Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives
Abstract
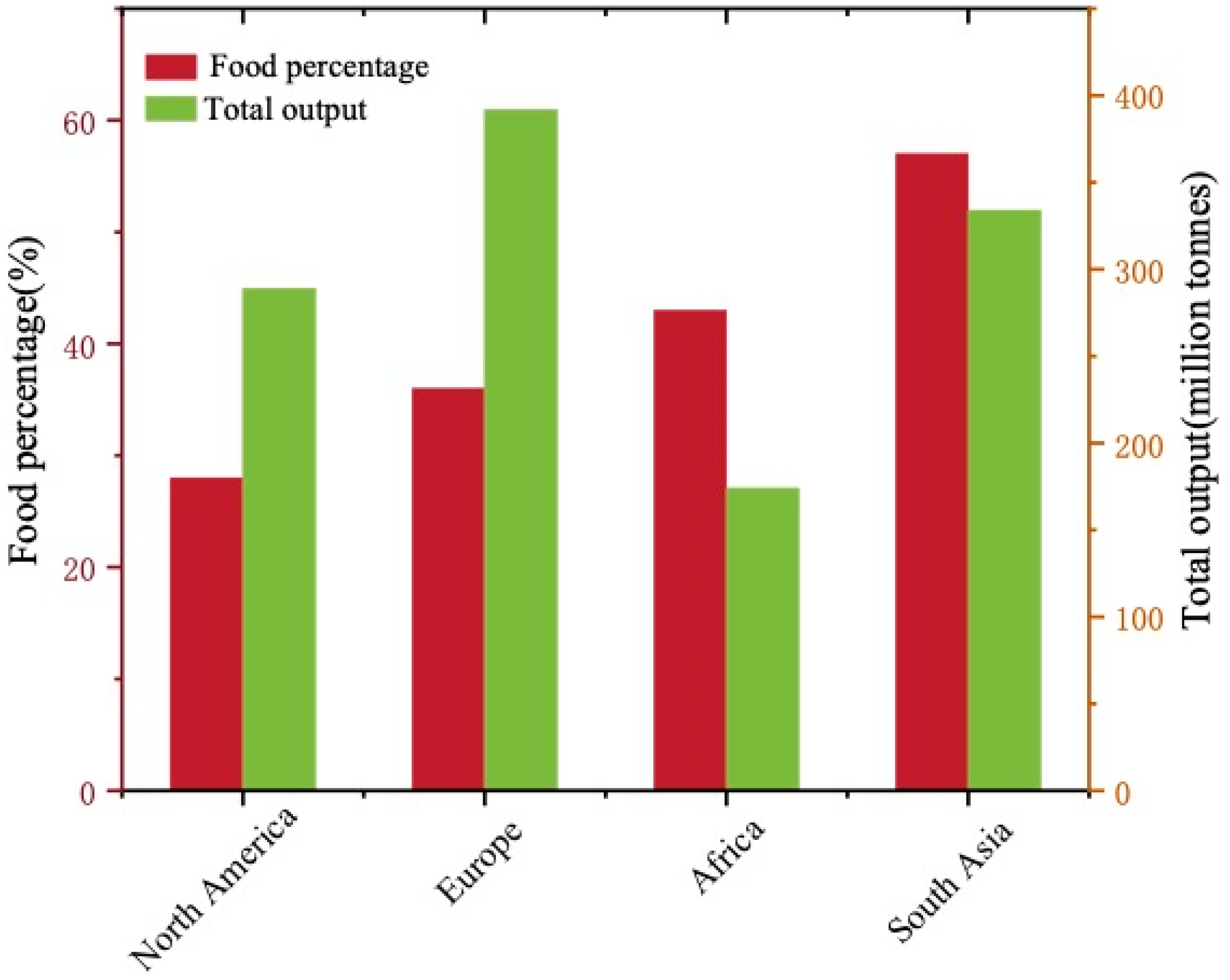
:1. Introduction
2. The Current State of AD of FW
2.1. Hydrogen Production
2.1.1. Temperature
2.1.2. pH
2.1.3. Nutrient and Metal Elements
2.1.4. Pre-Treatment
2.1.5. Co-Digestion
2.2. Organic Acid
2.2.1. Temperature
2.2.2. pH
2.2.3. Nutrient and Metal Elements
2.2.4. Pre-Treatment
2.2.5. Co-Digestion
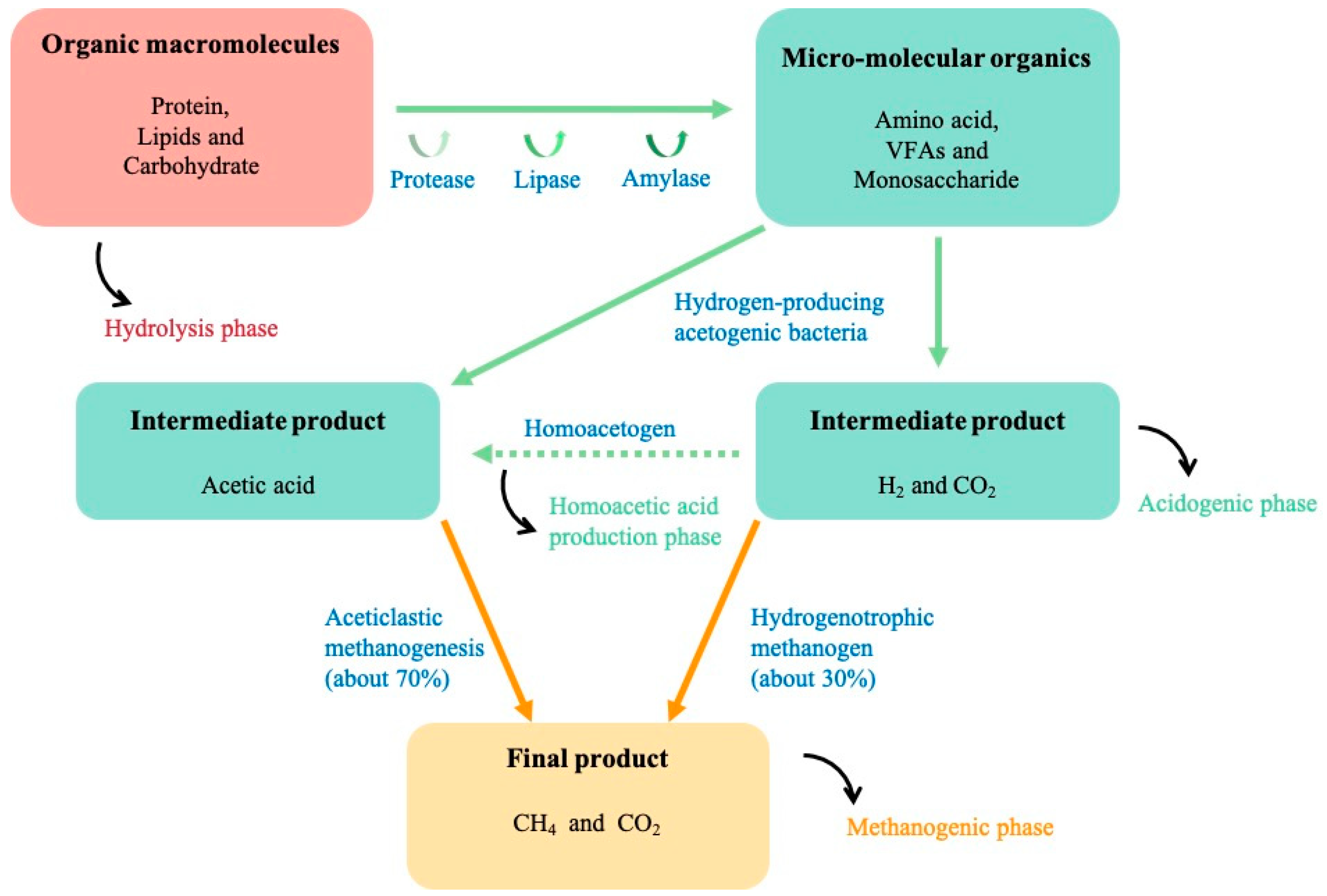
2.3. Methane
2.3.1. Temperature
2.3.2. pH
2.3.3. Nutrient and Metal Elements
2.3.4. Pre-Treatment
2.3.5. Co-Digestion
3. Studies of Anaerobic Microorganisms
3.1. Single Isolation Strains
3.2. Application of DGGE Technology
3.3. Application of FISH Technology
3.4. Application of Cloning Library Technology
3.5. Application of TRFLP Technology
| Feedstocks | Scale | Dominant Phylum (Bacteria) | Dominant Genus/Species (Bacteria) | Dominant Order (Archaea) | Dominant Genus/Species (Archaea) | Sequencing Platform | Ref. |
|---|---|---|---|---|---|---|---|
| FW + Anaerobic sludge | 500 mL reactor | ND | ND | ND | Methanosarcina mazei Methanobacterium sp. Methanoculleus marisniqri | DGGE | [104] |
| FW+ brown water | 5 L CSTR reactor | Bacteroidetes Chloroflexi Proteobacteria Firmicutes | Lactobacillus sp. Acetobacter peroxydans Fusobacterium sp. | Methanosarcinales Methanomicrobiales | Methanoculleus Methanosarcina Methanosaeta | FISH | [108] |
| Household waste | 45 L reactor | Bacteroidetes Chloroflexi Firmicutes Spirochaetes Thermotogae Actinobacteria Proteobacteria | ND | Thermoplasma Crenarchaeota Methanosarcinales Methanomicrobiales | Methanosarcina, Methanoculleus Methanobacterium. | Cloning library analysis | [107] |
| FW+ Fresh cow manure | 0.75 L CSTR reactor | Bacteroidetes Firmicutes Proteobacteria Spirochaetes | Clostridia Bacteroidetes Petrimonas Bacteroides | ND | Methanosarcina Methanobrevibacter Methanobacterium Methanoculleus | TRFLP | [111] |
| FW + sludge | 11 L STRs reactors | ND | ND | Methanosarcinales | Methanosarcina | TRFLP | [112] |
3.6. Application of High-Throughput Sequencing Technology
| Feedstocks | Scale | Dominant Phylum (Bacteria) | Dominant Genus (Bacteria) | Dominant Order (Archaea) | Dominant Genus (Archaea) | Sequencing Platform | Ref. |
|---|---|---|---|---|---|---|---|
| corn straw + chicken manure | 1 L bottle | Bacteroidetes Firmicutes Protecobacteria Chloroflexi Tenericutes | Order: Bacteroidales Clostridiales Xanthomonadales Lactobacillales Spirochaetales | Methanosarcinales Thermoplasmatales Methanobacteriales Methanomicrobiales | ND | Illumina | [118] |
| Anaerobic sludge + food wastewater | 50 L CSTR reactor | Firmicutes Bacteroidetes Chloroflexi Actinobacteria Synergistetes | Order: Sphingobacteriales Bacillales Synergistales Thermotogales Clostridiales | Methanobacteriaceae Methanosaetaceae Methanosaetaceae Methanomicrobiaceae | Methanobacterium Methanosaeta Methanoculleus Methanolobus Methanosphaera | Illumina | [119] |
| FW + Sludge | 400 mL anaerobic bottles | Chloroflexi Bacteroidetes Synergistetes Proteobacteria Firmicutes | Sutterella Treponema Phascolarctobacterium Bifidobacterium Bacteroides | Methanomicrobiales Thermoplasmatales Methanobacteriales Methanosarcinales | Methanosarcina, Methanoculleus Methanospirillum Methanobacterium Methanosaeta | Illumina | [88] |
| Anaerobic sludge | 118 mL reactor | Actinobacteria Bacteroidetes Chloroflexi Firmicutes Spirochaetes | Coprococcus Mesotoga Cloacamonas Clostridium Treponema | Methanosacrinales Methanomicrobiales Thermotogae Methanobacteriales | Methanothrix Methanoculleus Methanolinea Methanosaeta Methanobacterium | Illumina | [120] |
| FW + Seed sludge | three 6 L glass reactors | Thermotogae Tenericutes Chloroflexi Bacteroidetes Firmicutes | Rikenellaceae Anaerolineaceae Clostridiales Gelria Barnesiella | Thermoplasmatales Methanosarcinales Methananomicrobiales Methanobacteriales | ND | 454 | [116] |
| FW + Sludge | 50 L CSTR reactor | Firmicutes Bacteroidetes Nitrospirae Spirochaetes | Actinomyces Fastidiosipila Proteiniphilum Mobilitalea Aminobacterium | Methanobacteriales Methanomicrobiales Methanosarcinales | Methanosaeta Methanosarcina Methanobacterium Methanospirillim | Illumina | [121] |
| FRW + DWW | 24 L AnCMBR | Bacteroidetes Fiemicutes Nitrospirae Proteobacteria Spirochaetes | ND | Methanobacterials Methanomicrobiales Methanosacrinales | ND | Illumina | [122] |
3.7. Combination of High-Throughput Sequencing Technology and Isotope Tracing Technology
4. Perspective for the Study of Anaerobic Microorganisms
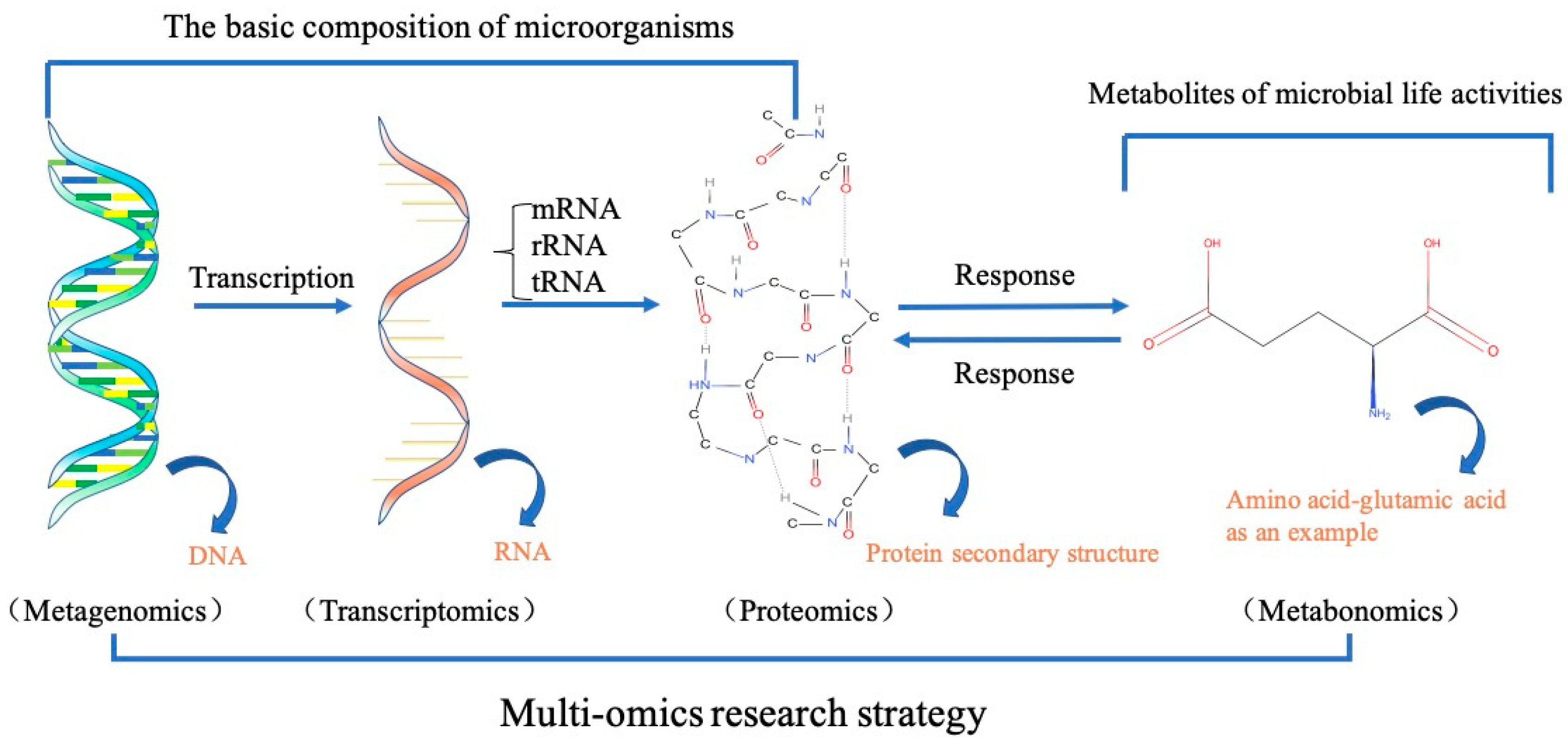
4.1. Integration of Multi-Omics for Understanding of the Microbial Community Activity during FWAD Process
4.2. Synthetic Biology for Manipulation of Functioning Microbial Consortia during FWAD Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| COD | chemical oxygen demand |
| DGGE | denaturing gradient gel electrophoresis |
| DNA-SIP | stable nucleic acid probing |
| FISH | fluorescence in situ hybridization |
| FW | food waste |
| FWAD | food waste anaerobic digestion |
| MSW | municipal solid wastes |
| OLR | organic loading rate |
| TRFLP | terminal restriction fragment length polymorphism |
| TSS | total suspended solids |
| VFAs | volatile fatty acids |
| VSS | volatile suspended solids |
References
- Lebersorger, S.; Schneider, F. Discussion on the methodology for determining food waste in household waste composition studies. Waste Manag. 2011, 31, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050, 3rd ed.; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- De Clercq, D.; Wen, Z.; Fan, F. Performance evaluation of restaurant food waste and biowaste to biogas pilot projects in China and implications for national policy. J. Environ. Manag. 2017, 189, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, M.; Yu, J.; Zhang, G. Food waste management in China: Status, problems and solutions. Shengtai Xuebao/Acta Ecol. Sin. 2012, 32, 4575–4584. [Google Scholar]
- Smith, M.; Shaw, P.; Williams, I. The potential for reducing avoidable food waste arisings from domestic households. In Proceedings of SUM 2014–Second Symposium on Urban Mining, 2nd ed.; CISA Publisher: Bergamo, Italy, 2014; pp. 73–89. [Google Scholar]
- Appel, F.; Ostermeyer-Wiethaup, A.; Balmann, A. Effects of the German Renewable Energy Act on structural change in agriculture–The case of biogas. Util. Policy 2016, 41, 172–182. [Google Scholar] [CrossRef]
- Pazera, A.; Slezak, R.; Krzystek, L.; Ledakowicz, S.; Bochmann, G.; Gabauer, W.; Helm, S.; Reitmeier, S.; Marley, L.; Gorga, F. Biogas in Europe: Food and beverage (FAB) waste potential for biogas production. Energy Fuels 2015, 29, 4011–4021. [Google Scholar] [CrossRef]
- Whiting, A.; Azapagic, A. Life cycle environmental impacts of generating electricity and heat from biogas produced by anaerobic digestion. Energy 2014, 70, 181–193. [Google Scholar] [CrossRef]
- Bees, A.; Williams, I. Explaining the differences in household food waste collection and treatment provisions between local authorities in England and Wales. Waste Manag. 2017, 70, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Tian, Z.; Wang, Z.; Wennersten, R.; Sun, Q. Comparison between the technologies for food waste treatment. Energy Procedia 2017, 105, 3915–3921. [Google Scholar] [CrossRef]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and biogas production potential of municipal solid wastes pretreated with a rotary drum reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Katami, T.; Yasuhara, A.; Shibamoto, T. Formation of dioxins from incineration of foods found in domestic garbage. Environ. Sci. Technol. 2004, 38, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Ting, C. Environmental impact analysis of two typical restaurant garbage regeneration technologies. J. Chin. J. Environ. Eng. 2010, 4, 189–194. [Google Scholar]
- Yuan, J.; Yang, Q.; Zhang, Z.; Li, G.; Luo, W.; Zhang, D. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. J. Environ. Sci. 2015, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Martins, O.; Dewes, T. Loss of nitrogenous compounds during composting of animal wastes. Bioresour. Technol. 1992, 42, 103–111. [Google Scholar] [CrossRef]
- Shimizu, S.; Fujisawa, A.; Mizuno, O.; Kameda, T.; Yoshioka, T. Fermentative Hydrogen Production from Food Waste without Inocula. In Proceedings of the 5th International Workshop on Water Dynamics, Sendai, Japan, 25–27 September 2007. [Google Scholar]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Ma, L.; Lai, S.; Zhao, S.; Chen, Y.; Liu, Y. Improved production of propionic acid driven by hydrolyzed liquid containing high concentration of l-lactic acid from co-fermentation of food waste and sludge. Bioresour. Technol. 2016, 220, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic digestion of food waste in a thermophilic leach bed reactor: Effect of pH and leachate recirculation rate on hydrolysis and volatile fatty acid production. Bioresour. Technol. 2017, 245, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Long-term anaerobic digestion of food waste stabilized by trace elements. Waste Manag. 2012, 32, 1509–1515. [Google Scholar] [CrossRef]
- Molino, A.; Nanna, F.; Ding, Y.; Bikson, B.; Braccio, G. Biomethane production by anaerobic digestion of organic waste. Fuel 2013, 103, 1003–1009. [Google Scholar] [CrossRef]
- Appels, L.; Van Assche, A.; Willems, K.; Degrève, J.; Van Impe, J.; Dewil, R. Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 4124–4130. [Google Scholar] [CrossRef]
- Coelho, N.M.G.; Droste, R.L.; Kennedy, K.J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res. 2011, 45, 2822–2834. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [Green Version]
- Zappi, A.; Hernandez, R.; Holmes, W.E. A review of hydrogen production from anaerobic digestion. Int. J. Environ. Sci. Technol. 2021, 18, 4075–4090. [Google Scholar] [CrossRef]
- Arslan, C.; Sattar, A.; Ji, C.; Sattar, S.; Yousaf, K.; Hashim, S. Optimizing the impact of temperature on bio-hydrogen production from food waste and its derivatives under no pH control using statistical modelling. Biogeosciences 2015, 12, 6503–6514. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.H.; Zhang, B.; Cai, W.M. Effect of temperature on hydrolysis and acidification of kitchen waste in two-phase anaerobic digestion. Environ. Sci. 2006, 8, 213–217. [Google Scholar]
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.-S. Methane production potential of leachate generated from Korean food waste recycling facilities: A lab-scale study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Jung, K.-W.; Kim, M.-S.; Shin, H.-S. Effect of initial pH independent of operational pH on hydrogen fermentation of food waste. Bioresour. Technol. 2011, 102, 8646–8652. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Wang, J. Effect of pH value on VFA concentration and composition during anaerobic fermentation of kitchen waste. China Environ. Sci. 2013, 33, 680–684. [Google Scholar]
- Yang, L.; Huang, Y.; Zhao, M.; Huang, Z.; Miao, H.; Xu, Z.; Ruan, W. Enhancing biogas generation performance from food wastes by high-solids thermophilic anaerobic digestion: Effect of pH adjustment. Int. Biodeter. Biodegr. 2015, 105, 153–159. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q. Buffering and nutrient effects of white mud from ammonia–soda process on thermophilic hydrogen fermentation from food waste. Int. J. Hydrogen Energy 2013, 38, 13564–13571. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Sheng, K.; Chen, X.; Pan, J.; Kloss, R.; Wei, Y.; Ying, Y. Effect of ammonia and nitrate on biogas production from food waste via anaerobic digestion. Biosyst. Eng. 2013, 116, 205–212. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrogen Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Wang, J.M.; Jiang, J.G.; Gong, C.X.; Zhang, Y.J.; Li, M.L. Effect of ultrasonic pretreatment on VFAs production from kitchen waste. China Environ. Sci. 2014, 34, 1207–1211. [Google Scholar]
- Jia, X.; Li, M.; Xi, B.; Zhu, C.; Yang, Y.; Xia, T.; Song, C.; Pan, H. Integration of fermentative biohydrogen with methanogenesis from fruit–vegetable waste using different pre-treatments. Energy Convers. Manag. 2014, 88, 1219–1227. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Guo, Q.; Qian, X.; Niu, D. Bio-hydrogen production from food waste and sewage sludge in the presence of aged refuse excavated from refuse landfill. Renew. Energy 2008, 33, 2573–2579. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X. Anaerobic co-digestion of kitchen waste and cattle manure for methane production. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1848–1856. [Google Scholar] [CrossRef]
- Tanisho, S.; Ishiwata, Y. Continuous hydrogen production from molasses by fermentation using urethane foam as a support of flocks. Int. J. Hydrogen Energy 1995, 20, 541–545. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process. Biochem. 2000, 35, 589–593. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, R.-C. Fermentative hydrogen production at ambient temperature. Int. J. Hydrogen Energy 2004, 29, 715–720. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Su, M.; di Pumpo, F.; Wandera, S.M.; Adani, F.; Dong, R. Bio-hydrolysis and bio-hydrogen production from food waste by thermophilic and hyperthermophilic anaerobic process. Bioresour. Technol. 2016, 216, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, J.T.; Bagley, D.M. Improving the yield from fermentative hydrogen production. Biotechnol. Lett. 2007, 29, 685–695. [Google Scholar] [CrossRef]
- Gottschalk, G. Regulation of bacterial metabolism. In Bacterial Metabolism, 2nd ed.; Springer: New York, NY, USA, 1986; pp. 178–207. [Google Scholar]
- Horiuchi, J.-I.; Shimizu, T.; Tada, K.; Kanno, T.; Kobayashi, M. Selective production of organic acids in anaerobic acid reactor by pH control. Bioresour. Technol. 2002, 82, 209–213. [Google Scholar] [CrossRef]
- Shin, H.-S.; Youn, J.-H.; Kim, S.-H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Kim, H.-W.; Kim, M.-S.; Shin, H.-S. Sewage sludge addition to food waste synergistically enhances hydrogen fermentation performance. Bioresour. Technol. 2011, 102, 8501–8506. [Google Scholar] [CrossRef]
- Zeshan, K.O.; Karthikeyan, P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Puyuelo, B.; Ponsá, S.; Gea, T.; Sánchez, A. Determining C/N ratios for typical organic wastes using biodegradable fractions. Chemosphere 2011, 85, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Pan, J.; Chen, X.; Sheng, K.; Yu, Y.; Zhang, C.; Ying, Y. Effect of ammonia on biohydrogen production from food waste via anaerobic fermentation. Int. J. Hydrogen Energy 2013, 38, 12747–12754. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef]
- Bien, J.B.; Malina, G.; Bien, J.D.; Wolny, L. Enhancing anaerobic fermentation of sewage sludge for increasing biogas generation. J. Environ. Sci. Health Part A 2004, 39, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xia, T.; Zhu, C.; Xi, B.; Jia, X.; Wei, Z.; Zhu, J. Effect of short-time hydrothermal pretreatment of kitchen waste on biohydrogen production: Fluorescence spectroscopy coupled with parallel factor analysis. Bioresour. Technol. 2014, 172, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Zhu, H.; Parker, W.; Basnar, R.; Proracki, A.; Falletta, P.; Béland, M.; Seto, P. Biohydrogen production by anaerobic co-digestion of municipal food waste and sewage sludges. Int. J. Hydrogen Energy 2008, 33, 3651–3659. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, C.; Wang, J.; Tian, S.; Zhang, Y. Effects of ultrasound pre-treatment on the amount of dissolved organic matter extracted from food waste. Bioresour. Technol. 2014, 155, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yinguang, C.; Chenchen, L. Waste activated sludge alkaline fermentation liquid as carbon source for biological nutrients removal in anaerobic followed by alternating aerobic-anoxic sequencing batch reactors. Chin. J. Chem. Eng. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Komemoto, K.; Lim, Y.; Nagao, N.; Onoue, Y.; Niwa, C.; Toda, T. Effect of temperature on VFA’s and biogas production in anaerobic solubilization of food waste. Waste Manag. 2009, 29, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hidaka, T.; Hagiwara, W.; Tsuno, H. Comparative performance and microbial diversity of hyperthermophilic and thermophilic co-digestion of kitchen garbage and excess sludge. Bioresour. Technol. 2009, 100, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, Y.; Zhang, H.; Jiang, S.; Zhou, Q.; Gu, G. Improved bioproduction of short-chain fatty acids (SCFAs) from excess sludge under alkaline conditions. Environ. Sci. Technol. 2006, 40, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Babel, S.; Fukushi, K.; Sitanrassamee, B. Effect of acid speciation on solid waste liquefaction in an anaerobic acid digester. Water Res. 2004, 38, 2417–2423. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 78–107. [Google Scholar]
- Borja, R.; Sánchez, E.; Weiland, P. Influence of ammonia concentration on thermophilic anaerobic digestion of cattle manure in upflow anaerobic sludge blanket (UASB) reactors. Process. Biochem. 1996, 31, 477–483. [Google Scholar] [CrossRef]
- Yirong, C.; Heaven, S.; Banks, C. Effect of a trace element addition strategy on volatile fatty acid accumulation in thermophilic anaerobic digestion of food waste. Waste Biomass Valorization 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Song, Z.X.; Wen, F.; Xu, J.P.; Xu, D.Y.; Cai, C.F. Effects of thermo-alkali pretreatment on the production and composition of volatile fatty acids in anaerobic fermentation of kitchen waste. J. Ecol. Rural Environ. 2015, 31, 244–248. (In Chinese) [Google Scholar]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrogen Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, J.; Du, G.; Chen, J. Enhancement of solubilization and acidification of waste activated sludge by pretreatment. Waste Manag. 2008, 28, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zuo, J.; Gan, L.; Li, P.; Liu, F.; Wang, K.; Chen, L.; Gan, H. Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. 2011, 23, 1403–1408. [Google Scholar] [CrossRef]
- Lim, J.W.; Wang, J.-Y. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013, 33, 813–819. [Google Scholar] [CrossRef]
- Liu, C.-F.; Yuan, X.-Z.; Zeng, G.-M.; Li, W.-W.; Li, J. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour. Technol. 2008, 99, 882–888. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, L.; Ma, F.; Huang, X.C.; Zhao, Z. Research Progress of Methanogens Methanogenic Metabolic Pathway and Its Ecological Factors. In Proceedings of the International Conference on Sustainable Energy and Environmental Engineering (ICSEEE 2012), Guangzhou, China, 29–30 December 2012. [Google Scholar]
- Trzcinski, A.P.; Stuckey, D.C. Treatment of municipal solid waste leachate using a submerged anaerobic membrane bioreactor at mesophilic and psychrophilic temperatures: Analysis of recalcitrants in the permeate using GC-MS. Water Res. 2010, 44, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Arsova, L. Anaerobic Digestion of Food Waste: Current Status, Problems and an Alternative Product; Columbia University: New York, NY, USA, May 2010. [Google Scholar]
- Van Lier, J.; Tilche, A.; Ahring, B.K.; Macarie, H.; Moletta, R.; Dohanyos, M.; Hulshoff Pol, L.; Lens, P.; Verstraete, W. New perspectives in anaerobic digestion. Water Sci. Technol. 2001, 43, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Sreekrishnan, T.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques—A review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar]
- Fricke, K.; Santen, H.; Wallmann, R.; Hüttner, A.; Dichtl, N. Operating problems in anaerobic digestion plants resulting from nitrogen in MSW. Waste Manag. 2007, 27, 30–43. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Xue, L.; Chen, C.; Liu, G.; Zhang, R. Effects of ammonia on anaerobic digestion of food waste: Process performance and microbial community. Energy Fuels 2016, 30, 5749–5757. [Google Scholar] [CrossRef]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speece, R.E. Anaerobic biotechnology for industrial wastewater treatment. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef]
- Naran, E.; Toor, U.A.; Kim, D.-J. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016, 113, 17–21. [Google Scholar] [CrossRef]
- Tamrat, A.; Amare, G. Co-digestion of cattle manure with organic kitchen waste to increase biogas production using rumen fluid as inoculums. J. Phys. Sci. 2013, 8, 443–450. [Google Scholar]
- Pace, N.R. A molecular view of microbial diversity and the biosphere. Science 1997, 276, 734–740. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Seol, E.-H.; Kim, J.R.; Park, S. Fermentative biohydrogen production by a new chemoheterotrophic bacterium Citrobacter sp. Y19. Int. J. Hydrogen Energy 2003, 28, 1353–1359. [Google Scholar] [CrossRef]
- Bryant, M.; Wolin, E.; Wolin, M.; Wolfe, R. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol. 1967, 59, 20–31. [Google Scholar] [CrossRef]
- Friedrich, M.; Springer, N.; Ludwig, W.; Schink, B. Phylogenetic Positions of Desulfofustis glycolicus gen. nov., sp. nov. and Syntrophobotulus glycolicus gen. nov., sp. nov., Two New Strict Anaerobes Growing with Glycolic Acid. Int. J. Syst. Bacteriol. 1996, 46, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Ferry, J.G. Methanogenesis: Ecology, Physiology, Biochemistry & Genetics, 3rd ed.; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Hallam, S.J.; Girguis, P.R.; Preston, C.M.; Richardson, P.M.; DeLong, E.F. Identification of methyl coenzyme M reductase A (mcrA) genes associated with methane-oxidizing archaea. Appl. Environ. Microbiol. 2003, 69, 5483–5491. [Google Scholar] [CrossRef] [Green Version]
- Freitag, T.E.; Toet, S.; Ineson, P.; Prosser, J.I. Links between methane flux and transcriptional activities of methanogens and methane oxidizers in a blanket peat bog. FEMS Microbiol. Ecol. 2010, 73, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Shin, H.-S. Effects of base-pretreatment on continuous enriched culture for hydrogen production from food waste. Int. J. Hydrogen Energy 2008, 33, 5266–5274. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Miao, H.; Huang, Z.; Gao, S.; Ruan, W. In situ volatile fatty acids influence biogas generation from kitchen wastes by anaerobic digestion. Bioresour. Technol. 2014, 163, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.F.; Ren, N.Q. Appilication of fluorescence in situ hybridization (FISH) in microbial ecology. Microbiol. Rep. 2003, 6, 117–122. (In Chinese) [Google Scholar]
- Betty, B. Fluorescence in Situ Hybridization, 1st ed.; Tianjin Science and Technology Translation Inc.: Tianjin, China, 2003. (In Chinese) [Google Scholar]
- Levén, L.; Eriksson, A.R.B.; Schnürer, A.J. Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste. FEMS Microbiol. Ecol. 2007, 59, 683–693. [Google Scholar] [CrossRef]
- Lim, J.; Chen, C.-L.; Ho, I.; Wang, J.-Y. Study of microbial community and biodegradation efficiency for single-and two-phase anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2013, 147, 193–201. [Google Scholar] [CrossRef]
- Schütte, U.M.; Abdo, Z.; Bent, S.J.; Shyu, C.; Williams, C.J.; Pierson, J.D.; Forney, L.J. Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Appl. Microbiol. Biotechnol. 2008, 80, 365–380. [Google Scholar] [CrossRef]
- Osborn, A.M.; Moore, E.R.; Timmis, K.N. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ. Microbiol. 2000, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Roos, M.M.; Zhong, Y.; Marsh, T.; Roman, M.B.; Ascencio, W.H.; Uribe, L.; Lorio, L.U.; Kirk, D.; Reinhold, D.M. Responses of anaerobic microorganisms to different culture conditions and corresponding effects on biogas production and solid digestate quality. Biomass Bioenergy 2016, 85, 84–93. [Google Scholar] [CrossRef]
- Blasco, L.; Kahala, M.; Tampio, E.; Ervasti, S.; Paavola, T.; Rintala, J.; Joutsjoki, V. Dynamics of microbial communities in untreated and autoclaved food waste anaerobic digesters. Anaerobe 2014, 29, 3–9. [Google Scholar] [CrossRef]
- Meyer, M.; Stenzel, U.; Hofreiter, M. Parallel tagged sequencing on the 454 platform. Nature 2008, 3, 267. [Google Scholar] [CrossRef]
- Wu, B.; Wang, X.; Deng, Y.-Y.; He, X.-L.; Li, Z.-W.; Li, Q.; Qin, H.; Chen, J.-T.; He, M.-X.; Zhang, M.J.B. Adaption of microbial community during the start-up stage of a thermophilic anaerobic digester treating food waste. Biosci. Biotechnol. Biochem. 2016, 80, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- Dennehy, C.; Lawlor, P.G.; Gardiner, G.E.; Jiang, Y.; Cormican, P.; McCabe, M.S.; Zhan, X. Process stability and microbial community composition in pig manure and food waste anaerobic co-digesters operated at low HRTs. Front. Environ. Sci. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: Performance and microbial characteristics analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Zhang, E.; Zhang, J.; Wang, W.; He, Y.; Liu, G.; Chen, C. Solid-State Co-digestion of NaOH-Pretreated Corn Straw and Chicken Manure Under Mesophilic Condition. Waste Biomass Valorization 2017, 9, 1027–1035. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, M.; Chen, Y.; Yu, M.; Ruan, W. Tolerance response to in situ ammonia stress in a pilot-scale anaerobic digestion reactor for alleviating ammonia inhibition. Bioresour. Technol. 2015, 198, 372–379. [Google Scholar] [CrossRef]
- Lim, J.W.; Ge, T.; Tong, Y.W. Monitoring of microbial communities in anaerobic digestion sludge for biogas optimisation. Waste Manag. 2018, 71, 334–341. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, L.; Zhao, X.; Qu, L.; Wu, D.; Peng, X. Investigation of foaming causes in three mesophilic food waste digesters: Reactor performance and microbial analysis. Sci. Rep. 2017, 7, 13701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.; Jeong, Y.; Seo, K.W.; Lee, S.; Smith, A.L.; Shin, S.G.; Cho, S.K.; Park, C. Effects of changes in temperature on treatment performance and energy recovery at mainstream anaerobic ceramic membrane bioreactor for food waste recycling wastewater treatment. Bioresour. Technol. 2018, 256, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Banks, C.; Zhang, Y.; Heaven, S.; Longhurst, P. Quantifying the percentage of methane formation via acetoclastic and syntrophic acetate oxidation pathways in anaerobic digesters. Waste Manag. 2018, 71, 749–756. [Google Scholar] [CrossRef]
- Jia, Z.J. Principle and application of DNA-based stable isotope probing—A review. J. Chin. Microbiol. 2011, 51, 1585–1594. (In Chinese) [Google Scholar]
- Zou, H.; Gao, M.; Yu, M.; Zhang, W.; Zhang, S.; Wu, C.; Tashiro, Y.; Wang, Q. Methane production from food waste via mesophilic anaerobic digestion with ethanol pre-fermentation: Methanogenic pathway and microbial community analyses. Bioresour. Technol. 2020, 297, 122450. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Daniel, R. Metagenomic analyses: Past and future trends. Appl. Environ. Microbiol. 2011, 77, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.D.S.; Ferreira, B.O.; Ribeiro, N.C.; Martins, R.; Leite, L.R.; Oliveira, G.; Colturato, L.F.; Chernicharo, C.A.; De Araujo, J.C. Metagenomic analysis and performance of a mesophilic anaerobic reactor treating food waste at various load rates. Environ. Technol. 2017, 38, 2153–2163. [Google Scholar] [CrossRef]
- Zhu, X.; Campanaro, S.; Treu, L.; Seshadri, R.; Ivanova, N.; Kougias, P.G.; Kyrpides, N.; Angelidaki, I. Metabolic dependencies govern microbial syntrophies during methanogenesis in an anaerobic digestion ecosystem. Microbiome 2020, 8, 22. [Google Scholar] [CrossRef]
| Reactor Volume | Final Products | Conditions | Results | Ref. |
|---|---|---|---|---|
| 550 mL digesters | H2 | Adjust the temperature to 55 °C | Achieve a maximum gas production of 82.47 mL/VS | [29] |
| 500 mL digesters | Organic acid | Adjust the temperature to 37 °C | VFA maximum output is 34.4 g/L | [30] |
| 500 mL serum bottles | CH4 | Adjust the temperature to 35 °C | Gas production increased by 32% over 55 °C | [31] |
| 635 mL fermenter | H2 | Adjust the pH to 8.0 | Maximum cumulative gas production is 1.3 L | [32] |
| 4.5 L glass reactor | Organic acid | Adjust the pH to 6.0 | Maximum acid production 40.89 g/L | [33] |
| 500 mL experiment bottle | CH4 | Adjust the pH to 8.0 | 7.57 times higher than pH uncontrolled | [34] |
| 500 mL glass digesters | H2 | Add ammonia soda | Maximum gas production is 145.4 mL H2/g-VS | [35] |
| 430 ± 2 mL working volume | Organic acid | Add trace elements and activated carbon | A faster consumption of propionic acid | [36] |
| 1 L batch reactors | CH4 | Adjust the ammonia concentration to 0.5 g/L | Maximum gas production is 314.7 mL/g | [37] |
| 4.5 L tank reactor | H2 | Ultrasonic pretreatment | Increase in hydrogen production by 75% | [38] |
| 1 L tank reactor | Organic acid | Ultrasonic pretreatment | VFAs increased by 27.2% | [39] |
| 500 mL serum bottles | CH4 | Alkali pretreatment | Maximum methane production rate is 6.63 mL/h | [40] |
| 250 mL serum bottles | H2 | Co-digestion with aged refuse and sewage sludge | Significantly increased hydrogen concentration by 26.6% | [41] |
| 5 L reactor | Organic acid | Co-digestion with waste-activated sludge | SCFA maximum is 690.9 mg COD/g-VS | [42] |
| 1 L reactor | CH4 | Co-digestion with cow dung | Maximum gas production is 233 mL/g-VSS | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xu, C.; Song, L.; Zhang, J. Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 9519. https://doi.org/10.3390/ijerph19159519
Wang S, Xu C, Song L, Zhang J. Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives. International Journal of Environmental Research and Public Health. 2022; 19(15):9519. https://doi.org/10.3390/ijerph19159519
Chicago/Turabian StyleWang, Shuijing, Chenming Xu, Liyan Song, and Jin Zhang. 2022. "Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives" International Journal of Environmental Research and Public Health 19, no. 15: 9519. https://doi.org/10.3390/ijerph19159519
APA StyleWang, S., Xu, C., Song, L., & Zhang, J. (2022). Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives. International Journal of Environmental Research and Public Health, 19(15), 9519. https://doi.org/10.3390/ijerph19159519







