 2011, 150, e37–e39. [Google Scholar] [CrossRef]
- Liang, J.J.; Fenstad, E.R.; Janish, C.D.; Sinak, L.J. Left ventricular non-compaction cardiomyopathy: Incidental diagnosis after ST-elevation myocardial infarction. Acute Card. Care 2016, 18, 25–27. [Google Scholar] [CrossRef]
- Gabrielli, F.A.; Lombardo, A.; Natale, L.; Galiuto, L.; Porcelli, A.; Rebuzzi, A.; Crea, F. Myocardial infarction in isolated ventricular non-compaction: Contrast echo and MRI. Int. J. Cardiol. 2006, 111, 315–317. [Google Scholar] [CrossRef]
- Celik, I.E.; Kilic, A.; Karadeniz, M. Anterior myocardial infarction in a patient with isolated left ventricular non-compaction. Cardiol. Young 2019, 29, 708–710. [Google Scholar] [CrossRef]
- Yavuzgil, O.; Gürgün, C.; Çinar, C.S.; Yüksel, A. Anterior myocardial infarction in an adult patient with left ventricular hypertrabeculation/noncompaction. Int. J. Cardiol. 2006, 106, 394–395. [Google Scholar] [CrossRef]
- Correia, E.; Santos, L.F.; Rodrigues, B.; Gama, P.; Cabral, C.; Santos, O. Noncompaction of the myocardium in a patient with acute myocardial infarction. Arq. Bras. Cardiol. 2010, 94, 125–127. [Google Scholar]
- Khalili, M.; Toufan, M.; Shahvalizadeh, R. Myocardial infarction in a patient with left ventricular noncompaction: A case report. Int. J. Gen. Med. 2012, 5, 661–665. [Google Scholar] [CrossRef][Green Version]
- Swinkels, B.M.; Boersma, L.V.A.; Rensing, B.J.; Jaarsma, W. Isolated left ventricular noncompaction in a patient presenting with a subacute myocardial infarction. Neth. Hear. J. 2007, 15, 109–111. [Google Scholar] [CrossRef][Green Version]
- Fettouhi, H.; Tamdy, A.; Ellouali, F.; Doghmi, N.; Zarzur, J.; Cherti, M. Crises convulsives révélant une non-compaction du VG compliquée d’un IDM apical [Convulsives crisis revealing left-ventricular non-compaction with apical myocardial infarction]. Ann. Cardiol. D’angéiol. 2011, 60, 159–164. [Google Scholar] [CrossRef]
- Salati, M.; Di Mauro, A.; Bregasi, A.; Mattioli, R. Coronary artery bypass graft and mitral valvuloplasty in a patient with isolated ventricular non-compaction. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 354–356. [Google Scholar] [CrossRef]
- Martini, B.; Sperotto, C.; Zhang, L. “Cardiac incidentaloma”: Left ventricular non-compaction in a kindred with familial coronary artery disease. Cardiol. J. 2007, 14, 407–410. [Google Scholar]
- Finsterer, J.; Stöllberger, C.; Bonner, E. Left ventricular hypertrabeculation/noncompaction associated with coronary heart disease and myopathy. Int. J. Cardiol. 2011, 148, e53–e55. [Google Scholar] [CrossRef]
- Tumolo, A.; Nguyen, D. Spectrum of Cardiac Arrhythmias in Isolated Ventricular Non-Compaction. J. Innov. Card. Rhythm Manag. 2017, 8, 2774–2783. [Google Scholar] [CrossRef]
- Steffel, J.; Kobza, R.; Namdar, M.; Wolber, T.; Brunckhorst, C.; Lüscher, T.F.; Jenni, R.; Duru, F. Electrophysiological findings in patients with isolated left ventricular non-compaction. Europace 2009, 11, 1193–1200. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Paolucci, M.; Cattafi, G.; Magenta, G.; Vecchi, M.R.; Schirru, M.; Lunati, M. La stratificazione del rischio di morte improvvisa: Dobbiamo considerare solo la frazione di eiezione? G Ital. Cardiol. 2008, 9 (Suppl. 1), 27S–32S. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Stämpfli, S.F.; Gotschy, A.; Kiarostami, P.; Özkartal, T.; Gruner, C.; Niemann, M.; Manka, R.; Tanner, F.C. Right ventricular involvement in left ventricular non-compaction cardiomyopathy. Cardiol. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Munroe, P.B.; et al. Prognostic Significance of Left Ventricular Non-compaction: A systematic review and meta-analysis of observational studies. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef] [PubMed]
- Sharain, K.; Anavekar, N.S. Outcomes in LV Noncompaction. Heterogeneity in Results or Heterogeneity in Diagnosing a Heterogeneous Disease? Circ. Cardiovasc. Imaging 2020, 13, e010268. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, C.; Barison, A.; Ivanov, A.; Andreini, D.; Amzulescu, M.S.; Mazurkiewicz, L.; De Luca, A.; Grzybowski, J.; Masci, P.G.; Marczak, M.; et al. Meta-Analysis of the Prognostic Role of Late Gadolinium Enhancement and Global Systolic Impairment in Left Ventricular Noncompaction. JACC Cardiovasc. Imaging 2019, 12, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar] [PubMed]
- Perrone, M.A.; Donatucci, B.; Salvati, A.; Gualtieri, P.; De Lorenzo, A.; Romeo, F.; Bernardini, S. Inflammation, oxidative stress and gene expression: The postprandial approach in professional soccer players to reduce the risk of muscle injuries and early atherosclerosis. Med. Sport 2019, 72, 234–243. [Google Scholar] [CrossRef]
- Perrone, M.A.; Intorcia, A.; Morgagni, R.; Marchei, M.; Sergi, D.; Pugliese, L.; Ferrante, P.; Chiocchi, M.; Borzi, M.; Romeo, F. Primary cardiac lymphoma: The role of multimodality imaging. J. Cardiovasc. Med. 2018, 19, 455–458. [Google Scholar] [CrossRef]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Heart J. 2020, 41, 1428–1436. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prandi, F.R.; Illuminato, F.; Galluccio, C.; Milite, M.; Macrini, M.; Di Landro, A.; Idone, G.; Chiocchi, M.; Sbordone, F.P.; Sergi, D.; et al. A Rare Case of Left Ventricular Non-Compaction with Coronary Artery Anomaly Complicated by ST-Elevation Myocardial Infarction and Subcutaneous Defibrillator Implantation. Int. J. Environ. Res. Public Health 2022, 19, 791. https://doi.org/10.3390/ijerph19020791
Prandi FR, Illuminato F, Galluccio C, Milite M, Macrini M, Di Landro A, Idone G, Chiocchi M, Sbordone FP, Sergi D, et al. A Rare Case of Left Ventricular Non-Compaction with Coronary Artery Anomaly Complicated by ST-Elevation Myocardial Infarction and Subcutaneous Defibrillator Implantation. International Journal of Environmental Research and Public Health. 2022; 19(2):791. https://doi.org/10.3390/ijerph19020791
Chicago/Turabian StylePrandi, Francesca Romana, Federica Illuminato, Chiara Galluccio, Marialucia Milite, Massimiliano Macrini, Alessio Di Landro, Gaetano Idone, Marcello Chiocchi, Francesco Paolo Sbordone, Domenico Sergi, and et al. 2022. "A Rare Case of Left Ventricular Non-Compaction with Coronary Artery Anomaly Complicated by ST-Elevation Myocardial Infarction and Subcutaneous Defibrillator Implantation" International Journal of Environmental Research and Public Health 19, no. 2: 791. https://doi.org/10.3390/ijerph19020791
APA StylePrandi, F. R., Illuminato, F., Galluccio, C., Milite, M., Macrini, M., Di Landro, A., Idone, G., Chiocchi, M., Sbordone, F. P., Sergi, D., Romeo, F., & Barillà, F. (2022). A Rare Case of Left Ventricular Non-Compaction with Coronary Artery Anomaly Complicated by ST-Elevation Myocardial Infarction and Subcutaneous Defibrillator Implantation. International Journal of Environmental Research and Public Health, 19(2), 791. https://doi.org/10.3390/ijerph19020791





