Laccase–TEMPO as an Efficient System for Doxorubicin Removal from Wastewaters
Abstract
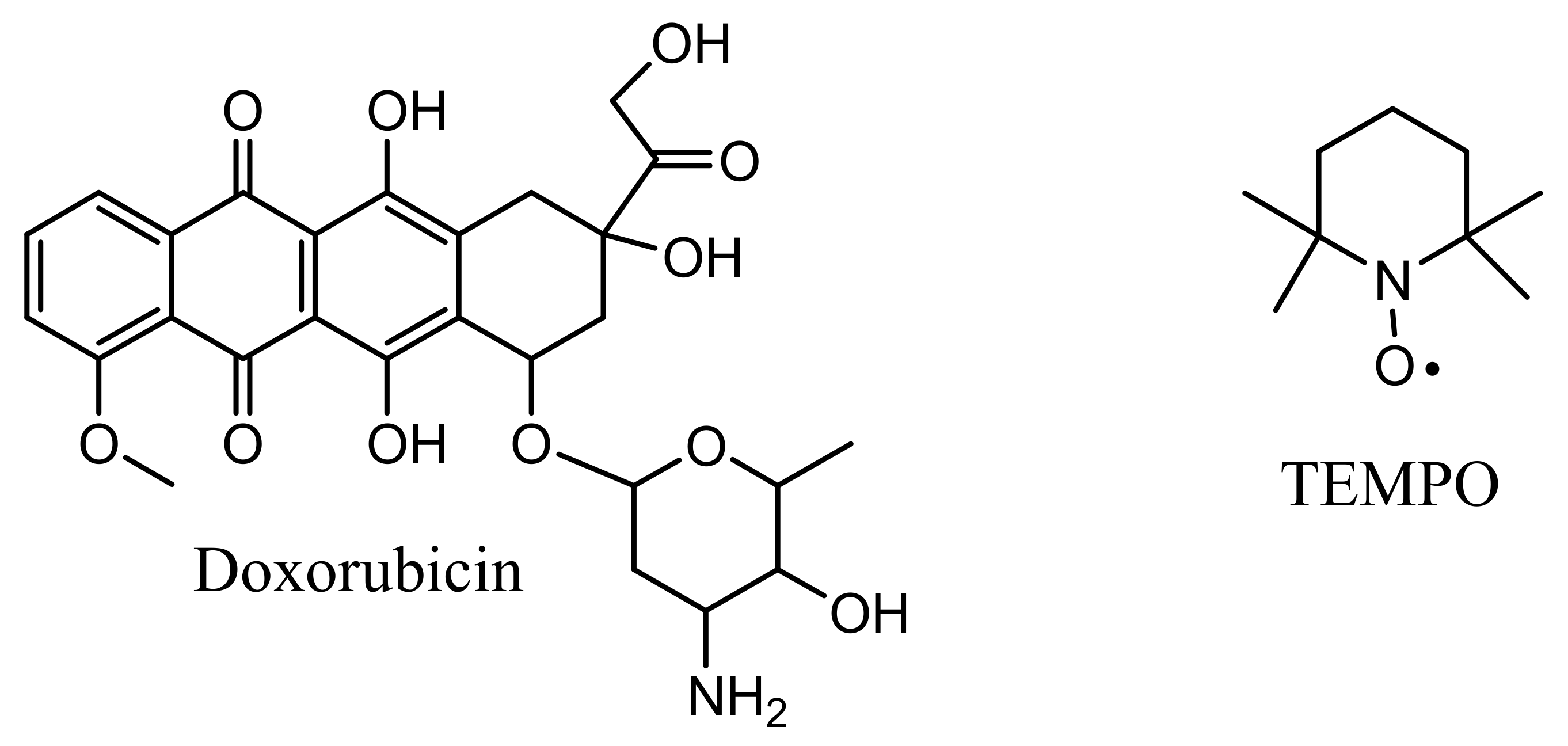
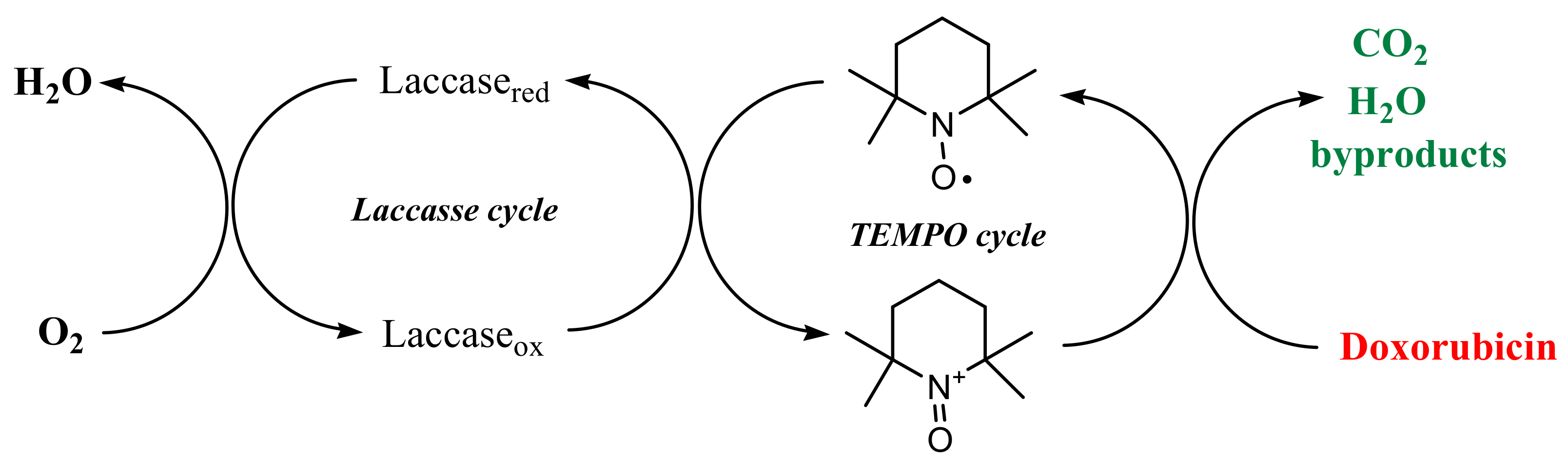
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Methods
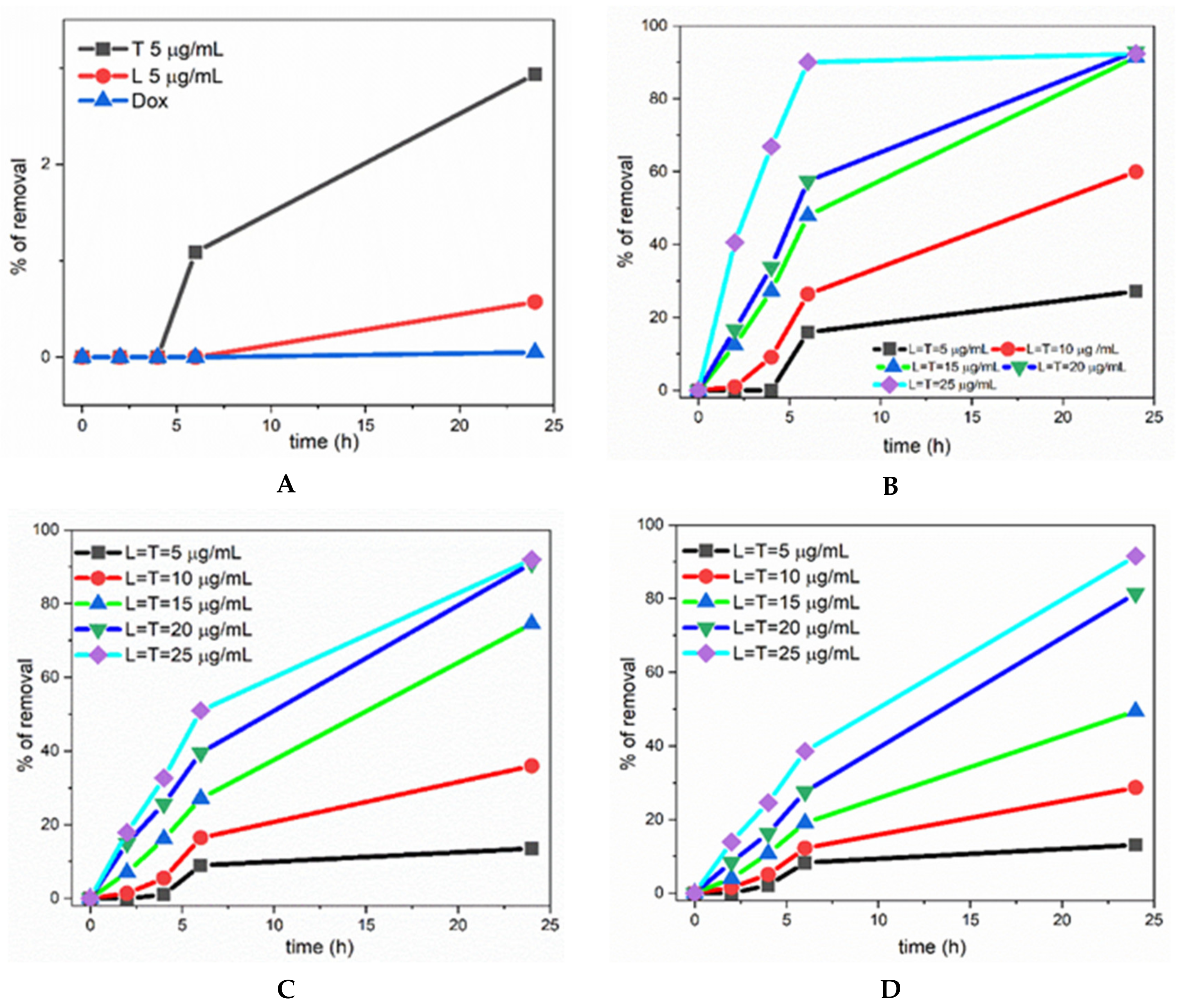
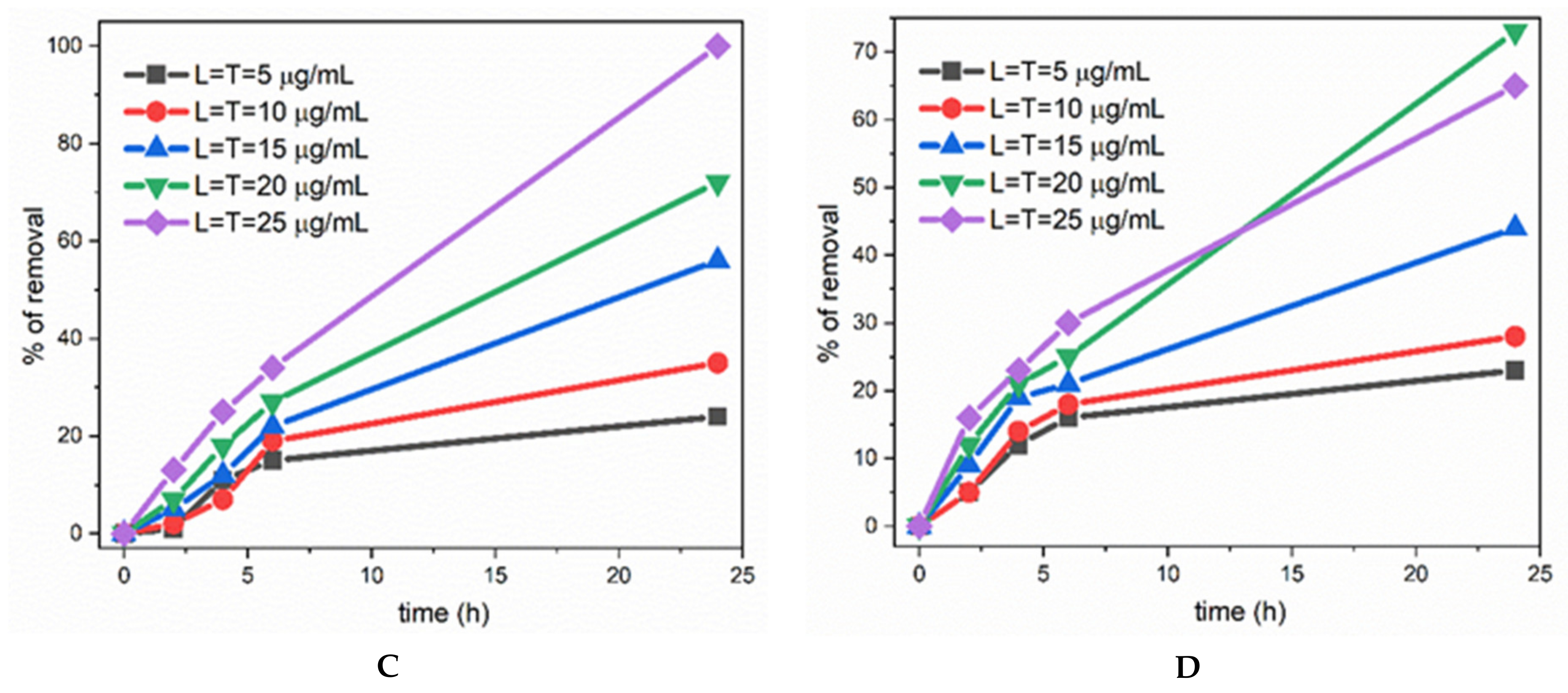
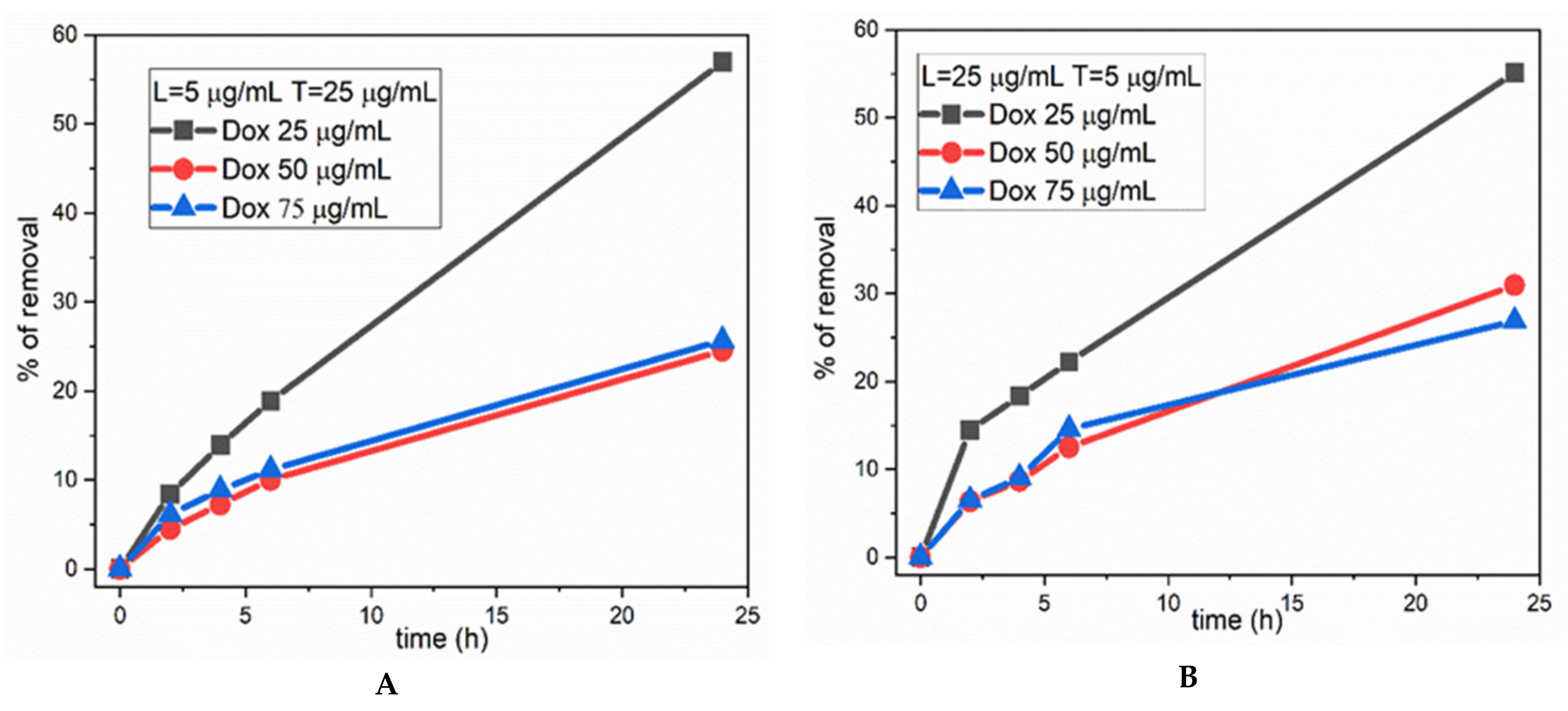
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Malla, S.; Niraula, N.P.; Liou, K.; Sohng, J.K. Improvement in doxorubicin productivity by overexpression of regulatory genes in Streptomyces peucetius. Res. Microbiol. 2010, 161, 109–117. [Google Scholar] [CrossRef]
- Yamada, Y. Dimerization of doxorubicin causes its precipitation. ACS Omega 2020, 5, 33235–33241. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [Green Version]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef]
- Voest, E.E.; Van Faassen, E.; Neijt, J.P.; Marx, J.J.M.; Van Asbeck, B.S. Doxorubicin-mediated free radical generation in intact human tumor cells enhances nitroxide electron paramagnetic resonance absorption intensity decay. Mag. Reson. Med. 1993, 30, 283–288. [Google Scholar] [CrossRef]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar]
- Mielczarek-Puta, M.; Roszkowski, P. Synthesis and anticancer effects of conjugates of doxorubicin and unsaturated fatty acids (LNA and DHA). Med. Chem. Res. 2019, 28, 2153–2164. [Google Scholar] [CrossRef] [Green Version]
- Raoul, J.L.; Heresbach, D.; Bretagne, J.F.; Ferrer, D.B.; Duvauferrier, R.; Bourguet, P.; Messner, M.; Gosselin, M. Chemoembolization of hepatocellular carcinomas. A study of biodistribution and pharmacokinetics of doxorubicin. Cancer 1992, 70, 585–590. [Google Scholar] [CrossRef]
- Beijnen, J.H.; Van der Houwen, O.A.; Underberg, W.J. Aspects of the degradation kinetics of doxorubicin in aqueous solution. Int. J. Pharm. 1986, 32, 123–131. [Google Scholar] [CrossRef]
- Feng, Y.; Colosi, L.M.; Gao, S.; Huang, Q.; Mao, L. Transformation and removal of tetrabromobisphenol a from water in the presence of natural organic matter via laccase-catalyzed reactions: Reaction rates, products, and pathways. Environ. Sci. Technol. 2013, 47, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Couto, S.R.; Herrera, J.L.T. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.H.; Cindy, C.Y.L.; Chun, Y.L.; Gek, K.C.; Sim, Y.C. A comparison of entrapped and covalently bonded laccase: Study of its leakage, reusability, and the catalytic efficiency in TEMPO-mediated glycerol oxidation. Biocatal. Biotrans. 2017, 36, 352–361. [Google Scholar] [CrossRef]
- Claus, H. Laccases: Structure, reactions, distribution. Micron 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Biores. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Singh, G.; Bhalla, A.; Kaur, P.; Capalash, N.; Sharma, P. Laccase from prokaryotes: A new source for an old enzyme. Rev. Environ. Sci. Biotechnol. 2011, 10, 309–326. [Google Scholar] [CrossRef]
- Moilanen, U.; Kellock, M.; Varnai, A.; Andberg, M.; Viikari, L. Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol. Biofuels 2014, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Mate, D.M.; Alcade, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microbial. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Li, Y.X.; Ausan, R. Comparison of TEMPO and its derivatives as mediators in laccase catalysed oxidation of alcohols. Tetrah. Lett. 2006, 62, 6659–6665. [Google Scholar] [CrossRef]
- Galai, S.; Korri-Youssoufi, H.; Marzouki, M.N. Characterization of yellow bacterial laccase SmLac/role of redox mediators in azo dye decolorization. J. Chem. Technol. Biotechnol. 2014, 89, 1741–1750. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Cai, Y. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 40, 126272. [Google Scholar] [CrossRef]
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase as an efficacious approach to remove anticancer drugs: A study of doxorubicin degradation, kinetic parameters, and toxicity assessment. J. Hazard. Mater. 2021, 409, 124520. [Google Scholar] [CrossRef] [PubMed]
- Suy, S.; Mitchell, J.B.; Samuni, A.; Mueller, S.; Kasid, U. Nitroxide tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer 2005, 103, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.C.; França, L.S.; de Araújo, C.F.; Ng, A.M.; de Andrade, C.M.; Andrade, A.C.; Santos, E.D.; Borges-Silva, M.D.; Macambira, S.G.; Noronha-Dutra, A.A.; et al. Protective effects of mito-TEMPO against doxorubicin cardiotoxicity in mice. Cancer Chemother. Pharmac. 2016, 77, 659–662. [Google Scholar] [CrossRef]
- Bragda, P.L.; Bekkumb, H.; Besemerc, A.C. TEMPO-mediated oxidation of polysaccharides: Survey of methods and applications. Top. Catal. 2004, 27, 49–66. [Google Scholar] [CrossRef]
- Rodrguez, A.D.; Martnez-Montero, L.; Lavandera, I.; Gotor, V.; Gotor-Ferndeza, V. Laccase/2,2,6,6-tetramethylpiperidinoxyl radical (TEMPO): An efficient catalytic system for selective oxidations of primary hydroxy and amino groups in aqueous and biphasic media. Adv. Synth. Catal. 2014, 356, 2321–2329. [Google Scholar] [CrossRef]
- Tromp, S.A.; Matijosyte, I.; Sheldon, R.A.; Arends, I.W.C.E.; Mul, G.; Kreutzer, M.T.; Moulijn, J.A.; Vries, S. Mechanism of laccase–TEMPO-catalyzed oxidation of benzyl alcohol. ChemCatChem 2010, 2, 827–833. [Google Scholar] [CrossRef]
- Silva, A.D.M.; Sousa, J.; Hultberg, M.; Figueiredo, S.A.; Freitas, O.M.; Delerue-Matos, C. Fluoxetine removal from aqueous solutions using a lignocellulosic substrate colonized by the whiterot fungus pleurotus ostreatus. Int. J. Environ. Res. Public Health 2022, 19, 2672. [Google Scholar] [CrossRef]
- Gross, J.; Tauber, K.; Fuchs, M.; Schmidt, N.G.; Rajagopalan, A.; Faber, K.; Fabian, W.M.F.; Pfeffer, J.; Haas, T.; Kroutil, W. Aerobic oxidation of isosorbide and isomannide employing TEMPO/laccase. Green Chem. 2014, 16, 2117–2121. [Google Scholar] [CrossRef] [Green Version]
- Reyes, C.; Poulin, A.; Nyström, G.; Schwarze, F.W.M.R.; Ribera, J. Enzyme activities of five white-rot fungi in the presence of nanocellulose. J. Fungi 2021, 7, 222. [Google Scholar] [CrossRef]
- Gao, Z.; Yi, Y.; Zhao, J.; Xia, Y.; Jiang, M.; Cao, F.; Zhou, H.; Wei, P.; Jia, H.; Yong, X. Co-immobilization of laccase and TEMPO onto amino-functionalized magnetic Fe3O4 nanoparticles and its application in acid fuchsin decolorization. Bioresour. Bioprocess. 2018, 5, 27. [Google Scholar] [CrossRef]
- Ran, F.; Zou, Y.; Xu, Y.; Liu, X.; Zhang, H. Fe3O4@MoS2@PEI-facilitated enzyme tethering for efficient removal of persistent organic pollutants in water. Chem. Eng. J. 2019, 375, 121947. [Google Scholar] [CrossRef]
- Patila, M.; Athanasiou, P.E.; Kortessis, L.; Potsi, G.; Kouloumpis, A.; Gournis, D.; Stamatis, H. Immobilization of laccase on hybrid super-structured nanomaterials for the decolorization of phenolic dyes. Processes 2022, 10, 233. [Google Scholar] [CrossRef]
- Levin, L.N.; Hernández-Luna, C.E.; Niño-Medina, G.; García-Rodríguez, J.P.; López-Sadin, I.; Méndez-Zamora, G.; Gutiérrez-Soto, G. Decolorization and detoxification of synthetic dyes by mexican strains of trametes sp. Int. J. Environ. Res. Public Health 2019, 16, 4610. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jinga, L.I.; Tudose, M.; Ionita, P. Laccase–TEMPO as an Efficient System for Doxorubicin Removal from Wastewaters. Int. J. Environ. Res. Public Health 2022, 19, 6645. https://doi.org/10.3390/ijerph19116645
Jinga LI, Tudose M, Ionita P. Laccase–TEMPO as an Efficient System for Doxorubicin Removal from Wastewaters. International Journal of Environmental Research and Public Health. 2022; 19(11):6645. https://doi.org/10.3390/ijerph19116645
Chicago/Turabian StyleJinga, Luiza Izabela, Madalina Tudose, and Petre Ionita. 2022. "Laccase–TEMPO as an Efficient System for Doxorubicin Removal from Wastewaters" International Journal of Environmental Research and Public Health 19, no. 11: 6645. https://doi.org/10.3390/ijerph19116645
APA StyleJinga, L. I., Tudose, M., & Ionita, P. (2022). Laccase–TEMPO as an Efficient System for Doxorubicin Removal from Wastewaters. International Journal of Environmental Research and Public Health, 19(11), 6645. https://doi.org/10.3390/ijerph19116645






