Psychophysiological and Metabolomics Responses of Adults during Horticultural Activities Using Soil Inoculated with Streptomyces rimosus: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
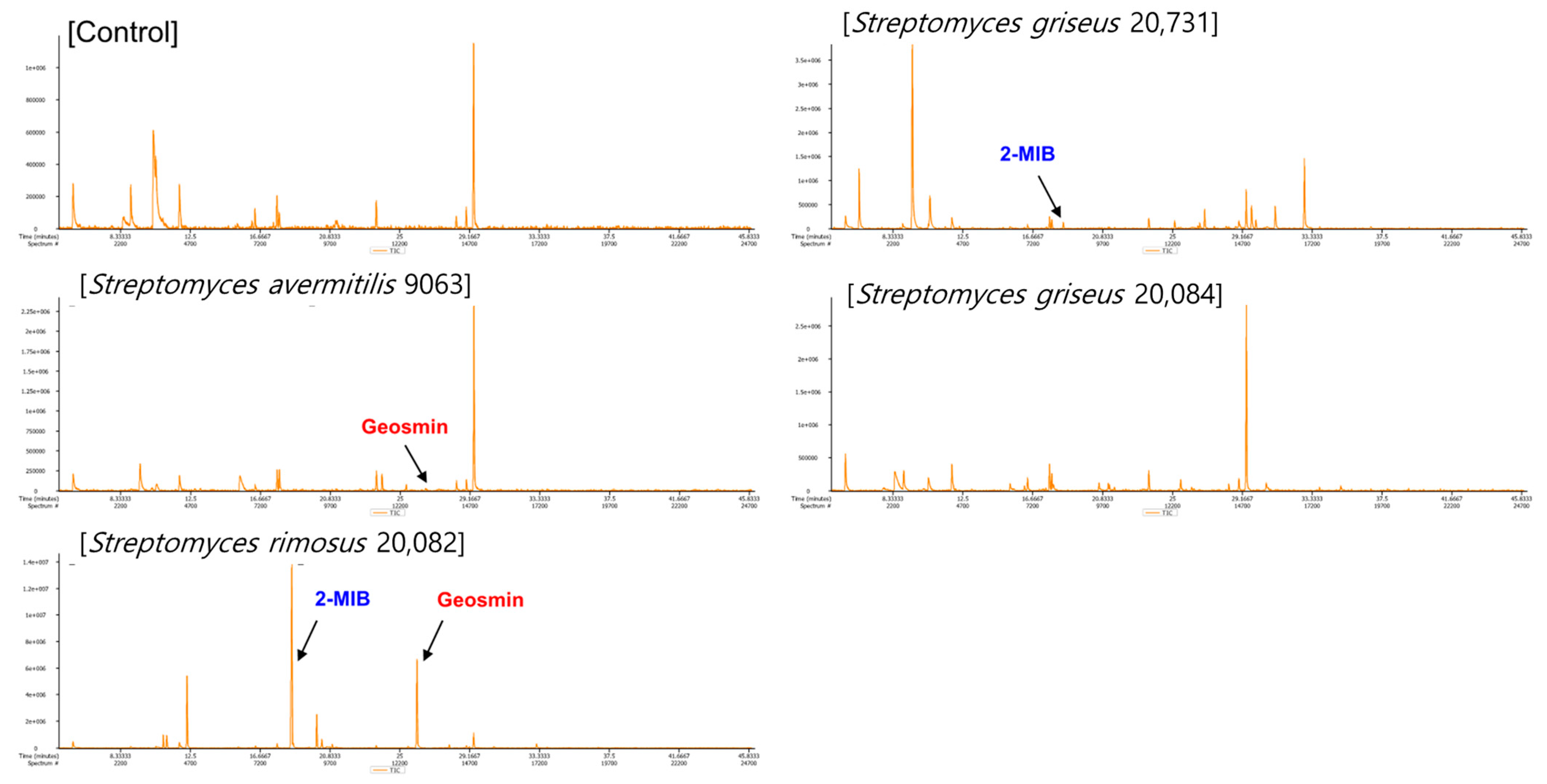
2.2. Selection of the Streptomyces Strain
2.3. Preparation of the Soil Sample
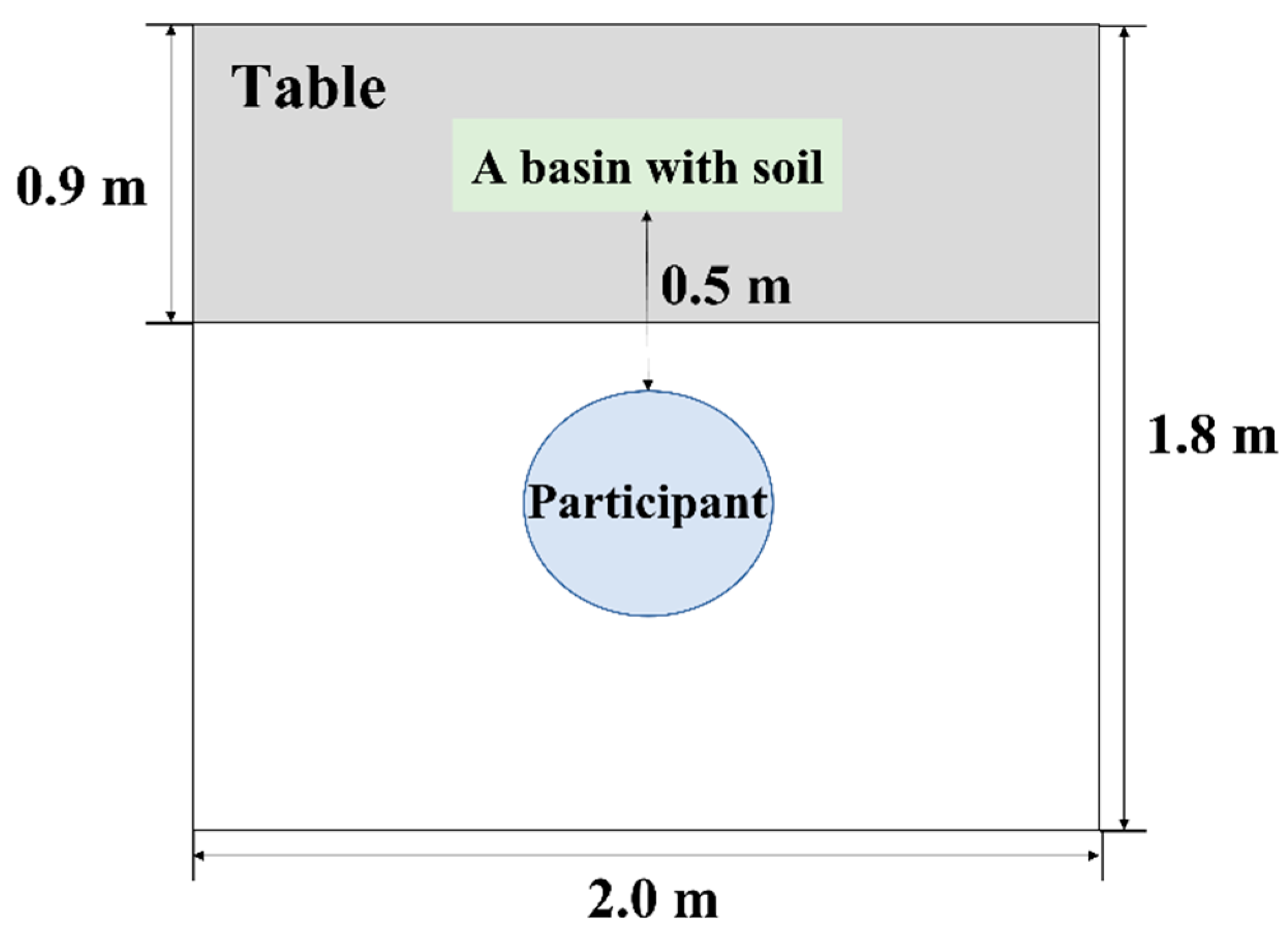
2.4. Experimental Environment
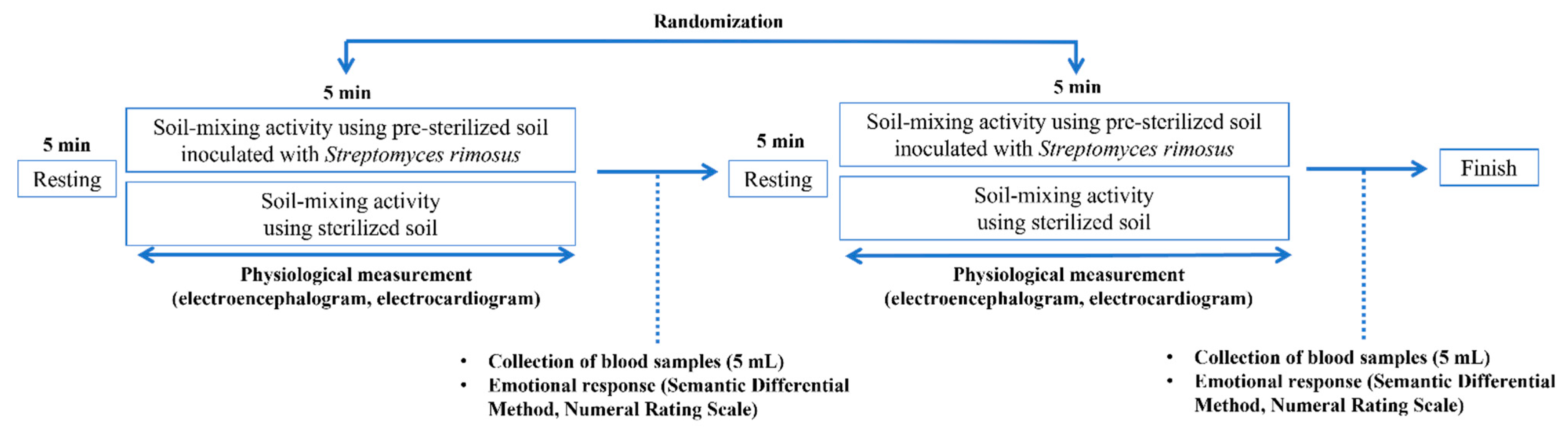
2.5. Experimental Protocol
2.6. Measurement
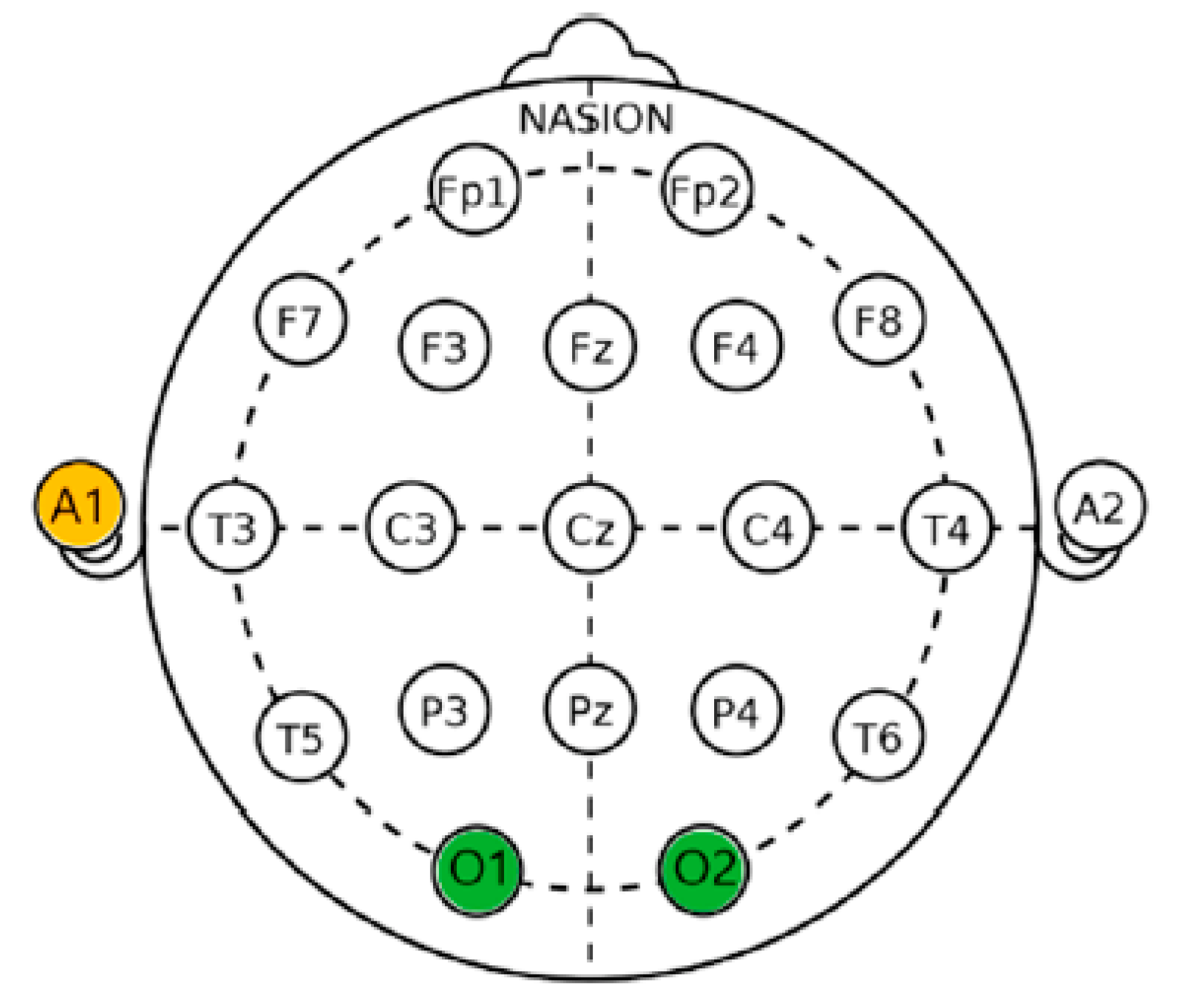
2.6.1. Psycho-Physiological Measurement
2.6.2. Soil Sample Extractions and Analysis of VOCs by GC–MS
2.6.3. Measurement of Blood Metabolites
2.6.4. Measurement of Brain-Derived Neurotrophic Factor (BDNF) and C-Reactive Protein (CRP) Levels in Serum
2.7. Data Analysis
3. Results
3.1. Demographic Information
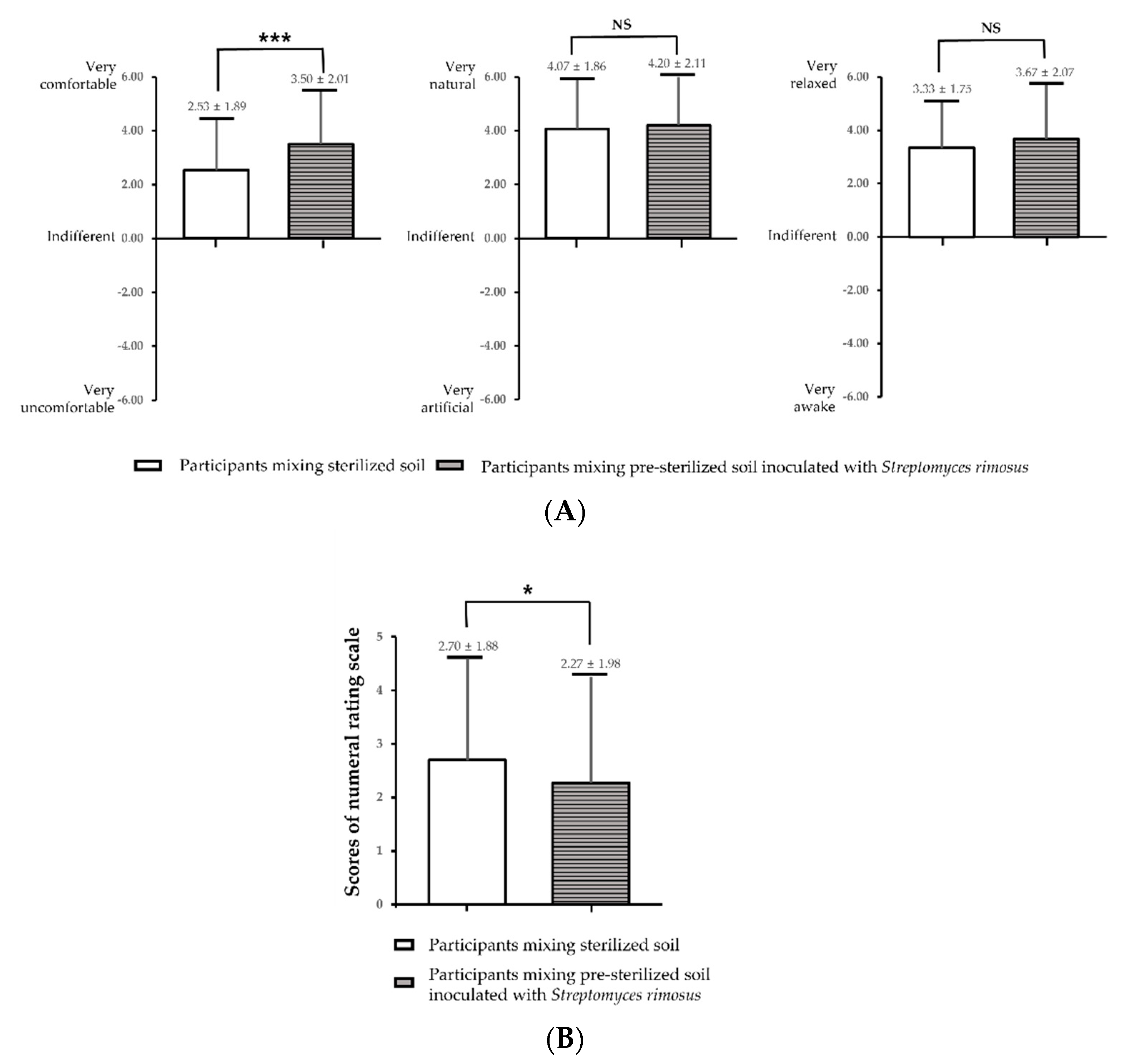
3.2. Psycho-Physiological Responses
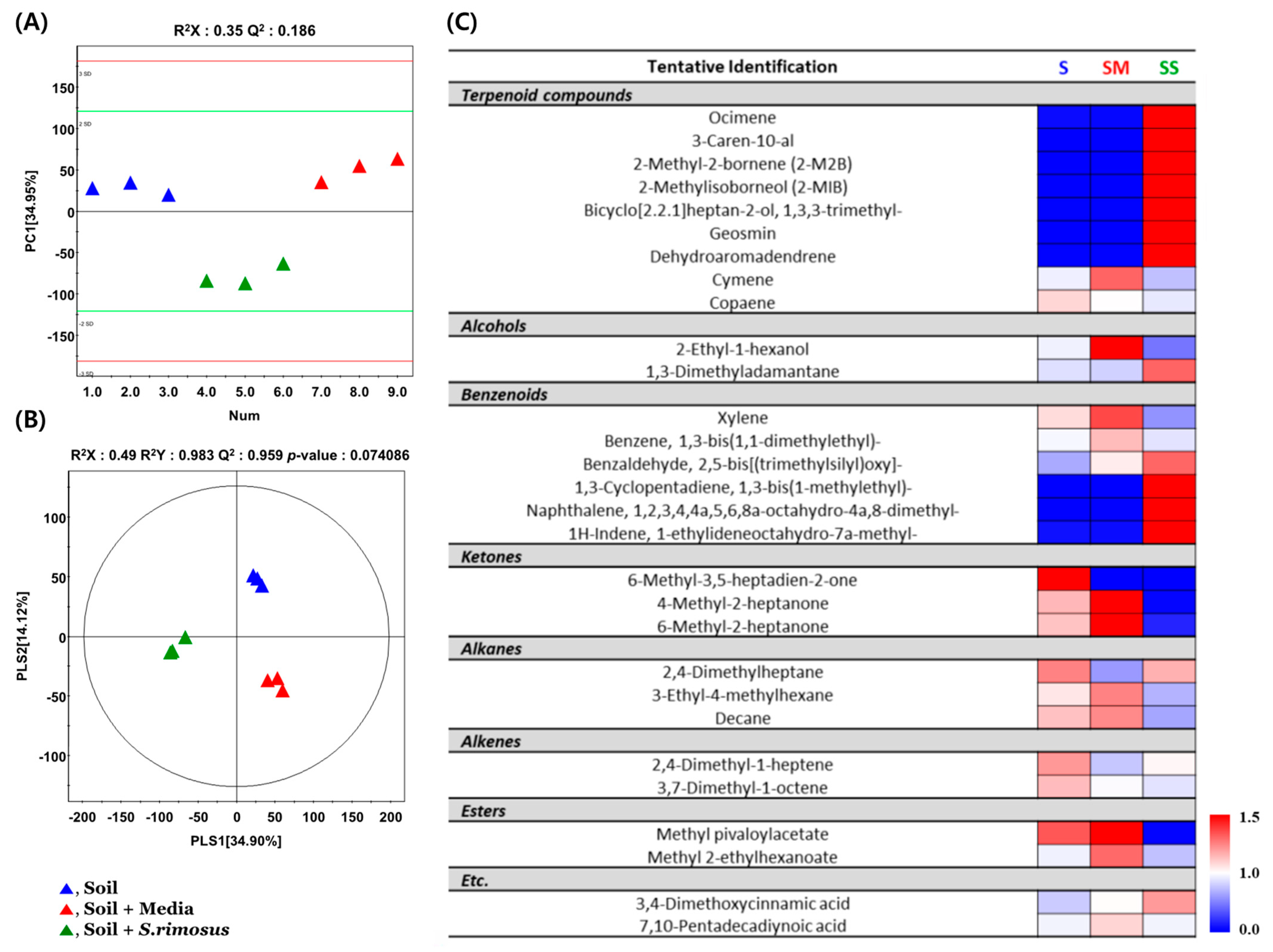
3.3. Volatolome Profiling of Soil Samples
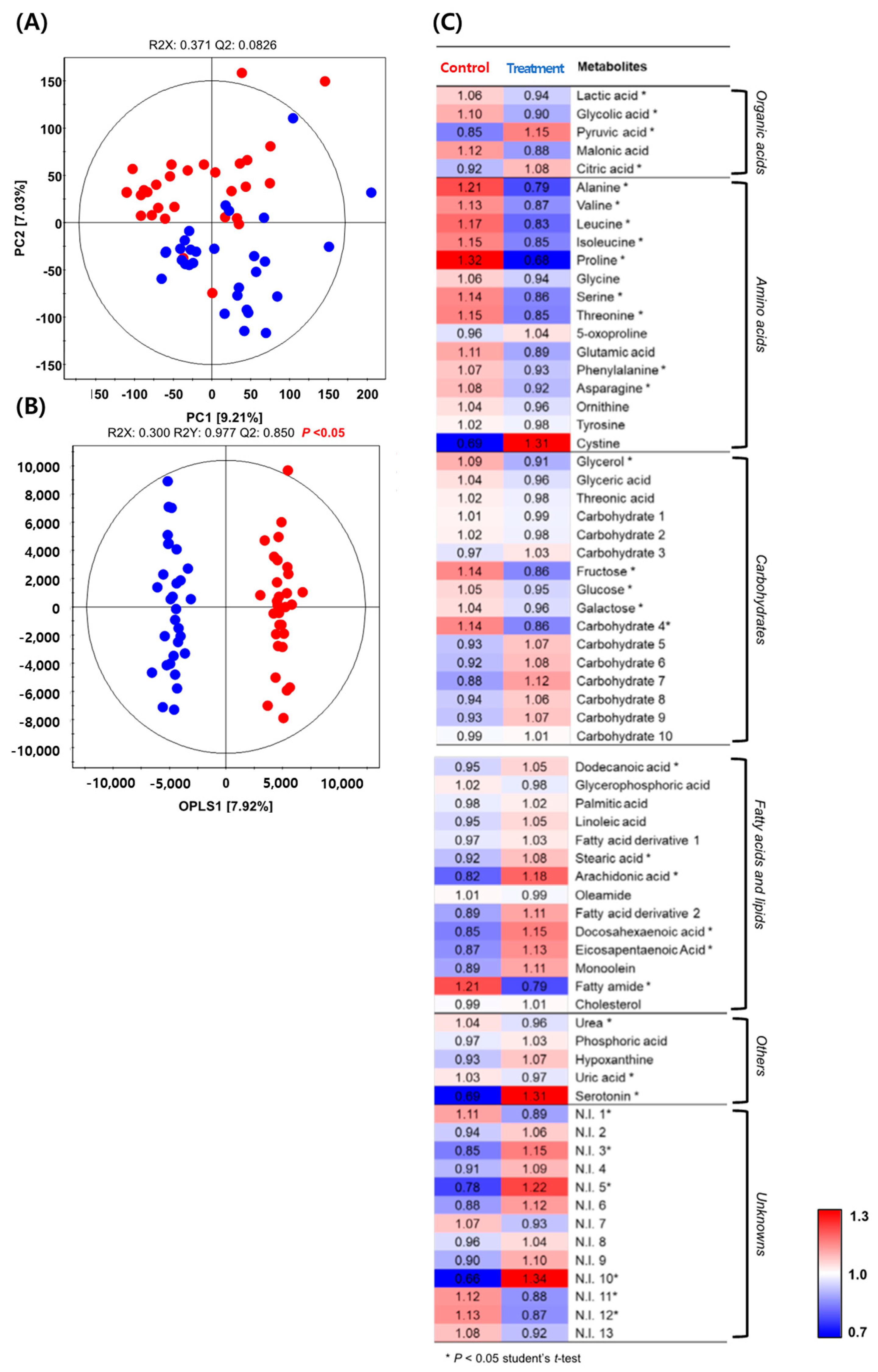
3.4. Metabolite Analysis and Correlation Analysis of Serum Metabolites after the Effect of the Soil Mixing Activity
3.5. Effect of BDNF and CRP Level in Serum after Each Soil Mixing Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Procópio, R.E.; Silva, I.R.; Martins, M.K.; Azevedo, J.L.; Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.J.; Andam, C.P. Within-species genomic variation and variable patterns of recombination in the tetracycline producer Streptomyces rimosus. Front. Microbiol. 2019, 10, 552. [Google Scholar] [CrossRef]
- Liato, V.; Aïder, M. Geosmin as a source of the earthy-musty smell in fruits, vegetables and water: Origins, impact on foods and water, and review of the removing techniques. Chemosphere 2017, 181, 9–18. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Kim, T.; Kim, J.E.; Yang, J.E.; Kim, S. Gender differences in electroencephalographic activity in response to the earthy odorants geosmin and 2-methylisoborneol. Appl. Sci. 2017, 7, 876. [Google Scholar] [CrossRef] [Green Version]
- Haese, G.; Humeau, P.; De Oliveira, F.; Le Callet, P.; Le Cloirec, P. Tastes and odors of water—Quantifying objective analyses: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2455–2501. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F.L.; Silva, V.V.; Dal Pizzol, C.; Spir, L.G.; Praes, C.E.; Maibach, H. Physiological effect of olfactory stimuli inhalation in humans: An overview. Int. J. Cosmet. Sci. 2014, 36, 117–123. [Google Scholar] [CrossRef]
- Breer, K.R. Sense of smell: Recognition and transduction of olfactory signals. Biochem. Soc. Trans. 2003, 31, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Strous, R.D.; Shoenfeld, Y. To smell the immune system: Olfaction, autoimmunity and brain involvement. Autoimmun. Rev. 2006, 6, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Olfactory system: Functional organization and involvement in neurodegenerative disease. Neurology 2010, 75, 1104–1109. [Google Scholar] [CrossRef]
- Touhara, K.; Vosshall, L.B. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef]
- Hongratanaworakit, T.; Buchbauer, G. Relaxing effect of ylang ylang oil on humans after transdermal absorption. Phytother. Res. 2006, 20, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Song, C.; Ikei, H.; Ohira, T.; Miyazaki, Y. Effect of olfactory stimulation by fresh rose flowers on autonomic nervous activity. J. Altern. Complement. Med. 2014, 20, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Kim, S.O.; Son, S.Y.; Kim, M.J.; Lee, C.H.; Park, S.A. Physiological responses of adults during soil-mixing activities based on the presence of soil microorganisms: A metabolomics approach. J. Am. Soc. Hortic. Sci. 2022, 147, 135–144. [Google Scholar] [CrossRef]
- Oh, Y.A.; Kim, S.O.; Park, S.A. Real foliage plants as visual stimuli to improve concentration and attention in elementary students. Int. J. Environ. Res. Public Health 2019, 16, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.O.; Jeong, J.E.; Oh, Y.A.; Kim, H.R.; Park, S.A. Comparing concentration levels and emotional states of children using electroencephalography during horticultural and nonhorticultural activities. HortScience 2021, 56, 324–329. [Google Scholar] [CrossRef]
- Tarkka, I.; Hallett, M. Cortical topography of premotor and motor potentials preceding self-paced, voluntary movement of dominant and non-dominant hands. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 36–43. [Google Scholar] [CrossRef]
- Son, K.; Song, J.; Um, S.; Lee, J.; Kwack, H. Effects of visual recognition of green plants on the changes of EEG in patients with schizophrenia. Acta Hortic. 2004, 639, 193–199. [Google Scholar] [CrossRef]
- Kim, S.O.; Oh, Y.A.; Park, S.A. Foliage plants improve concentration and emotional condition of elementary school students performing an intensive assignment. HortScience 2020, 55, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Jasper, H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–495. [Google Scholar]
- Iijima, M.; Osawa, M.; Nishitani, N.; Iwata, M. Effects of incense on brain function: Evaluation using electroencephalograms and event–related potentials. Neuropsychobiology 2009, 59, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- Lyu, A.; Yang, L.; Wu, M.; Zhang, J.; Li, G. High efficacy of the volatile organic compounds of Streptomyces yanglinensis 3–10 in suppression of Aspergillus contamination on peanut kernels. Front. Microbiol. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, S.; Singh, D.; Oh, J.Y.; Jeon, E.J.; Ryu, H.S.; Lee, D.W.; Kim, B.S.; Lee, C.H. Comparative evaluation of microbial diversity and metabolite profiles in doenjang, a fermented soybean paste, during the two different industrial manufacturing processes. Food Chem. 2017, 221, 1578–1586. [Google Scholar] [CrossRef]
- Park, S.; Son, S.Y.; Lee, A.; Park, H.G.; Lee, W.L.; Lee, C.H. Metabolite profiling revealed that a gardening activity program improves cognitive ability correlated with BDNF levels and serotonin metabolism in the elderly. Int. J. Environ. Res. Public Health 2020, 17, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Kim, S. Effect of olfactory stimulation of isomeric aroma compounds,(+)-limonene and terpinolene on human electroencephalographic activity. Eur. J. Integr. Med. 2015, 7, 561–566. [Google Scholar] [CrossRef]
- Suliman, S.; Hemmings, S.M.; Seedat, S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Front. Integr. Neurosci. 2013, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsych. Dis. Treat. 2009, 5, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, B.S.; Steiner, J.; Molendijk, M.L.; Dodd, S.; Nardin, P.; Gonçalves, C.A.; Jacka, F.; AKöhler, C.; Karmakar, C.; Carvalho, A.F.; et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: A systematic review and meta-analysis. Lancet Psychiatr. 2016, 3, 1147–1156. [Google Scholar] [CrossRef]
- Leung, B.M.; Nwoke, C. Association between C-reactive protein and mood disorder in a representative sample of the Canadian population: Analysis of CHMS data 2013–2014. Can. J. Public Health 2020, 111, 743–751. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest volatile organic compounds and their effects on human health: A state-of-the-art review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1–23. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, C.S. The Chemistry of Fragrances—From Perfumer to Consumer, 2nd ed.; Quest International: Irvine, CA, USA, 2006. [Google Scholar]
- Diego, M.A.; Jones, N.A.; Field, T.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; McAdam, V.; Galamaga, R.; Galamaga, M. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int. J. Neurosci. 1998, 96, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M.; Cisneros, W.; Feijo, L.; Vera, Y.; Gil, K.; Grina, D.; Claire, H.Q. Lavender fragrance cleansing gel effects on relaxation. Int. J. Neurosci. 2005, 115, 207–222. [Google Scholar] [CrossRef]
- Kutlu, A.K.; Yilmaz, E.; Cecen, D. Effects of aroma inhalation on examination anxiety. Teach. Learn. Nurs. 2008, 3, 125–130. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Silber, B.Y.; Schmitt, J.A.J. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407. [Google Scholar] [CrossRef]
- Sircus, M. Compendium Surviving Cancer-Natural Allopathic Medicine; Lulu Press, Inc.: Morrisville, NC, USA, 2014. [Google Scholar]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef] [Green Version]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.W.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A high-resolution in vivo atlas of the human Brain′s serotonin system. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Watakabe, A.; Komatsu, Y.; Sadakane, O.; Shimegi, S.; Takahata, T.; Higo, N.; Tochitani, S.; Hashikawa, T.; Naito, T.; Osaki, H.; et al. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb. Cortex 2009, 19, 1915–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 363, 1–25. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Yao, Y.M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef]

| Variable | |
|---|---|
| Gender | % (N) |
| Male | 36.7 (11) |
| Female | 63.3 (19) |
| Mean (SD) | |
| Age (years) | 28.6 (8.4) |
| Height (cm) | 168.0 (8.4) |
| Body weight (kg) | 62.9 (12.6) |
| Body mass index 1 (kg·m−2) | 22.1 (3.3) |
| Soil Mixing Activity | ASEF50 1 | |
|---|---|---|
| O1 | O2 | |
| Mean ± SD 2 | ||
| Using soil with S. rimosus added after sterilization | 10.376 ± 0.137 | 10.399 ± 0.120 |
| Using sterilized soil | 10.350 ± 0.107 | 10.352 ± 0.121 |
| Significance 3 | 0.132 | 0.008 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-O.; Kim, M.J.; Choi, N.-Y.; Kim, J.H.; Oh, M.S.; Lee, C.H.; Park, S.-A. Psychophysiological and Metabolomics Responses of Adults during Horticultural Activities Using Soil Inoculated with Streptomyces rimosus: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 12901. https://doi.org/10.3390/ijerph191912901
Kim S-O, Kim MJ, Choi N-Y, Kim JH, Oh MS, Lee CH, Park S-A. Psychophysiological and Metabolomics Responses of Adults during Horticultural Activities Using Soil Inoculated with Streptomyces rimosus: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12901. https://doi.org/10.3390/ijerph191912901
Chicago/Turabian StyleKim, Seon-Ok, Min Ji Kim, Na-Yoon Choi, Jin Hee Kim, Myung Sook Oh, Choong Hwan Lee, and Sin-Ae Park. 2022. "Psychophysiological and Metabolomics Responses of Adults during Horticultural Activities Using Soil Inoculated with Streptomyces rimosus: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 19: 12901. https://doi.org/10.3390/ijerph191912901
APA StyleKim, S.-O., Kim, M. J., Choi, N.-Y., Kim, J. H., Oh, M. S., Lee, C. H., & Park, S.-A. (2022). Psychophysiological and Metabolomics Responses of Adults during Horticultural Activities Using Soil Inoculated with Streptomyces rimosus: A Pilot Study. International Journal of Environmental Research and Public Health, 19(19), 12901. https://doi.org/10.3390/ijerph191912901









