A Case of Successful Use of the “Anchoring Technique” for Percutaneous Treatment of Left Ventricular Assist Device Graft Occlusion
Abstract
:1. Introduction
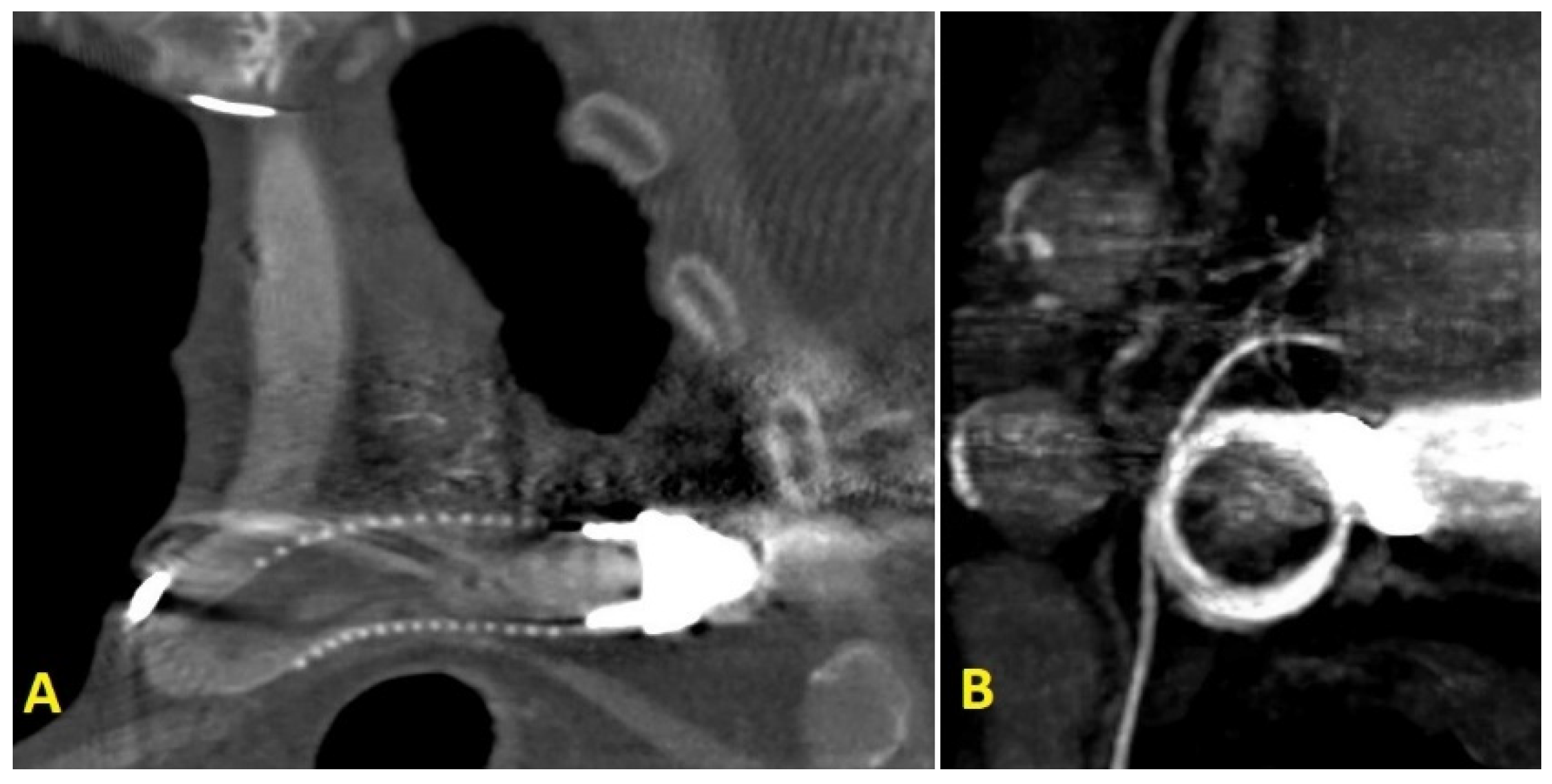
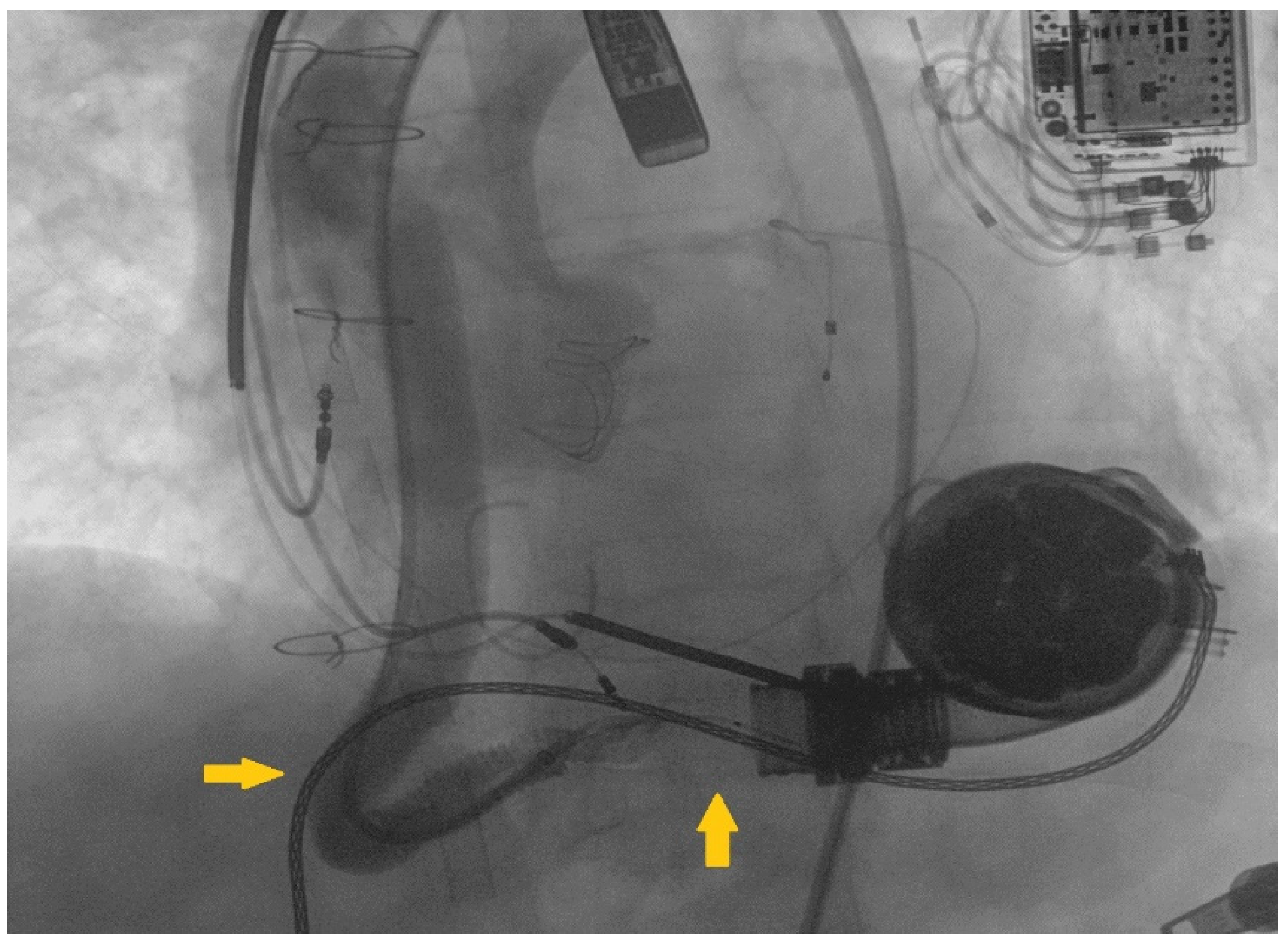
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure. JACC Heart Fail 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Stio, R.E.; Montalto, A.; Feccia, M.; Intorcia, A.; Buffa, V.; Cesario, V.; Petroni, G.; De Felice, F.; Musumeci, F. Extracorporeal membrane oxygenation-assisted emergency percutaneous treatment of left ventricular assist device graft occlusion. ESC Heart Fail 2021, 8, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Milwidsky, A.; Alvarez Villela, M.; Wiley, J.; Sanina, C.; Patel, S.R.; Sutton, N.; Latib, A.; Sims, D.B.; Forest, S.J.; Shin, J.J.; et al. Outflow graft obstruction in patients with the HM 3 LVAD: A percutaneous approach. Catheter. Cardiovasc. Interv. 2021, 98, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Scandroglio, A.M.; Kaufmann, F.; Pieri, M.; Kretzschmar, A.; Müller, M.; Pergantis, P.; Dreysse, S.; Falk, V.; Krabatsch, T.; Potapov, E.V. Diagnosis and Treatment Algorithm for Blood Flow Obstructions in Patients with Left Ventricular Assist Device. J. Am. Coll. Cardiol. 2016, 67, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Banerjee, S.; Brilakis, E.S. Applications of the distal anchoring technique in coronary and peripheral interventions. J. Invasive Cardiol. 2011, 23, 291–294. [Google Scholar] [PubMed]
- Kalathiya, R.J.; Grinstein, J.; Uriel, N.; Shah, A.P. Percutaneous Transcatheter Therapies for the Management of Left Ventricular Assist Device Complications. J. Invasive Cardiol. 2017, 29, 151–162. [Google Scholar] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Pagani, F.D.; Kormos, R.L.; Myers, S.; Acker, M.A.; Rogers, J.; Slaughter, M.S.; Stevenson, L.W. Pump thrombosis in the Thoratec Heart-Mate II device: An update analysis of the INTERMACS registry. J. Heart Lung Transplant. 2015, 34, 1515–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.T.; O’Malley, T.J.; Maynes, E.J.; Vishnevsky, A.; Morris, R.J.; Samuels, L.E.; Massey, H.T.; Tchantchaleishvili, V. Survival outcomes of stenting outflow graft stenosis in continuous-flow left ventricular assist devices: A systematic review. Heart Fail Rev. 2020, 25, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, C.; Ramasami, N. Techniques to enhance guide catheter support. Catheter. Cardiovasc. Interv. 2008, 72, 505–512. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stio, R.E.; Montalto, A.; Intorcia, A.; Polizzi, V.; Feccia, M.; Musto, C.; Pennacchi, M.; Paolucci, L.; Stumpo, R.; D’Avino, E.; et al. A Case of Successful Use of the “Anchoring Technique” for Percutaneous Treatment of Left Ventricular Assist Device Graft Occlusion. Int. J. Environ. Res. Public Health 2022, 19, 2441. https://doi.org/10.3390/ijerph19042441
Stio RE, Montalto A, Intorcia A, Polizzi V, Feccia M, Musto C, Pennacchi M, Paolucci L, Stumpo R, D’Avino E, et al. A Case of Successful Use of the “Anchoring Technique” for Percutaneous Treatment of Left Ventricular Assist Device Graft Occlusion. International Journal of Environmental Research and Public Health. 2022; 19(4):2441. https://doi.org/10.3390/ijerph19042441
Chicago/Turabian StyleStio, Rocco Edoardo, Andrea Montalto, Alfredo Intorcia, Vincenzo Polizzi, Mariano Feccia, Carmine Musto, Mauro Pennacchi, Luca Paolucci, Regina Stumpo, Emilio D’Avino, and et al. 2022. "A Case of Successful Use of the “Anchoring Technique” for Percutaneous Treatment of Left Ventricular Assist Device Graft Occlusion" International Journal of Environmental Research and Public Health 19, no. 4: 2441. https://doi.org/10.3390/ijerph19042441
APA StyleStio, R. E., Montalto, A., Intorcia, A., Polizzi, V., Feccia, M., Musto, C., Pennacchi, M., Paolucci, L., Stumpo, R., D’Avino, E., De Felice, F., Gabrielli, D., & Musumeci, F. (2022). A Case of Successful Use of the “Anchoring Technique” for Percutaneous Treatment of Left Ventricular Assist Device Graft Occlusion. International Journal of Environmental Research and Public Health, 19(4), 2441. https://doi.org/10.3390/ijerph19042441





