Effects of Multicomponent Training Followed by a Detraining Period on Frailty Level and Functional Capacity of Older Adults with or at Risk of Frailty: Results of 10-Month Quasi-Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Evaluations
2.3. Multicomponent Training Program: Eelder-Fit
2.4. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Sample
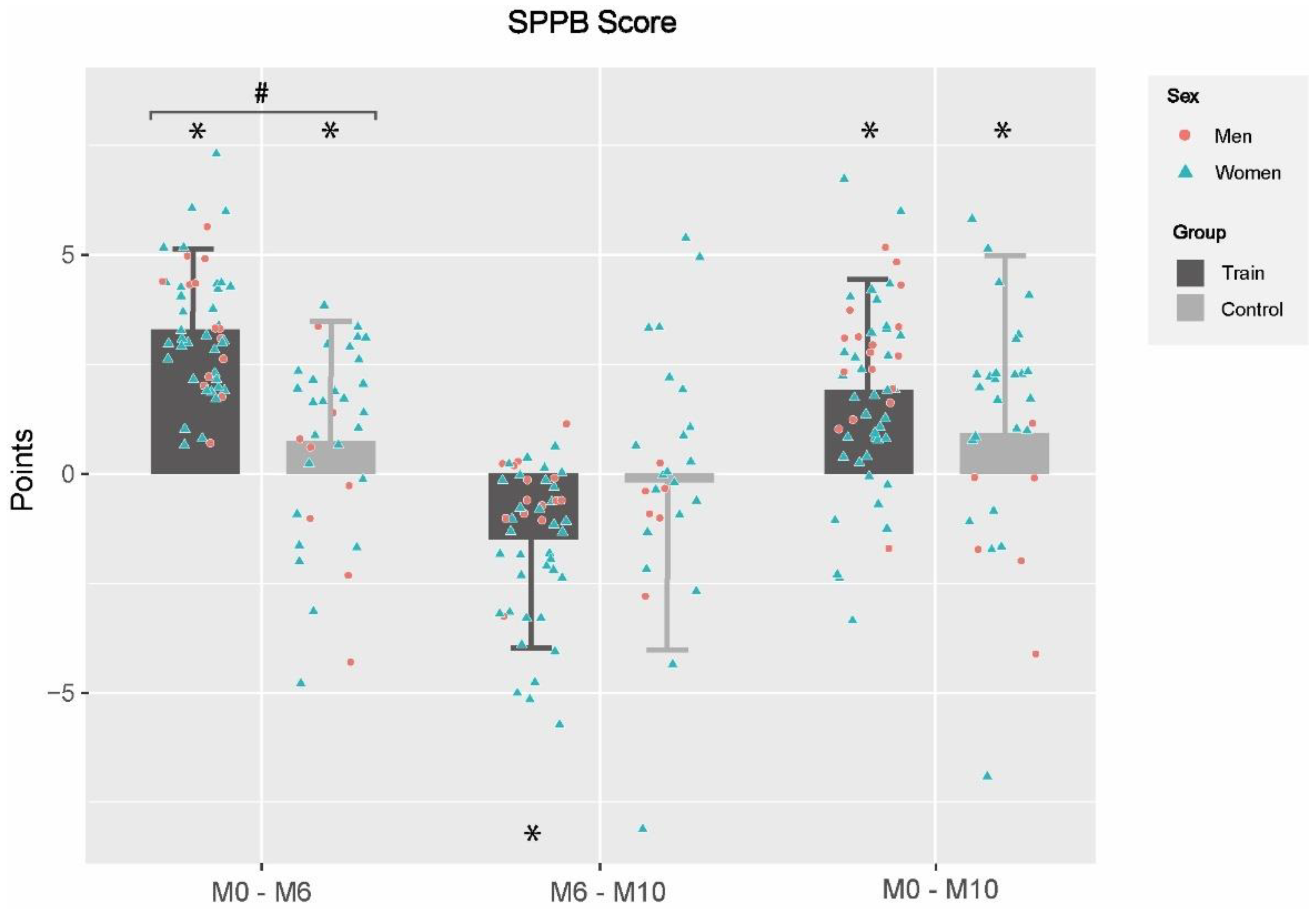
3.2. Effects of Multicomponent Training Program and Detraining Period on Functional Capacity
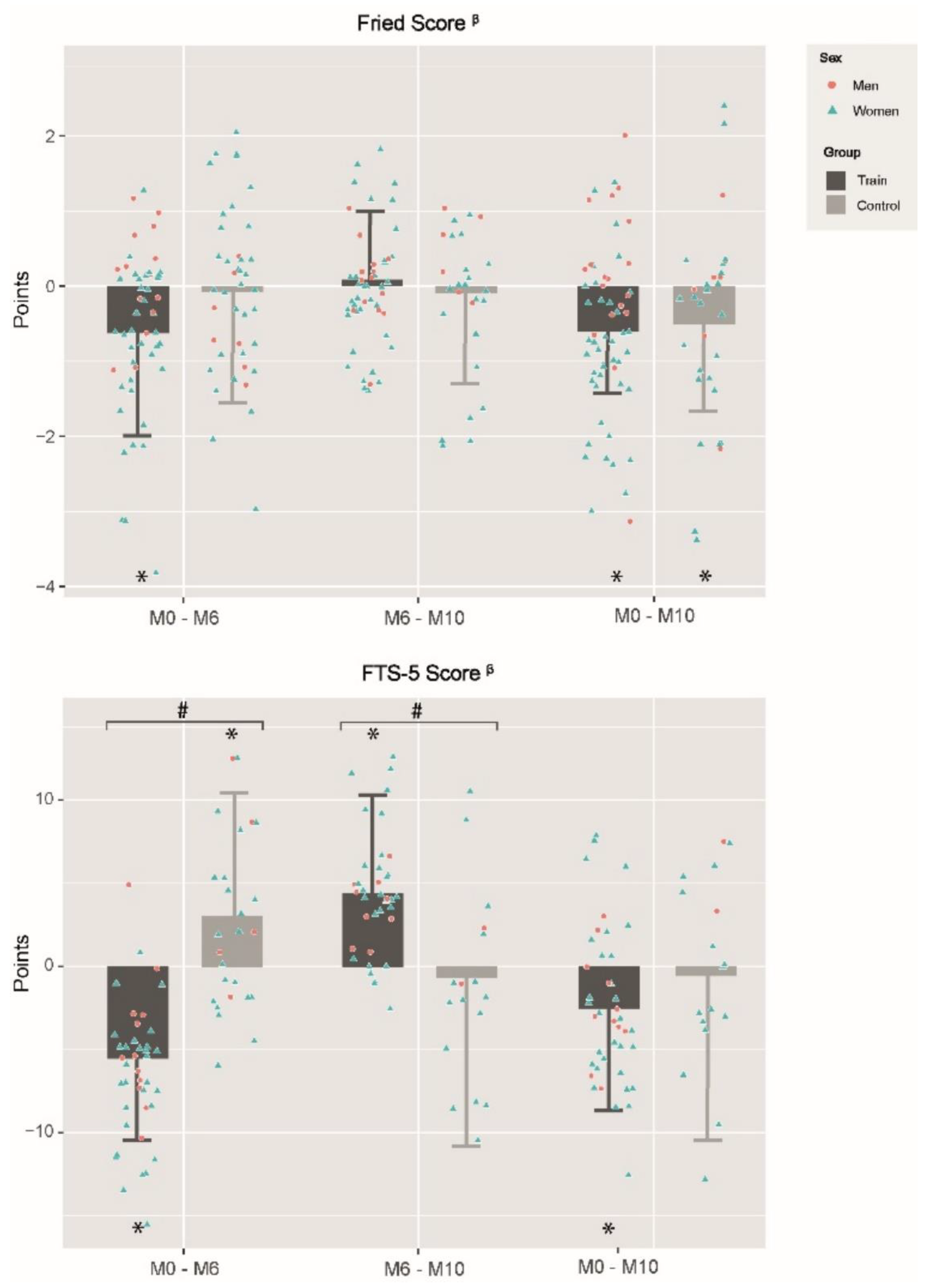
3.3. Effects of MCT Program and Detraining Period on Frailty Level
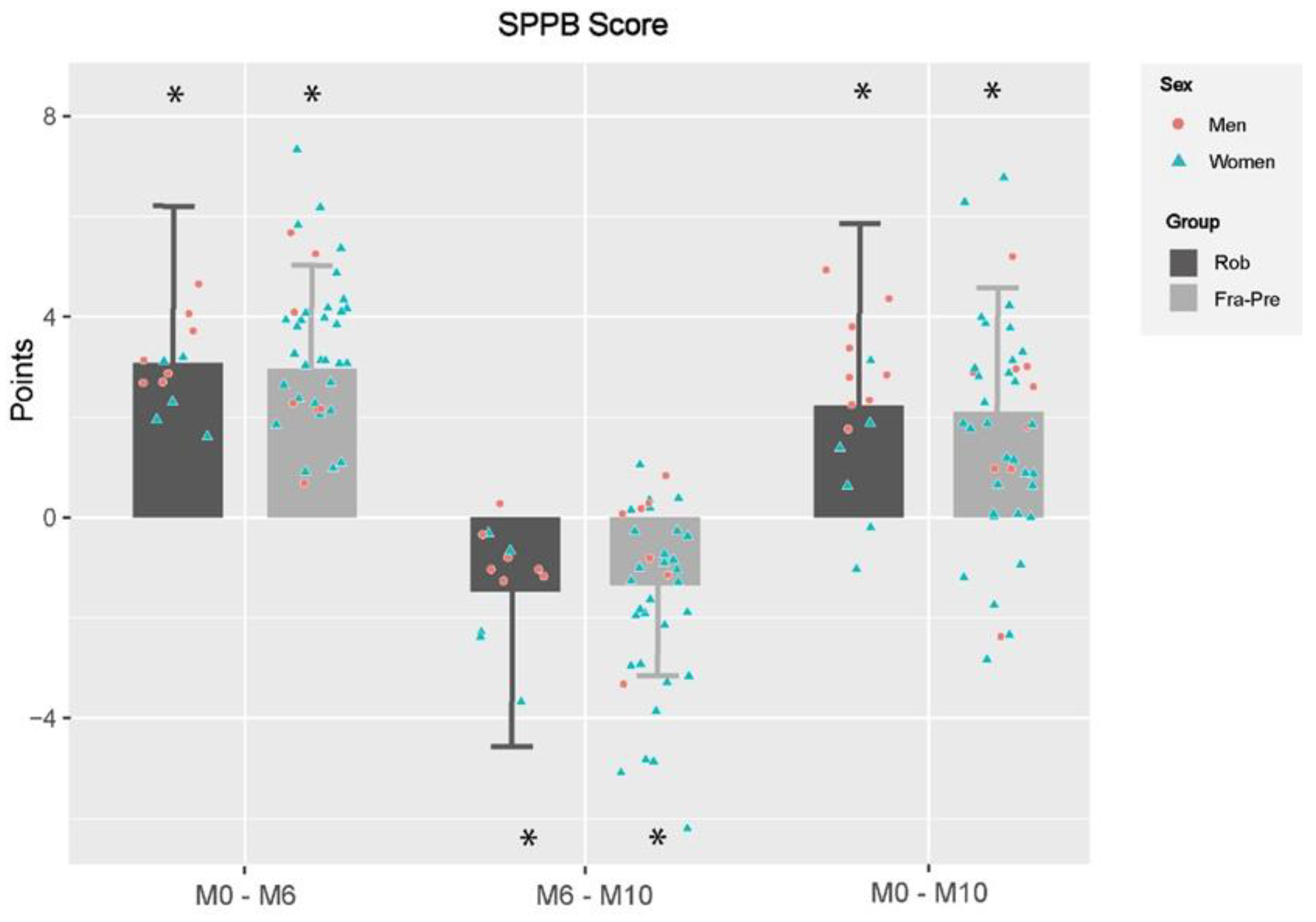
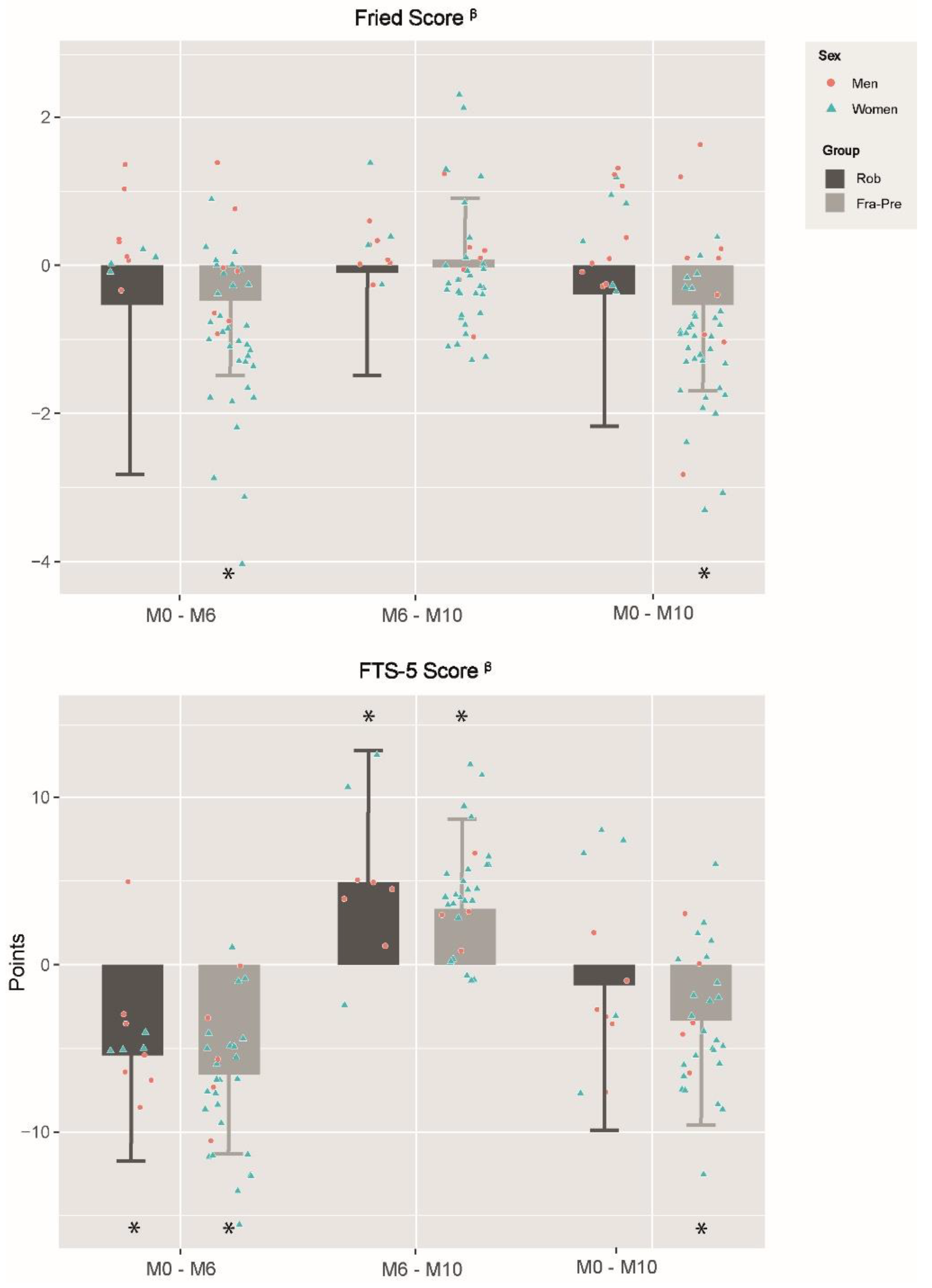
3.4. Effects of Frailty Status in Training and Detraining Effects on Functional Capacity and Frailty Level
4. Discussion
4.1. Training Effects on Functional Capacity
4.2. Training Effects on Frailty Level
4.3. Detraining Effects on Functional Capacity
4.4. Detraining Effects on Frailty
4.5. Effects of Frailty Status on Training and Detraining Effects on Functional Capacity and Frailty Level
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patrizio, E.; Calvani, R.; Marzetti, E.; Cesari, M. Physical Functional Assessment in Older Adults. J. Frailty Aging 2021, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.M.; Cassels, A.K. The world report on ageing and health. Gerontologist 2016, 56, S163–S166. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Stadnyk, K.; MacKnight, C.; McDowell, I.; Hebert, R.; Hogan, D.B. A brief clinical instrument to classify frailty in elderly people. Lancet 1999, 353, 205–206. [Google Scholar] [CrossRef]
- Fried, L.P.; Xue, Q.L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; William Molloy, D.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.; Bock, J.O.; Saum, K.U.; Matschinger, H.; Brenner, H.; Holleczek, B.; Haefeli, W.E.; Heider, D.; König, H.H. Frailty and healthcare costs-longitudinal results of a prospective cohort study. Age Ageing 2018, 47, 233–241. [Google Scholar] [CrossRef]
- García-Nogueras, I.; Aranda-Reneo, I.; Peña-Longobardo, L.M.; Oliva-Moreno, J.; Abizanda, P. Use of health resources and healthcare costs associated with frailty: The FRADEA study. J. Nutr. Health Aging 2017, 21, 207–214. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J. Am. Geriatr. Soc. 2012, 60, 1478–1486. [Google Scholar] [CrossRef]
- García-García, F.J.; Carcaillon, L.; Fernandez-Tresguerres, J.; Alfaro, A.; Larrion, J.L.; Castillo, C.; Rodriguez-Mañas, L. A New Operational Definition of Frailty: The Frailty Trait Scale. J. Am. Med. Dir. Assoc. 2014, 15, 371.e7–371.e13. [Google Scholar] [CrossRef]
- García-García, F.J.; Carnicero, J.A.; Losa-Reyna, J.; Alfaro-Acha, A.; Castillo-Gallego, C.; Rosado-Artalejo, C.; Gutiérrrez-Ávila, G.; Rodriguez-Mañas, L. Frailty Trait Scale–Short Form: A Frailty Instrument for Clinical Practice. J. Am. Med. Dir. Assoc. 2020, 21, 1260–1266. [Google Scholar] [CrossRef]
- Treacy, D.; Hassett, L. The Short Physical Performance Battery. J. Physiother. 2018, 64, 61. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M. Multicomponent physical exercise program: Vivifrail. Nutr. Hosp. 2019, 36, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Perracini, M.R.; Mello, M.; De Oliveira Máximo, R.; Bilton, T.L.; Ferriolli, E.; Lustosa, L.P.; Da Silva Alexandre, T. Diagnostic Accuracy of the Short Physical Performance Battery for Detecting Frailty in Older People. Phys. Ther. 2020, 100, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Arrieta, H.; Rezola-Pardo, C.; Zarrazquin, I.; Echeverria, I.; Yanguas, J.J.; Iturburu, M.; Gil, S.M.; Rodriguez-Larrad, A.; Irazusta, J. A multicomponent exercise program improves physical function in long-term nursing home residents: A randomized controlled trial. Exp. Gerontol. 2018, 103, 94–100. [Google Scholar] [CrossRef]
- Carta, M.G.; Cossu, G.; Pintus, E.; Zaccheddu, R.; Callia, O.; Conti, G.; Pintus, M.; Aviles Gonzalez, C.I.; Massidda, M.V.; Mura, G.; et al. Moderate Exercise Improves Cognitive Function in Healthy Elderly People: Results of a Randomized Controlled Trial. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 75–80. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Liu, Y.; Ye, L. Exercise interventions for older people at risk for frailty: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25940. [Google Scholar] [CrossRef]
- Esain, I.; Gil, S.M.; Bidaurrazaga-Letona, I.; Rodriguez-Larrad, A. Effects of 3 months of detraining on functional fitness and quality of life in older adults who regularly exercise. Aging Clin. Exp. Res. 2019, 31, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, Á.I.; Gómez-Cabello, A.; Gómez-Bruton, A.; Moradell, A.; Navarrete-Villanueva, D.; Pérez-Gómez, J.; González-Gross, M.; Ara, I.; Casajús, J.A.; Vicente-Rodríguez, G. Effects of multicomponent training and detraining on the fitness of older adults with or at risk of frailty: Results of a 10-month quasi-experimental study. Eur. J. Sport Sci. 2022. published online first. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Larrad, A.; Arrieta, H.; Rezola-Pardo, C.; Esain, I.; Mendia-Oria, P.; Irazusta, J. Loss of benefits after cessation of exercise interventions in nursing home residents: Randomized controlled trial follow-up. Geriatr. Nurs. 2021, 42, 621–627. [Google Scholar] [CrossRef]
- Markotegi, M.; Irazusta, J.; Sanz, B.; Rodriguez-Larrad, A. Effect of the COVID-19 pandemic on the physical and psychoaffective health of older adults in a physical exercise program. Exp. Gerontol. 2021, 155, 111580. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, Á.I.; Gómez-Cabello, A.; Moradell, A.; Navarrete-Villanueva, D.; Pérez-Gómez, J.; Ara, I.; Pedrero-Chamizo, R.; Subías-Perié, J.; Muniz-Pardos, B.; Casajús, J.A.; et al. How to Improve the Functional Capacity of Frail and Pre-Frail Elderly People? Health, Nutritional Status and Exercise Intervention. The EXERNET-Elder 3.0 Project. Sustainability 2020, 12, 6246. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1965, 9, 179–186. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Mini nutritional assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Interv. Geriatr. 1997, 4, 15–32. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=2861439 (accessed on 5 May 2020).
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Moradell, A.; Rodríguez-Gómez, I.; Fernández-García, Á.I.; Navarrete-Villanueva, D.; Marín-Puyalto, J.; Pérez-Gómez, J.; Villa-Vicente, J.G.; González-Gross, M.; Ara, I.; Casajús, J.A.; et al. Associations between daily movement distribution, bone structure, falls, and fractures in older adults: A compositional data analysis study. Int. J. Environ. Res. Public Health 2021, 18, 3757. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, V.T.; Renström, F.; Wright, A.; Gradmark, A.; Catt, M.; Chen, K.Y.; Löf, M.; Bluck, L.; Pomeroy, J.; Wareham, N.J.; et al. Estimation of daily energy expenditure in pregnant and Non-Pregnant women using a Wrist-Worn Tri-Axial accelerometer. PLoS ONE 2011, 6, e22922. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, W.; Lang, P.O.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health benefits of multicomponent training programmes in seniors: A systematic review. Int. J. Clin. Pract. 2016, 70, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Moneo AB, B.; Mensat, M.M.; Muñoz, A.R.; Casas-Herrero, A.; Rodriguez-Mañas, L.; Izquierdo, M. Positive effects of resistance training in frail elderly patients with dementia after long-term physical restraint. Age 2014, 36, 801–811. [Google Scholar] [CrossRef]
- Cadore, E.L.; Pinto, R.S.; Bottaro, M.; Izquierdo, M. Strength and endurance training prescription in healthy and frail elderly. In Aging Dis.; 2014; 5, pp. 183–195. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4037310/ (accessed on 21 December 2020). [CrossRef]
- De Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials Physical functioning, physical health and activity. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef]
- Losa-Reyna, J.; Baltasar-Fernandez, I.; Alcazar, J.; Navarro-Cruz, R.; Garcia-Garcia, F.J.; Alegre, L.M.; Alfaro-Acha, A. Effect of a short multicomponent exercise intervention focused on muscle power in frail and pre frail elderly: A pilot trial. Exp. Gerontol. 2019, 115, 114–121. [Google Scholar] [CrossRef]
- Tarazona-Santabalbina, F.J.; Gómez-Cabrera, M.C.; Pérez-Ros, P.; Martínez-Arnau, F.M.; Cabo, H.; Tsaparas, K.; Salvador-Pascual, A.; Rodriguez-Mañas, L.; Viña, J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2016, 17, 426–433. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The effectiveness of exercise interventions for the management of frailty: A systematic review. J. Aging Res. 2011, 2011, 569194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frändin, K.; Grönstedt, H.; Helbostad, J.L.; Bergland, A.; Andresen, M.; Puggaard, L.; Harms-Ringdahl, K.; Granbo, R.; Hellström, K. Long-Term Effects of Individually Tailored Physical Training and Activity on Physical Function, Well-Being and Cognition in Scandinavian Nursing Home Residents: A Randomized Controlled Trial. Gerontology 2016, 62, 571–580. [Google Scholar] [CrossRef] [PubMed]
- McGough, E.L.; Kelly, V.E.; Logsdon, R.G.; McCurry, S.M.; Cochrane, B.B.; Engel, J.M.; Teri, L. Associations between physical performance and executive function in older adults with mild cognitive impairment: Gait speed and the timed “up & go” test. Phys. Ther. 2011, 91, 1198–1207. Available online: https://academic.oup.com/ptj/article-abstract/91/8/1198/2735116 (accessed on 19 April 2021). [PubMed]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Viccaro, L.J.; Perera, S.; Studenski, S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011, 59, 887–892. [Google Scholar] [CrossRef]
- Moradell, A.; Navarrete-Villanueva, D.; Fernández-García, Á.I.; Sagarra-Romero, L.; Marín-Puyalto, J.; Pérez-Gómez, J.; Gesteiro, E.; Ara, I.; Casajus, J.A.; Gómez-Cabello, A.; et al. Effects of a multicomponent exercise program, a detraining period and dietary intake prediction of body composition of frail and pre-frail older adults from the exernet elder 3.0 study. Sustainability 2020, 12, 9894. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Uchida, M.C.; Picca, A.; Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Marzetti, E. Evidence-based recommendations for resistance and power training to prevent frailty in community-dwellers. Aging Clin. Exp. Res. 2021, 33, 2069–2086. [Google Scholar] [CrossRef]
- Esain, I.; Rodriguez-Larrad, A.; Bidaurrazaga-Letona, I.; Gil, S.M. Health-related quality of life, handgrip strength and falls during detraining in elderly habitual exercisers. Health Qual. Life Outcomes 2017, 15, 226. [Google Scholar] [CrossRef]
- Ansai, J.H.; Aurichio, T.R.; Gonçalves, R.; Rebelatto, J.R. Effects of two physical exercise protocols on physical performance related to falls in the oldest old: A randomized controlled trial. Geriatr. Gerontol. Int. 2016, 16, 492–499. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; López Sáez De Asteasu, M.; Morley, J.E.; Cano-Gutierrez, C.A.; Izquierdo, M. Performance of the Short Physical Performance Battery in Identifying the Frailty Phenotype and Predicting Geriatric Syndromes in Community-Dwelling Elderly. J. Nutr. Health Aging 2021, 25, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Manas, A.; Gomez-Redondo, P.; Valenzuela, P.L.; Morales, J.S.; Lucia, A.; Ara, I. Unsupervised home-based resistance training for community-dwelling older adults: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 69, 101368. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.J.; van Assen, M.A.L.M.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M.G.A. The tilburg frailty indicator: Psychometric properties. J. Am. Med. Dir. Assoc. 2010, 11, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Bustos, A.; Carnicero-Carreño, J.A.; Sanchez-Sanchez, J.L.; Garcia-Garcia, F.J.; Alonso-Bouzón, C.; Rodríguez-Mañas, L. Associations between frailty trajectories and frailty status and adverse outcomes in community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2022, 13, 230–239. [Google Scholar] [CrossRef]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Cadore, E.L.; Ramirez-Velez, R.; Izquierdo, M. Inter-individual variability in response to exercise intervention or usual care in hospitalized older adults. J. Cachexia Sarcopenia Muscle 2019, 10, 1266–1275. [Google Scholar] [CrossRef]
- Izquierdo, M.; Rodriguez-Mañas, L.; Casas-Herrero, A.; Martinez-Velilla, N.; Cadore, E.L.; Sinclair, A.J. Is It Ethical Not to Precribe Physical Activity for the Elderly Frail? J. Am. Med. Dir. Assoc. 2016, 17, 779–781. [Google Scholar] [CrossRef]
| Strength & Power Session Periodization | Phase | PHASE 1 Familiarization (Weeks 1–4) | PHASE 2 Strength (Weeks 5–14) | PHASE 3 Coordination and Power (Weeks 15–21) | PHASE 4 Functional and Power (Weeks 22–24) | ||||||||||||||||||||
| Goals | Cause training adaptations | Increase strength levels | Enhance intermuscular coordination | Increase power | Improve performance DLA | ||||||||||||||||||||
| Learn technical executions | Increase muscle endurance | Increase muscle endurance and strength level | Increase strength levels | Increase power and coordination | |||||||||||||||||||||
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| Type of session | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | PW | PW | PW | PW | PW | PW | PW | |
| Sessions/week | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | |
| Nº Ex * | 6(2) | 6(2) | 7(2) | 7(2) | 7 ●(2) | 7(2) | 7(2) | 8 ‡(2) | 8 ●(2) | 8(2) | 8 ‡(2) | 8 ●(2) | 8(2) | 8 ‡(2) | 7 ●(2) | 7 | 7 ● | 7 | 6 ●(6) | 6 ‡(6) | 7(7) | 6 ●(6) | 6 ‡(6) | 6(6) | |
| Sets | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | |
| Rep & Speed | 8↓ | 8↓ | 10↓ | 10↓ | 10→ | 12→ | 15→ | 12→ | 12→ | 15→ | 12→ | 12→ | 15→ | 12→ | 12→ | 15→ | 12→ | 15→ | 12↑ | 12↑ | 15↑ | 12↑ | 12↑ | 15↑ | |
| Balance ex (s) | 15 | 15 | 20 | 20 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | - | - | - | 20 | 20 | 20 | 30 | 30 | 30 | |
| Set Rest time (s) | 90 | 90 | 90 | 90 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 75 | 75 | 75 | 75 | 90(20a) | 90(20a) | 90(20a) | 90(30a) | 90(30a) | 90(30a) | |
| Aerobic Endurance & Functional Session Periodization | Phase | PHASE 1 Familiarization (Weeks 1–4) | PHASE 2 Development (Weeks 5–14) | PHASE 3 Maintenance (Weeks 15–21) | PHASE 4# Functional and Power (Weeks 22–24) | ||||||||||||||||||||
| Goals | Increase aerobic capacity (VO2 max) | Increase aerobic capacity (VO2 max) | Increase aerobic capacity (VO2 max) | Improve performance DLA | |||||||||||||||||||||
| Improve coordination and functional performance | Improve coordination and functional performance | Improve coordination and functional performance | Increase power and coordination | ||||||||||||||||||||||
| Enhance motor skills and dynamic balance | Enhance motor skills and dynamic balance | Enhance motor skills and dynamic balance | |||||||||||||||||||||||
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| Type of session | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | AE | FUN | FUN | FUN | |
| Sessions/week | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | |
| Nº Ex | 7 | 7 | 7 | 7 | 7 ● | 7 | 7 | 7‡ | 7 ● | 7 | 7 | 7 ‡ | 7 ● | 7 | 7 | 7 ‡ | 7 ● | 7 | 7 | 7 ‡ | 7 | 6 | 6 | 6 | |
| Sets | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Set time (s) | 30 | 30 | 45 | 45 | 60 | 60 | 60 | 60 | 75 | 75 | 75 | 75 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 60 | 75 | 90 | |
| Set Rest time (s) | 60 | 60 | 90 | 90 | 90 | 90 | 75 | 75 | 75 | 75 | 60 | 60 | 60 | 60 | 90(30b) | 90(30b) | 90(45b) | 90(45b) | 90(60b) | 90(60b) | 90(60b) | 60(30a) | 75(45a) | 90(60a) | |
| Total WTs | 7 | 7 | 10.5 | 10.5 | 14 | 14 | 14 | 14 | 17.5 | 17.5 | 17.5 | 17.5 | 21 | 21 | 28 | 28 | 31.5 | 31.5 | 35 | 35 | 35 | 18 | 24 | 30 | |
| Ratio (WT:RT) (s) | 1:2 | 1:2 | 1:2 | 1:2 | 1:1.5 | 1:1.5 | 1:1.25 | 1:1.25 | 1:1 | 1:1 | 1.25:1 | 1.25:1 | 1.5:1 | 1.5:1 | 2:1 | 2:1 | 2.25:1 | 2.25:1 | 2.5:1 | 2.5:1 | 2.5:1 | 1.5:1 | 2:1 | 2.5:1 | |
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| STRENGTH, POWER & FUNCTIONAL TRAINING METHODOLOGY | Phase | PHASE 1 Familiarization (Weeks 1–4) | PHASE 2 Strength (Weeks 5–14) | PHASE 3 Coordination and Power (Weeks 15–21) | PHASE 4 Functional and Power (Weeks 22–24) | ||||||||||||||||||||
| Goals | Cause training adaptations | Increase strength levels | Enhance intermuscular coordination | Increase power | Improve performance DLA | ||||||||||||||||||||
| Learn technical executions | Increase muscle endurance | Increase muscle endurance and strength level | Increase strength levels | Increase power and coordination | |||||||||||||||||||||
| Equipment | Elastic resistance bands, free weights (dumbbells, weighted anklets and medicine balls) and fitballs | ||||||||||||||||||||||||
| Strength and Power Exercises | Exercises involving large muscle groups through single movements of lower or upper limbs | Exercises involving large muscle groups through single movements of lower or upper limbs | Exercises involving large muscle groups combined multi-joint movements of lower and upper limbs | Exercises involving large muscle groups through single movements of lower or upper limbs | Exercises involving large muscle groups combined multi-joint movements of lower and upper limbs | ||||||||||||||||||||
| Light weights lifted at low speed | Medium and heavier weights lifted at moderate speed | Medium weights lifted at fast as possible in the concentric phase | |||||||||||||||||||||||
| Performed exercises: Chest press and fly, shoulder press, flexion and abduction, triceps pushdown, kickbacks and overhead extensions, biceps curl, pull-down, high and low back row, pull apart, lower-back extension, trunk rotation, abdominal crunch through sit position, different types of squats, quadriceps extension, leg curl, hip abduction, adduction, flexion and extension and calf raise | |||||||||||||||||||||||||
| Balance Exercises | Static balance exercises | Static balance exercises decreasing limb involvement, base support and input of information from the senses in addition to induce variations in the center of gravity | Balance training included in the strength exercise executions | Static balance exercises decreasing limb involvement, base support and input of information from the senses in addition to induce variations in the center of gravity | |||||||||||||||||||||
| Double leg stance with feet together, single leg stances, semi-tandem and tandem stance | Double leg stance with feet together, single leg stance, semi-tandem and tandem stance with or without the movement of some objects or parts of the body | Double leg stance with feet together, single leg stance, semi-tandem and tandem stance with or without the movement of some objects or parts of the body | |||||||||||||||||||||||
| Functional Exercises | Exercises consisting of dynamic movements that simulated ADL | ||||||||||||||||||||||||
| Shopping, walking avoiding obstacles, bringing and serving food and drink, climbing up and down stairs, walking fast to “take the public transport” and getting up from the floor | |||||||||||||||||||||||||
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
AEROBIC ENDURANCE TRAINING METHODOLOGY | Phase | PHASE 1 Familiarization (weeks 1–4) | PHASE 2 Development (weeks 5–14) | PHASE 3 Maintenance (weeks 15–21) | |||||||||||||||||||||
| Goals | Increase aerobic capacity | Increase aerobic capacity | Increase aerobic capacity | ||||||||||||||||||||||
| Improve coordination and functional performance | Improve coordination and functional performance | Improve coordination and functional performance | |||||||||||||||||||||||
| Enhance motor skills and dynamic balance | Enhance motor skills and dynamic balance | Enhance motor skills and dynamic balance | |||||||||||||||||||||||
| Equipment | Psychomotricity material, agility ladders, static cycles, steps, dumbbells, weighted anklets, balls and balloons. | ||||||||||||||||||||||||
| Aerobic Exercises | Basic exercises with an increase in speed or frequency. The load was also increased by hardening the resistance level in cycling or including slight free weights while performing exercises | ||||||||||||||||||||||||
| Walking, step exercises and stationary cycle for legs, arms or both. | |||||||||||||||||||||||||
| Dynamic Balance-Agility Exercises | Difficulty progressively increased involving both motor (perturbing the center of gravity throughout different types of displacement, changes of direction and/or velocity), load (including slight free weights while performing exercises) and cognitive tasks (dual- and multi-task activities) | ||||||||||||||||||||||||
| Walking with change of direction, toe and heel walking, tandem gait, and | |||||||||||||||||||||||||
| Motor and Coordination Skills | Eye-Hand, Eye-Leg or Eye-Hand-Leg coordination. Difficulty should progressively increase involving both motor and cognitive tasks (dual- and multi-task activities) | ||||||||||||||||||||||||
| Static or dynamic skills-handling with ball (bounce, passes and receptions, throws, turns, changes of direction) and balloon (keep control of the balloon with hands and/or legs) | |||||||||||||||||||||||||
| Characteristics | Whole Sample (n = 106) | Control (n = 46) | Training (n = 60) | p Value CON vs. TRAIN |
|---|---|---|---|---|
| Age (years) | 80.5 ± 6.0 | 79.7 ± 5.8 | 81.1 ± 6.2 | 0.216 |
| Sex | ||||
| Males | 25 (29.1) | 9 (19.6) | 19 (31.7) | 0.161 |
| Females | 63 (70.9) | 37 (80.4) | 41 (68.3) | |
| Functional capacity & ADL performance | ||||
| SPPB (p) | 7.7 ± 1.7 | 7.8 ± 1.7 | 7.5 ± 1.8 | 0.389 |
| IADL score | 10.2 ± 4.1 | 10.1 ± 3.8 | 10.3 ± 4.4 | 0.858 |
| Barthel Index score | 95.5 ± 7.3 | 95.0 ± 8.4 | 96.0 ± 6.4 | 0.515 |
| Frailty level | ||||
| Fried (p) | 1.6 ± 1.3 | 1.6 ± 1.2 | 1.5 ± 1.3 | 0.828 |
| n robusts (fried criteria) | 23 (21.7) | 8 (17.4) | 15 (25.0) | |
| n pre-frails (fried criteria) | 73 (68.9) | 33 (71.7) | 40 (66.7) | |
| n frails (fried criteria) | 10 (9.4) | 5 (10.9) | 5 (8.3) | |
| FTS-5 (p) | 18.8 ± 6.9 | 18.3 ± 7.3 | 19.1 ± 6.7 | 0.612 |
| Physical Activity and Sedentary Behaviour * | ||||
| ST & SB (min/day) | 1333.5 ± 66.1 | 1334.1 ± 64.0 | 1331.5 ± 63.1 | 0.915 |
| LPA (min/day) | 89.4 ± 51.8 | 89.7 ± 52.0 | 93.6 ± 52.4 | 0.954 |
| MVPA (min/day) | 17.1 ± 20.7 | 16.2 ± 19.8 | 14.9 ± 15.4 | 0.625 |
| Body composition measurement | ||||
| BMI | 29.6 ± 5.3 | 29.5 ± 5.5 | 29.8 ± 5.1 | 0.821 |
| Weight (kg) | 72.4 ± 13.6 | 69.5 ± 13.0 | 74.4 ± 13.8 | 0.077 |
| Height | 155.6 ± 10.4 | 152.8 ± 11.8 | 157.7 ± 8.7 | 0.017 |
| % BF | 37.7 ± 6.5 | 38.3 ± 6.6 | 37.3 ± 6.5 | 0.478 |
| Cognitive impairment | ||||
| Minimental score | 25.8 ± 4.2 | 25.8 ± 4.5 | 25.63 ± 4.7 | 0.957 |
| Malnutrition | ||||
| MNA | 24.4 ± 3.4 | 24.7 ± 3.1 | 24.0 ± 4.7 | 0.277 |
| Post-Training vs. Pre-Training M0–M6 | Post-Detraining vs. Post-Training M6–M10 | Post-Detraining vs. Pre-Training M0–M10 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON (n = 35) | TRAIN (n = 51) | Group Effects | CON (n = 27) | TRAIN (n = 49) | Group Effects | CON (n = 31) | TRAIN (n = 56) | Group Effects | |||||||
| Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | |
| Romberg test (pt) | −0.3 ± 1.28 | 0.021 | 0.7 ± 1.1 | <0.001 | <0.001 | −0.4 ± 2.08 | 0.084 | −0.4 ± 1.4 | 0.013 | 0.970 | −0.3 ± 1.9 | 0.164 | 0.1 ± 1.4 | 0.721 | 0.160 |
| 4-m Gait speed test (s) β | −0.4 ± 1.7 | 0.036 | −1.5 ± 1.4 | <0.001 | <0.001 | 0.2 ± 2.11 | 0.532 | 0.6 ± 1.5 | <0.001 | 0.125 | −0.3 ± 2.2 | 0.248 | −1.0 ± 1.6 | <0.001 | 0.009 |
| Chair stand test (s) β | −4.0 ± 5.5 | <0.001 | −6.6 ± 4.3 | <0.001 | <0.001 | 1.1 ± 4.5 | 0.033 | 1.5 ± 3.2 | <0.001 | 0.129 | −3.4 ± 5.4 | <0.001 | −4.8 ± 3.8 | <0.001 | 0.046 |
| Post-Training vs. Pre-Training M0–M6 | Post-Detraining vs. Post-Training M6–M10 | Post-Detraining vs. Pre-Training M0–M10 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON (n = 26) | TRAIN (n = 41) | Group Effects | CON (n = 17) | TRAIN (n = 38) | Group Effects | CON (n = 17) | TRAIN (n = 41) | Group Effects | |||||||
| Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | |
| Romberg test (FTS-5 score) β | 1.7 ± 4.8 | 0.005 | −2.2 ± 3.7 | <0.001 | <0.001 | 0.7 ± 8.2 | 0.518 | 1.6 ± 4.8 | 0.014 | 0.457 | −0.1 ± 7.3 | 0.940 | −0.5 ± 4.6 | 0.425 | 0.693 |
| BMI (kg/m2) | −0.3 ± 1.8 | 0.200 | −0.1 ± 1.4 | 0.628 | 0.433 | 0.3 ± 1.1 | 0.100 | 0.1 ± 0.7 | 0.415 | 0.292 | −0.1 ± 2.3 | 0.756 | −0.3 ± 1.4 | 0.183 | 0.637 |
| PASE (pt) | −22.7 ± 65.6 | 0.006 | 16.1 ± 50.2 | 0.010 | <0.001 | −14.2 ± 69.42 | 0.132 | −25.2 ± 41.8 | <0.001 | 0.295 | −16.4 ± 67.7 | 0.067 | −9.6 ± 41.8 | 0.082 | 0.481 |
| 4-m Gait speed test (s) β | −0.3 ± 1.8 | 0.139 | −1.6 ± 1.4 | <0.001 | <0.001 | −0.1 ± 2.0 | 0.786 | 0.7 ± 1.3 | <0.001 | 0.009 | −0.3 ± 2.7 | 0.410 | −1.1 ± 1.6 | <0.001 | 0.051 |
| Grip strength (kg) | 1.3 ± 6.2 | 0.085 | 2.7 ± 5.4 | <0.001 | 0.102 | 0.5 ± 4.5 | 0.413 | 0.9 ± 3.4 | 0.060 | 0.570 | 2.0 ± 7.3 | 0.037 | 3.1 ± 5.1 | <0.001 | 0.289 |
| SPPB | Post-Training vs. Pre-Training M0–M6 | Post-Detraining vs. Post-Training M6–M10 | Post-Detraining vs. Pre-Training M0–M10 | |||||||||||||
| ROB (n = 12) | FRA-PRE (n = 39) | Group Effects | ROB (n = 12) | FRA-PRE (n = 37) | Group Effects | ROB (n = 15) | FRA-PRE (n = 41) | Group Effects | ||||||||
| Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | ||
| Romberg test (pt) | 0.8 ± 1.1 | <0.001 | 0.8 ± 0.7 | <0.001 | 0.943 | −1.1 ± 2.0 | <0.001 | −0.3 ± 1.3 | 0.106 | 0.034 | −0.1 ± 1.9 | 0.591 | 0.4 ± 1.3 | 0.016 | 0.089 | |
| 4-m Gait speed test (s) β | −1.8 ± 2.1 | <0.001 | −1.6 ± 1.3 | <0.001 | 0.625 | 0.7 ± 2.2 | 0.034 | 0.6 ± 1.4 | 0.007 | 0.767 | −1.1 ± 2.1 | <0.001 | −1.1 ± 1.4 | <0.001 | 0.954 | |
| Chair stand test (s) β | −6.7 ± 7.3 | <0.001 | −6.7 ± 4.6 | <0.001 | 0.949 | 0.4 ± 4.7 | 0.539 | 2.0 ± 3.1 | <0.001 | 0.077 | −5.9 ± 6.0 | <0.001 | −4.6 ± 4.0 | <0.001 | 0.230 | |
| FTS-5 | ROB (n = 11) | FRA-PRE (n = 30) | Group Effects | ROB (n = 8) | FRA-PRE (n = 30) | Group Effects | ROB (n = 11) | FRA-PRE (n = 30) | Group Effects | |||||||
| Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | Change | p Value | Change | p Value | p Value | ||
| Romberg test (SPPB score) β | −2.4 ± 3.5 | <0.001 | −2.9 ± 2.5 | <0.001 | 0.463 | 3.5 ± 6.8 | 0.003 | 1.1 ± 4.5 | 0.155 | 0.085 | 0.5 ± 6.3 | 0.619 | −2.1 ± 4.5 | 0.004 | 0.041 | |
| BMI (kg/m2) | 0.1 ± 1.9 | 0.750 | −0.2 ± 1.3 | 0.461 | 0.530 | 0.1 ± 1.1 | 0.703 | 0.1 ± 0.7 | 0.339 | 0.846 | −0.4 ± 2.3 | 0.226 | −0.1 ± 1.6 | 0.632 | 0.489 | |
| PASE (pt) | 20.5 ± 87.8 | 0.138 | 11.9 ± 60.3 | 0.209 | 0.623 | −23.1 ± 69.9 | 0.046 | −36.3 ± 46.2 | <0.001 | 0.353 | −6.6 ± 67.0 | 0.529 | −12.2 ± 50.1 | 0.124 | 0.672 | |
| 4-m Gait speed test (s) β | −1.8 ± 1.9 | <0.001 | −1.9 ± 1.3 | <0.001 | 0.859 | 0.7 ± 2.2 | 0.059 | 0.7 ± 1.5 | 0.004 | 0.952 | −1.2 ± 2.3 | 0.002 | −1.2 ± 1.6 | <0.001 | 0.951 | |
| Grip strength (kg) | 1.5 ± 7.4 | 0.207 | 2.8 ± 4.6 | <0.001 | 0.303 | 1.7 ± 4.8 | 0.037 | 0.4 ± 3.4 | 0.431 | 0.169 | 2.0 ± 7.5 | 0.083 | 3.1 ± 4.9 | <0.001 | 0.394 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, Á.I.; Moradell, A.; Navarrete-Villanueva, D.; Subías-Perié, J.; Pérez-Gómez, J.; Ara, I.; González-Gross, M.; Casajús, J.A.; Vicente-Rodríguez, G.; Gómez-Cabello, A. Effects of Multicomponent Training Followed by a Detraining Period on Frailty Level and Functional Capacity of Older Adults with or at Risk of Frailty: Results of 10-Month Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 12417. https://doi.org/10.3390/ijerph191912417
Fernández-García ÁI, Moradell A, Navarrete-Villanueva D, Subías-Perié J, Pérez-Gómez J, Ara I, González-Gross M, Casajús JA, Vicente-Rodríguez G, Gómez-Cabello A. Effects of Multicomponent Training Followed by a Detraining Period on Frailty Level and Functional Capacity of Older Adults with or at Risk of Frailty: Results of 10-Month Quasi-Experimental Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12417. https://doi.org/10.3390/ijerph191912417
Chicago/Turabian StyleFernández-García, Ángel Iván, Ana Moradell, David Navarrete-Villanueva, Jorge Subías-Perié, Jorge Pérez-Gómez, Ignacio Ara, Marcela González-Gross, José Antonio Casajús, Germán Vicente-Rodríguez, and Alba Gómez-Cabello. 2022. "Effects of Multicomponent Training Followed by a Detraining Period on Frailty Level and Functional Capacity of Older Adults with or at Risk of Frailty: Results of 10-Month Quasi-Experimental Study" International Journal of Environmental Research and Public Health 19, no. 19: 12417. https://doi.org/10.3390/ijerph191912417
APA StyleFernández-García, Á. I., Moradell, A., Navarrete-Villanueva, D., Subías-Perié, J., Pérez-Gómez, J., Ara, I., González-Gross, M., Casajús, J. A., Vicente-Rodríguez, G., & Gómez-Cabello, A. (2022). Effects of Multicomponent Training Followed by a Detraining Period on Frailty Level and Functional Capacity of Older Adults with or at Risk of Frailty: Results of 10-Month Quasi-Experimental Study. International Journal of Environmental Research and Public Health, 19(19), 12417. https://doi.org/10.3390/ijerph191912417










