SARS-CoV-2 Infection in Pregnancy: Placental Histomorphological Patterns, Disease Severity and Perinatal Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects Identification
2.2. Placental Histopathological Examination
2.3. Immunohistochemistry (IHC) Analysis
2.4. Statistical Analysis
3. Results
3.1. Maternal Characteristics, Obstetric and Neonatal Outcomes
3.2. Placental Histomorphological Alteration and Immunohistochemical Findings
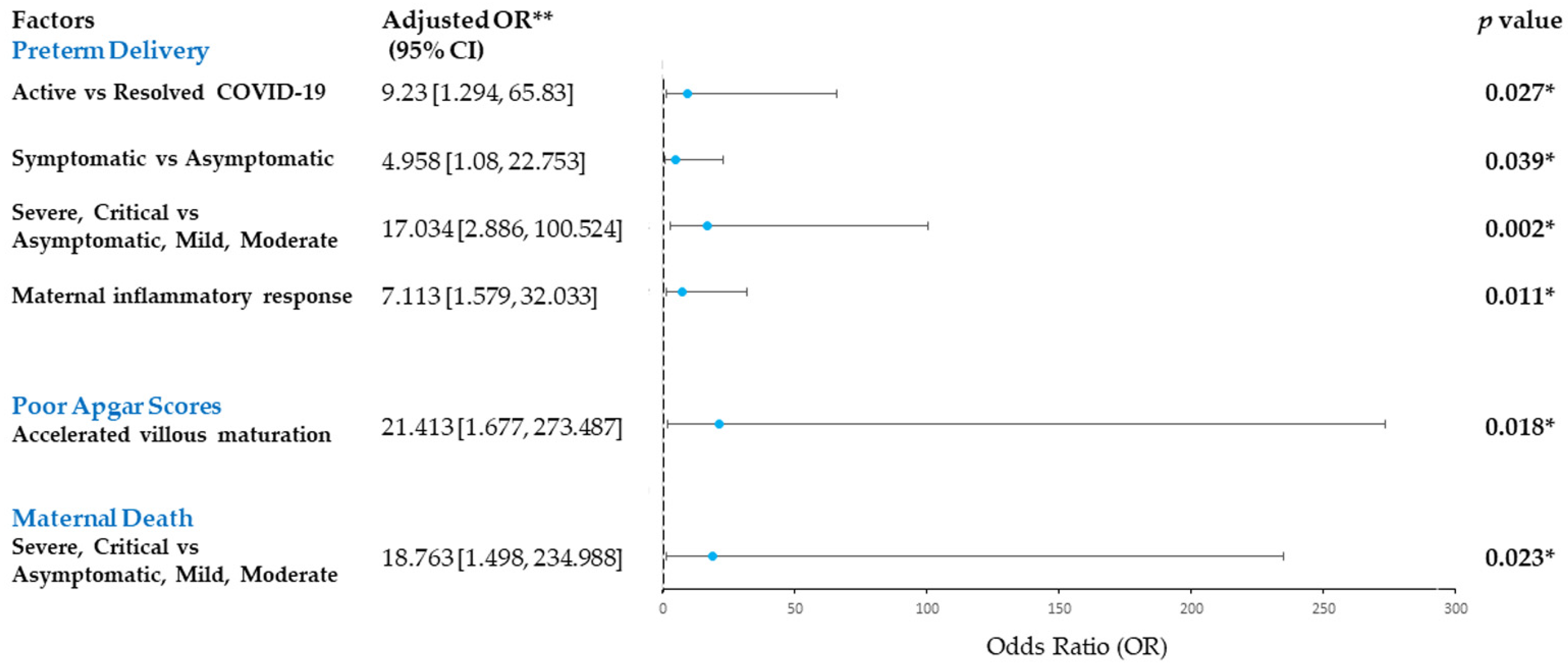
3.3. Risk Factors for Adverse Maternal and Perinatal Outcomes in COVID-19-Infected Pregnancies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Xu, Y.; Theis, K.; Arenas-Hernandez, M.; Miller, D.; Peyvandipour, A.; Galaz, J.; Levenson, D.; Bhatti, G.; et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022, 13, 320. [Google Scholar] [CrossRef]
- Collin, J.; Byström, E.; Carnahan, A.; Ahrne, M. Public Health Agency of Sweden’s Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet. Gynecol. Scand. 2020, 99, 819–822. [Google Scholar] [CrossRef]
- Huntley, B.J.F.; Huntley, E.S.; Di Mascio, D.; Chen, T.; Berghella, V.; Chauhan, S.P. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A systematic review. Obstet. Gynecol. 2020, 136, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Lokken, E.M.; Taylor, G.G.; Huebner, E.M.; Vanderhoeven, J.; Hendrickson, S.; Coler, B.; Sheng, J.S.; Walker, C.L.; McCartney, S.A.; Kretzer, N.M.; et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am. J. Obstet. Gynecol. 2021, 225, 75.e1–75.e16. [Google Scholar] [CrossRef] [PubMed]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: National cohort study. Am. J. Obstet. Gynecol. 2021, 225, 522.e1–522.e11. [Google Scholar] [CrossRef]
- Moodley, A.; Payton, K.S.E. The term newborn: Congenital infections. Clin. Perinatol. 2021, 48, 485–511. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1635–1640. [Google Scholar] [CrossRef]
- Zaigham, M.; Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020, 99, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resta, L.; Vimercati, A.; Cazzato, G.; Mazzia, G.; Cicinelli, E.; Colagrande, A.; Fanelli, M.; Scarcella, S.V.; Ceci, O.; Rossi, R. SARS-CoV-2 and placenta: New insights and perspectives. Viruses 2021, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Mourad, M.; Jacob, T.; Sadovsky, E.; Bejerano, S.; Simone, G.S.-D.; Bagalkot, T.R.; Zucker, J.; Yin, M.T.; Chang, J.Y.; Liu, L.; et al. Placental response to maternal SARS-CoV-2 infection. Sci. Rep. 2021, 11, 14390. [Google Scholar] [CrossRef] [PubMed]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; La Rosa, M. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, 37, 861–865. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012, 15, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Tikellis, C.; Thomas, M. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2010, 84, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Aljhdali, H.M.; Abdullah, L.S.; Alhazmi, D.A.; Almosallam, A.M.; Bondagji, N.S. Practice of Placenta Submission for Histopathological Examination, Experience of a Teaching/Tertiary Care Hospital in Saudi Arabia. Cureus 2021, 13, e17364. [Google Scholar] [CrossRef]
- Menter, T.; Mertz, K.D.; Jiang, S.; Chen, H.; Monod, C.; Tzankov, A.; Waldvogel, S.; Schulzke, S.M.; Hösli, I.; Bruder, E. Placental pathology findings during and after SARS-CoV-2 infection: Features of villitis and malperfusion. Pathobiology 2021, 88, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Almohammadi, N.H. A review of the main placenta histopathological findings reported in coronavirus disease 2019. J. Taibah Univ. Med. Sci. 2022, 17, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, T.M.; Bulfamante, G.; Cheng, K.; Collins, R.R.; Debelenko, L.; De Luca, D.; Facchetti, F.; et al. Hofbauer Cells and COVID-19 in Pregnancy. Arch. Pathol. Lab. Med. 2021, 145, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.H.; Moreno, W.; Zofkie, A.C.; Macdonald, L.; McIntire, D.D.; Collins, R.R.J.; Spong, C.Y. Pregnancy Outcomes Among Women with and without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw. Open 2020, 3, e2029256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Salafia, C.; Heyman, T.; Lederman, S.; Dygulska, B. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am. J. Obstet. Gynecol. MFM 2020, 2, 100197. [Google Scholar] [CrossRef]
- Rad, H.S.; Röhl, J.; Stylianou, N.; Allenby, M.C.; Bazaz, S.R.; Warkiani, M.E.; Guimaraes, F.S.F.; Clifton, V.L.; Kulasinghe, A. The Effects of COVID-19 on the Placenta During Pregnancy. Front. Immunol. 2021, 12, 743022. [Google Scholar] [CrossRef] [PubMed]
- Rebutini, P.Z.; Zanchettin, A.C.; Stonoga, E.T.S.; Prá, D.M.M.; de Oliveira, A.L.P.; Dezidério, F.D.S.; Fonseca, A.S.; Dagostini, J.C.H.; Hlatchuk, E.C.; Furuie, I.N.; et al. Association Between COVID-19 Pregnant Women Symptoms Severity and Placental Morphologic Features. Front. Immunol. 2021, 12, 685919. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Khong, T.Y.; Tan, G.C. The effects of COVID-19 on placenta and pregnancy: What do we know so far? Diagnostics 2021, 11, 94. [Google Scholar] [CrossRef]
- Suhren, J.-T.; Meinardus, A.; Hussein, K.; Schaumann, N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta 2022, 117, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.O.; Huang, Y.; Tsoi, M.T.F.; Tang, A.; Wong, S.Y.S.; Wei, W.I.; Hui, D.S.C. Epidemiology, clinical spectrum, viral kinetics and impact of COVID-19 in the Asia-Pacific Region. Respirology 2021, 26, 322–333. [Google Scholar] [CrossRef]
- Wong, Y.P.; Cheah, F.C.; Wong, K.K.; Shah, S.A.; Phon, S.E.; Ng, B.K.; Lim, P.S.; Khong, T.Y.; Tan, G.C. Gardnerella vaginalis infection in pregnancy: Effects on placental development and neonatal outcomes. Placenta 2022, 120, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClymont, E.; Albert, A.Y.; Alton, G.D.; Boucoiran, I.; Castillo, E.; Fell, D.B.; Kuret, V.; Poliquin, V.; Reeve, T.; Scott, H.; et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA 2022, 327, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Heath, P.; Ladhani, S.; et al. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020, 25, 100446. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatrics 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Tan, G.C.; Azliana, A.F.; Zainul-Rashid, M.R.; Chandramaya, S.F.; Farouk, W.I.; Nurwardah, A.; Wong, Y.P. Vascular endothelial growth factor expression in placenta of hypertensive disorder in pregnancy. Indian J. Pathol. Microbiol. 2017, 60, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.S.; Ghazali, R.; Othman, H.; Ismail, N.A.M.; Othman, A.S.; Laim, N.M.S.T.; Wong, Y.P.; Tan, G.C. Endocan expression in placenta of women with hypertension. J. Obstet. Gynaecol. Res. 2019, 45, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Shaaya, E.S.; Yahaya, A.; Mustangin, M.; Alfian, N.; Aizuddin, A.N.; Wong, Y.P.; Tan, G.C. Placental Cyclophilin A Expression in Pregnancies Complicated with Hypertension. Int. J. Environ. Res. Public Health 2022, 19, 5448. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Tan, G.C.; Wong, K.K.; Anushia, S.; Cheah, F.C. Gardnerella vaginalis in perinatology: An overview of the clinicopathological correlation. Malays. J. Pathol. 2018, 40, 267–286. [Google Scholar]
- Cheah, F.-C.; Lai, C.H.; Tan, G.C.; Swaminathan, A.; Wong, K.K.; Wong, Y.P.; Tan, T.-L. Intrauterine Gardnerella vaginalis Infection Results in Fetal Growth Restriction and Alveolar Septal Hypertrophy in a Rabbit Model. Front. Pediatr. 2021, 8, 593802. [Google Scholar] [CrossRef]
- Shchegolev, A.I.; Kulikova, G.V.; Lyapin, V.M.; Shmakov, R.G.; Sukhikh, G.T. The Number of Syncytial Knots and VEGF Expression in Placental Villi in Parturient Woman with COVID-19 Depends on the Disease Severity. Bull. Exp. Biol. Med. 2021, 171, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Jaiman, S.; Romero, R.; Pacora, P.; Jung, E.; Bhatti, G.; Yeo, L.; Kim, Y.M.; Kim, B.; Kim, C.J.; Kim, J.-S.; et al. Disorders of placental villous maturation in fetal death. J. Périnat. Med. 2021, 49, 412–430. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Shazniza Shaaya, E.; Halim, S.A.A.; Leong, K.W.; Ku, K.B.P.; Lim, P.S.; Tan, G.C.; Wong, Y.P. Candida Chorioamnionitis in Mothers with Gestational Diabetes Mellitus: A Report of Two Cases. Int. J. Environ. Res. Public Health 2021, 18, 7450. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.J.; De Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch. Pathol. Lab. Med. 2021, 145, 517–528. [Google Scholar] [PubMed]
- Bertero, L.; Borella, F.; Botta, G.; Carosso, A.; Cosma, S.; Bovetti, M.; Carosso, M.; Abbona, G.; Collemi, G.; Papotti, M.; et al. Placenta histopathology in SARS-CoV-2 infection: Analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021, 479, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1–382.e18. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.; Czeresnia, R.M.; Cheek, E.H.; Quinton, R.A.; Chakraborty, R.; Enninga, E.A.L. Expression of Immune Checkpoint Receptors in Placentae with Infectious and Non-Infectious Chronic Villitis. Front. Immunol. 2021, 12, 705219. [Google Scholar] [CrossRef]
- Wong, Y.P.; Wagiman, N.; De Tan, J.W.; Hanim, B.S.; Rashidan, M.S.H.; Fong, K.M.; Norhazli, N.N.; Qrisha, Y.; Alam Shah, R.N.R.; Mustangin, M.; et al. Loss of CXC-Chemokine Receptor 1 Expression in Chorioamnionitis Is Associated with Adverse Perinatal Outcomes. Diagnostics 2022, 12, 882. [Google Scholar] [CrossRef]
- Martinez-Perez, O.; Prats Rodriguez, P.; Muner Hernandez, M.; Encinas Pardilla, M.B.; Perez Perez, N.; Vila Hernandez, M.R.; Villalba Yarza, A.; Nieto Velasco, O.; Del Barrio Fernandez, P.G.; Forcen Acebal, L.; et al. The association between SARS-CoV-2 infection and preterm delivery: A prospective study with a multivariable analysis. BMC Pregnancy Childbirth 2021, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- Areia, A.L.; Mota-Pinto, A. Inflammation and Preterm Birth: A Systematic Review. Reprod. Med. 2022, 3, 101–111. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Khalil, A.; Alameddine, S.; D’Angelo, E.; Galliani, C.; Matarrelli, B.; Buca, D.; Liberati, M.; Rizzo, G.; D’Antonio, F. Placental histopathology after SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100468. [Google Scholar] [CrossRef] [PubMed]


| Clinicopathological Features | COVID-19 Cases n = 47 (%) | Controls n = 47 (%) | p-Value | |
|---|---|---|---|---|
| Maternal age (years) | 31.45 ± 4.58 | 31.47 ± 3.68 | 0.158 | |
| Gestational age (weeks) | 36.18 ± 3.72 | 36.38 ± 3.69 | 0.931 | |
| Ethnicity | Malay | 42 (89.3) | 46 (97.9) | 0.235 |
| Chinese | 2 (4.3) | 0 (0.0) | ||
| Others | 3 (6.4) | 1 (2.1) | ||
| Delivery mode | Caesarean section | 37 (78.7) | 17 (36.2) | <0.001 * |
| Assisted delivery | 2 (4.3) | 0 (0.0) | ||
| Vaginal delivery | 8 (17.0) | 30 (63.8) | ||
| Comorbidity | No | 22 (46.8) | 21 (44.7) | 1.000 |
| 1 comorbid | 17 (36.2) | 14 (29.8) | ||
| More than 1 comorbid | 8 (17.0) | 12 (25.5) | ||
| Severity of COVID-19 | Asymptomatic | 19 (40.4) | N/A | N/A |
| Mild | 14 (29.8) | |||
| Moderate | 5 (10.6) | |||
| Severe | 7 (14.9) | |||
| Critical | 2 (4.3) | |||
| Maternal death | Yes | 4 (8.5) | 0 (0.0) | 0.117 |
| No | 43 (91.5) | 47 (100.0) | ||
| Infection-to-delivery interval (days) | 34.98 ± 55.08 | N/A | N/A | |
| Newborn birth weight (grams) | 2500.26 ± 636.83 | 2557.32 ± 703.10 | 0.339 | |
| Fetal growth restriction/small for gestation | 20 (42.6) | 9 (19.1) | 0.025 * | |
| Placental weight (grams) | 509.68 ± 108.45 | 507.67 ± 113.72 | 0.595 | |
| Fetal-placental weight ratio | 4.90 ± 0.87 | 5.13 ± 1.21 | 0.083 | |
| Apgar score (1 min) | 7.74 ± 2.52 | 7.94 ± 1.74 | 0.035 * | |
| Apgar score (5 min) | 8.83 ± 2.32 | 9.23 ± 1.03 | 0.002 * | |
| Neonatal death | Yes | 3 (6.4) | 1 (2.1) | 0.617 |
| No | 44 (93.6) | 46 (97.9) | ||
| Clinicopathological Features | COVID-19 Cases | COVID-19 Cases | |||||
|---|---|---|---|---|---|---|---|
| Active n = 33 (%) | Resolved n = 14 (%) | p-Value | Asymptomatic n = 19 (%) | Symptomatic n = 28 (%) | p-Value | ||
| Maternal age (years) | 32.18 ± 4.10 | 29.71 ± 5.31 | 0.274 | 32.42 ± 4.97 | 30.79 ± 4.26 | 0.776 | |
| Gestational age (weeks) | 35.37 ± 4.11 | 38.08 ± 1.38 | <0.001 * | 37.68 ± 1.24 | 35.16 ± 4.46 | <0.001 * | |
| Ethnicity | Malay | 29 (87.9) | 13 (92.9) | 0.427 | 18 (94.7) | 24 (85.7) | 0.376 |
| Chinese | 1 (3.0) | 1 (7.1) | 1 (5.3) | 1 (3.6) | |||
| Others | 3 (9.1) | 0 (0.0) | 0 (0.0) | 3 (10.7) | |||
| Delivery mode | Caesarean section | 29 (87.9) | 8 (57.1) | 0.007 * | 15 (78.9) | 22 (78.6) | 1.000 |
| Assisted delivery | 2 (6.1) | 0 (0.0) | 1 (5.3) | 1 (3.6) | |||
| Vaginal delivery | 2 (6.1) | 6 (42.9) | 3 (15.8) | 5 (17.9) | |||
| Comorbidity | No | 16 (48.5) | 5 (35.7) | 0.470 | 7 (36.8) | 14 (50.0) | 0.601 |
| 1 comorbid | 8 (24.2) | 6 (42.9) | 7 (36.8) | 7 (25.0) | |||
| More than 1 comorbid | 9 (27.3) | 3 (21.4) | 5 (26.3) | 7 (25.0) | |||
| Severity of COVID-19 | Asymptomatic | 14 (42.4) | 5 (35.7) | 0.124 | N/A | N/A | N/A |
| Mild | 8 (24.2) | 6 (42.9) | |||||
| Moderate | 2 (6.1) | 3 (21.4) | |||||
| Severe | 7 (21.2) | 0 (0.0) | |||||
| Critical | 2 (6.1) | 0 (0.0) | |||||
| Maternal death | Yes | 4 (12.1) | 0 (0.0) | 0.302 | 0 (0.0) | 4 (14.3) | 0.137 |
| No | 29 (87.9) | 14 (100.0) | 19 (100.0) | 24 (85.7) | |||
| Infection-to-delivery interval (days) | 4.67 ± 5.57 | 106.43 ± 53.18 | <0.001 * | 39.53 ± 68.60 | 31.89 ± 44.80 | 0.054 | |
| Newborn birth weight (g) | 2443.09 ± 728.11 | 2635.00 ± 319.71 | 0.015 * | 2708.95 ± 416.29 | 2358.64 ± 723.85 | 0.054 | |
| Placental weight (g) | 504.09 ± 125.23 | 522.86 ± 52.39 | 0.032 * | 518.68 ± 64.72 | 503.57 ± 130.96 | 0.012 * | |
| Fetal-placental weight ratio | 4.84 ± 0.97 | 5.06 ± 0.59 | 0.022 * | 5.23 ± 0.60 | 4.68 ± 0.96 | 0.040 * | |
| Apgar score (1 min) | 7.24 ± 2.87 | 8.93 ± 0.27 | <0.001 * | 8.63 ± 1.17 | 7.14 ± 3.00 | <0.001 * | |
| Apgar score (5 min) | 8.39 ± 2.65 | 9.86 ± 0.36 | <0.001 * | 9.68 ± 0.75 | 8.25 ± 2.81 | <0.001 * | |
| Neonatal death | Yes | 3 (9.1) | 0 (0.0) | 0.544 | 0 (0.0) | 3 (10.7) | 0.262 |
| No | 30 (90.9) | 14 (100.0) | 0.274 | 19 (100.0) | 25 (89.3) | ||
| Histological Features | COVID-19 Cases n = 47 (%) | Controls n = 47 (%) | p-Value | COVID-19 Cases | p-Value | ||
|---|---|---|---|---|---|---|---|
| Active n = 33 (%) | Resolved n = 14 (%) | ||||||
| Maternal vascular malperfusion | Accelerated villous maturation/distal villous hypoplasia | 8 (17.0) | 7 (14.9) | 1.000 | 7 (21.2) | 0 (0.0) | 0.086 |
| Syncytial knots (mean ± SD) | 34.16 ± 12.55 | 31.52 ± 9.72 | 0.022 * | 35.17 ± 13.62 | 31.77 ± 9.61 | 0.091 | |
| Villous infarction | 3 (6.4) | 6 (12.8) | 0.486 | 3 (9.1) | 3 (21.4) | 0.344 | |
| Decidual vasculopathy | 22 (46.8) | 8 (17.0) | 0.004 * | 17 (51.5) | 5 (35.7) | 0.358 | |
| Maternal vascular thrombosis | 9 (19.1) | 1 (2.1) | 0.015 * | 9 (27.3) | 0 (0.0) | 0.042 * | |
| Fetal vascular malperfusion | Avascular villi | 3 (6.4) | 0 (0.0) | 0.242 | 2 (6.1) | 1 (7.1) | 1.000 |
| Fetal vascular thrombosis | 3 (6.4) | 0 (0.0) | 0.242 | 2 (6.1) | 1 (7.1) | 1.000 | |
| Chorangiosis | 7 (14.9) | 0 (0.0) | 0.012 * | 4 (12.1) | 3 (21.4) | 0.410 | |
| Chronic inflammation | Chronic deciduitis | 5 (10.6) | 1 (2.1) | 0.203 | 3 (9.1) | 2 (14.3) | 0.627 |
| Chronic villitis, low grade | 5 (10.6) | 1 (2.1) | 0.030 * | 5 (15.2) | 0 (0.0) | 0.193 | |
| Chronic villitis, high grade | 6 (12.8) | 1 (2.1) | 3 (9.1) | 3 (21.4) | |||
| Chronic histiocytic intervillositis | 10 (21.3) | 2 (4.3) | 0.027 * | 6 (18.2) | 4 (28.6) | 0.456 | |
| Maternal inflammatory response | Acute subchorionitis | 21 (44.7) | 11 (23.4) | 0.056 | 15 (45.5) | 6 (42.9) | 0.765 |
| Acute chorioamnionitis | 2 (4.3) | 6 (12.8) | 1 (3.0) | 1 (7.1) | |||
| Fetal inflammatory response | Chorionic vasculitis &/umbilical phlebitis | 4 (8.5) | 3 (6.4) | 0.255 | 1 (3.0) | 2 (14.3) | 0.208 |
| Umbilical arteritis | 3 (6.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Others | Oedematous villi | 5 (10.6) | 9 (19.1) | 0.386 | 4 (12.1) | 1 (7.1) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, Y.P.; Tan, G.C.; Omar, S.Z.; Mustangin, M.; Singh, Y.; Salker, M.S.; Abd Aziz, N.H.; Shafiee, M.N. SARS-CoV-2 Infection in Pregnancy: Placental Histomorphological Patterns, Disease Severity and Perinatal Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 9517. https://doi.org/10.3390/ijerph19159517
Wong YP, Tan GC, Omar SZ, Mustangin M, Singh Y, Salker MS, Abd Aziz NH, Shafiee MN. SARS-CoV-2 Infection in Pregnancy: Placental Histomorphological Patterns, Disease Severity and Perinatal Outcomes. International Journal of Environmental Research and Public Health. 2022; 19(15):9517. https://doi.org/10.3390/ijerph19159517
Chicago/Turabian StyleWong, Yin Ping, Geok Chin Tan, Siti Zarqah Omar, Muaatamarulain Mustangin, Yogesh Singh, Madhuri S. Salker, Nor Haslinda Abd Aziz, and Mohamad Nasir Shafiee. 2022. "SARS-CoV-2 Infection in Pregnancy: Placental Histomorphological Patterns, Disease Severity and Perinatal Outcomes" International Journal of Environmental Research and Public Health 19, no. 15: 9517. https://doi.org/10.3390/ijerph19159517
APA StyleWong, Y. P., Tan, G. C., Omar, S. Z., Mustangin, M., Singh, Y., Salker, M. S., Abd Aziz, N. H., & Shafiee, M. N. (2022). SARS-CoV-2 Infection in Pregnancy: Placental Histomorphological Patterns, Disease Severity and Perinatal Outcomes. International Journal of Environmental Research and Public Health, 19(15), 9517. https://doi.org/10.3390/ijerph19159517







