Validity of the International Physical Activity Questionnaire Long Form for Assessing Physical Activity and Sedentary Behavior in Subjects with Chronic Stroke
Abstract
:1. Introduction
2. Materials and Methods
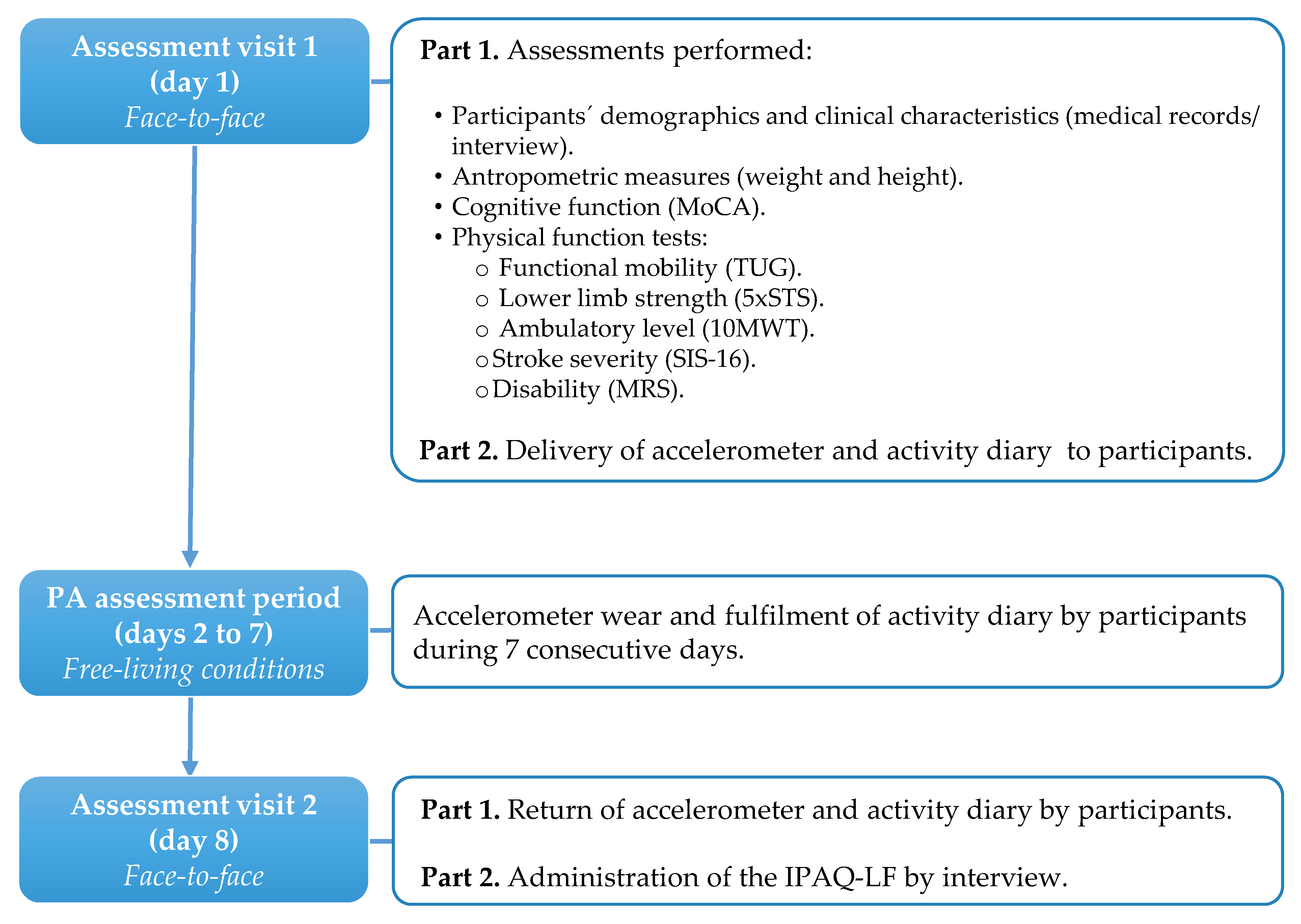
2.1. Study Design and Participants
2.2. Procedures
2.2.1. Accelerometry
2.2.2. Self-Reported Physical Activity
2.2.3. Physical Function Assessment
2.3. Sample Size Estimation
2.4. Statistical Analysis
3. Results
3.1. Validity against Accelerometry
3.2. Validity against Physical Function
3.3. IPAQ-LF Sensitivity and Specificity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L. Anthology of stroke epidemiology in the 20th and 21st centuries: Assessing the past, the present, and envisioning the future. Int. J. Stroke 2019, 14, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Cerniauskaite, M.; Quintas, R.; Koutsogeorgou, E.; Meucci, P.; Sattin, D.; Leonardi, M.; Raggi, A. Quality-of-life and disability in patients with stroke. Am. J. Phys. Med. Rehabil. 2012, 91, S39. [Google Scholar] [CrossRef]
- Cavalcanti, P.; Campos, T.; Araujo, J. Actigraphic analysis of the sleep-wake cycle and physical activity level in patients with stroke: Implications for clinical practice. Chronobiol. Int. 2012, 29, 1267–1272. [Google Scholar] [CrossRef]
- English, C.; Manns, P.J.; Tucak, C.; Bernhardt, J. Physical activity and sedentary behaviors in people with stroke living in the community: A systematic review. Phys. Ther. 2014, 94, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Fini, N.A.; Holland, A.E.; Keating, J.; Simek, J.; Bernhardt, J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys. Ther. 2017, 97, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Butler, E.N.; Evenson, K.R. Prevalence of physical activity and sedentary behavior among stroke survivors in the United States. Top. Stroke Rehabil. 2014, 21, 246–255. [Google Scholar] [CrossRef] [Green Version]
- English, C.; Healy, G.N.; Coates, A.; Lewis, L.; Olds, T.; Bernhardt, J. Sitting and activity time in people with stroke. Phys. Ther. 2016, 96, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, M.S.; Colley, R.C.; Saunders, T.J.; Healy, G.N.; Owen, N. Physiological and health implications of a sedentary lifestyle. Appl. Physiol. Nutr. Metab. 2010, 35, 725–740. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Bushnell, C.D.; Adams, R.J.; Appel, L.J.; Braun, L.T.; Chaturvedi, S.; Creager, M.A.; Culebras, A.; Eckel, R.H.; Hart, R.G.; et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2011, 42, 517–584. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kawajiri, H.; Kamisaka, K.; Kamiya, K.; Akao, K.; Asai, C.; Inuzuka, K.; Yamada, S. Predictive impact of daily physical activity on new vascular events in patients with mild ischemic stroke. Int. J. Stroke 2015, 10, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Fitzsimons, C.; Baker, G. Should we reframe how we think about physical activity and sedentary behaviour measurement? Validity and reliability reconsidered. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, J.L.; Ashe, M.C.; Biddle, S.J.; Brown, W.J.; Buman, M.P.; Chastin, S.; Gardiner, P.A.; Inoue, S.; Jefferis, B.J.; Oka, K.; et al. Sedentary time in older adults: A critical review of measurement, associations with health, and interventions. Br. J. Sports Med. 2017, 51, 1539. [Google Scholar] [CrossRef] [Green Version]
- Compagnat, M.; Mandigout, S.; Chaparro, D.; Daviet, J.C.; Salle, J.Y. Validity of the actigraph GT3x and influence of the sensor positioning for the assessment of active energy expenditure during four activities of daily living in stroke subjects. Clin. Rehabil. 2018, 32, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, V.A.; Knarr, B.A.; Reisman, D.S.; Higginson, J.S. Frontal plane compensatory strategies associated with self-selected walking speed in individuals post-stroke. Clin. Biomech. 2014, 29, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Cleland, C.; Ferguson, S.; Ellis, G.; Hunter, R.F. Validity of the international physical activity questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med. Res. Methodol. 2018, 18, 176. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, P.; Friedenreich, C.; Matthews, C.E. The role of measurement error in estimating levels of physical activity. Am. J. Epidemiol. 2007, 166, 832–840. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.J.; Wullems, J.A.; Stebbings, G.K.; Morse, C.I.; Stewart, C.E.; Onambele-Pearson, G.L. Reliability and validity of the international physical activity questionnaire compared to calibrated accelerometer cut-off points in the quantification of sedentary behaviour and physical activity in older adults. PLoS ONE 2018, 13, e0195712. [Google Scholar] [CrossRef] [Green Version]
- Silsbury, Z.; Goldsmith, R.; Rushton, A. Systematic review of the measurement properties of self-report physical activity questionnaires in healthy adult populations: Figure 1. BMJ Open 2015, 5, e008430. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Van Holle, V.; de Bourdeaudhuij, I.; Deforche, B.; van Cauwenberg, J.; van Dyck, D. Assessment of physical activity in older belgian adults: Validity and reliability of an adapted interview version of the long international physical activity questionnaire (IPAQ-L). BMC Public Health 2015, 15, 433. [Google Scholar] [CrossRef] [Green Version]
- Cerin, E.; Barnett, A.; Cheung, M.; Sit, C.H.P.; Macfarlane, D.J.; Chan, W. Reliability and validity of the IPAQ-L in a sample of Hong Kong urban older adults: Does neighborhood of residence matter? J. Aging Phys. Act. 2012, 20, 402–420. [Google Scholar] [CrossRef]
- Wanner, M.; Probst-Hensch, N.; Kriemler, S.; Meier, F.; Autenrieth, C.; Martin, B.W. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev. Med. Rep. 2016, 3, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Blikman, T.; Stevens, M.; Bulstra, S.K.; van den Akker-Scheek, I.; Reininga, I.H.F. Reliability and validity of the Dutch version of the international physical activity questionnaire in patients after total hip arthroplasty or total knee arthroplasty. J. Orthop. Sports Phys. Ther. 2013, 43, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, S.A.; Guler, S.A.; Khalil, N.; Camp, P.G.; Guenette, J.A.; Swigris, J.J.; Ryerson, C.J. Minimal important difference for physical activity and validity of the international physical activity questionnaire in interstitial lung disease. Ann. Am. Thorac. Soc. 2019, 16, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Fillipas, S.; Cicuttini, F.; Holland, A.E.; Cherry, C.L. The international physical activity questionnaire overestimates moderate and vigorous physical activity in HIV-infected individuals compared with accelerometry. J. Assoc. Nurses AIDS Care 2010, 21, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.S.; Hernon, J.; Clark, A.; Saxton, J.M. Validation of the IPAQ against different accelerometer cut-points in older cancer survivors and adults at risk of cancer. J. Aging Phys. Act. 2018, 26, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Helmerhorst, H.J.F.; Brage, S.; Warren, J.; Besson, H.; Ekelund, U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- English, C.; Healy, G.N.; Coates, A.; Lewis, L.K.; Olds, T.; Bernhardt, J. Sitting time and physical activity after stroke: Physical ability is only part of the story. Top. Stroke Rehabil. 2016, 23, 36–42. [Google Scholar] [CrossRef]
- Viosca, E.; Martínez, J.L.; Almagro, P.L.; Gracia, A.; González, C. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch. Phys. Med. Rehabil. 2005, 86, 1234–1238. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Cumming, T.B.; Churilov, L.; Linden, T.; Bernhardt, J. Montreal cognitive assessment and mini-mental state examination are both valid cognitive tools in stroke. Acta Neurol. Scand. 2013, 128, 122–129. [Google Scholar] [CrossRef]
- Chan, P.P.; Tou, J.I.S.; Tse, M.M.; Ng, S.S. Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Clark, R.A.; Bower, K.J.; Williams, G.; Pua, Y.-H. Five times sit-to-stand following stroke: Relationship with strength and balance. Gait Posture 2020, 78, 35–39. [Google Scholar] [CrossRef]
- Bushnell, C.; Bettger, J.P.; Cockroft, K.M.; Cramer, S.C.; Edelen, M.O.; Hanley, D.; Katzan, I.L.; Mattke, S.; Nilsen, D.M.; Piquado, T.; et al. Chronic stroke outcome measures for motor function intervention trials: Expert panel recommendations. Circ. Cardiovasc. Qual. Outcomes 2015, 8, S163–S169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, P.W.; Lai, S.M.; Bode, R.K.; Perera, S.; DeRosa, J. Stroke impact scale-16: A brief assessment of physical function. Neurology 2003, 60, 291–296. [Google Scholar] [CrossRef]
- Broderick, J.P.; Adeoye, O.; Jordan, E. Evolution of the modified rankin scale and its use in future stroke trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Rhoda, A.; Conradsson, D.M. Levels and patterns of physical activity in stroke survivors with different ambulation status living in low-income areas of Cape Town, South Africa. Top. Stroke Rehabil. 2020, 27, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- English, C.; Janssen, H.; Crowfoot, G.; Bourne, J.; Callister, R.; Dunn, A.; Oldmeadow, C.; Ong, L.K.; Palazzi, K.; Patterson, A.; et al. Frequent, short bouts of light-intensity exercises while standing decreases systolic blood pressure: Breaking up sitting time after stroke (BUST-Stroke) Trial. Int. J. Stroke 2018, 13, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, W.; Riveros, C.; Askim, T.; Bussmann, J.B.J.; Callisaya, M.L.; Chastin, S.F.M.; Dean, C.M.; Ezeugwu, V.E.; Jones, T.M.; Kuys, S.S.; et al. Identifying factors associated with sedentary time after stroke. Secondary analysis of pooled data from nine primary studies. Top. Stroke Rehabil. 2019, 26, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Esliger, D.W.; Copeland, J.L.; Barnes, J.D.; Tremblay, M.S. Standardizing and optimizing the use of accelerometer data for free-living physical activity monitoring. J. Phys. Act. Health 2005, 2, 366–383. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.A.; Shumway-Cook, A.; Ciol, M.A.; Kartin, D. Participation in community walking following stroke: Subjective versus objective measures and the impact of personal factors. Phys. Ther. 2011, 91, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the computer science and applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef] [Green Version]
- IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms. Available online: https://docs.google.com/viewer?a=v&pid=sites&srcid=ZGVmYXVsdGRvbWFpbnx0aGVpcGFxfGd4OjE0NDgxMDk3NDU1YWRlZTM (accessed on 17 November 2020).
- Lavelle, G.; Noorkoiv, M.; Theis, N.; Korff, T.; Kilbride, C.; Baltzopoulos, V.; Shortland, A.; Levin, W.; Ryan, J.M. Validity of the international physical activity questionnaire short form (IPAQ-SF) as a measure of physical activity (PA) in young people with cerebral palsy: A cross-sectional study. Physiotherapy 2020, 107, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Flansbjer, U.-B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Mong, Y.; Teo, T.W.; Ng, S.S. 5-repetition sit-to-stand test in subjects with chronic stroke: Reliability and validity. Arch. Phys. Med. Rehabil. 2010, 91, 407–413. [Google Scholar] [CrossRef]
- Wolf, S.L.; Catlin, P.A.; Gage, K.; Gurucharri, K.; Robertson, R.; Stephen, K. Establishing the reliability and validity of measurements of walking time using the Emory functional ambulation profile. Phys. Ther. 1999, 79, 1122–1133. [Google Scholar] [CrossRef]
- Lin, J.-H.; Hsu, M.-J.; Hsu, H.-W.; Wu, H.-C.; Hsieh, C.-L. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke 2010, 41, 2021–2025. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-Y.; Ou, Y.-C.; Chiang, T.-R. Psychometric comparisons of four disease-specific health-related quality of life measures for stroke survivors. Clin. Rehabil. 2015, 29, 816–829. [Google Scholar] [CrossRef]
- Quinn, T.J.; Dawson, J.; Walters, M.R.; Lees, K.R. Variability in modified rankin scoring across a large cohort of international observers. Stroke 2008, 39, 2975–2979. [Google Scholar] [CrossRef] [Green Version]
- Weimar, C.; Kurth, T.; Kraywinkel, K.; Wagner, M.; Busse, O.; Haberl, R.L.; Diener, H.-C. Assessment of functioning and disability after ischemic stroke. Stroke 2002, 33, 2053–2059. [Google Scholar] [CrossRef] [Green Version]
- Kohn, M.; Senyak, J. Sample Size Calculators. Available online: https://data.ucsf.edu/research/sample-size (accessed on 1 December 2020).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Odor, P.M.; Bampoe, S.; Cecconi, M. Cardiac output monitoring: Validation studies—How results should be presented. Curr. Anesthesiol. Rep. 2017, 7, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Van Poppel, M.N.M.; Chinapaw, M.J.M.; Mokkink, L.B.; van Mechelen, W.; Terwee, C.B. Physical activity questionnaires for adults: A systematic review of measurement properties. Sports Med. 2010, 40, 565–600. [Google Scholar] [CrossRef] [PubMed]
- Compagnat, M.; Batcho, C.S.; David, R.; Vuillerme, N.; Salle, J.Y.; Daviet, J.C.; Mandigout, S. Validity of the walked distance estimated by wearable devices in stroke individuals. Sensors 2019, 19, 2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, S.A.; Hallsworth, K.; Plötz, T.; Ford, G.A.; Rochester, L.; Trenell, M.I. Physical activity, sedentary behaviour and metabolic control following stroke: A cross-sectional and longitudinal study. PLoS ONE 2013, 8, e55263. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Phusuttatam, T.; Saengsuwan, J.; Kittipanya-ngam, P. Development and preliminary validation of a stroke physical activity questionnaire. Stroke Res. Treat. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Avan, A.; Digaleh, H.; di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the global burden of disease study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Purroy, F.; Vena, A.; Forné, C.; de Arce, A.M.; Dávalos, A.; Fuentes, B.; Arenillas, J.F.; Krupinski, J.; Gómez-Choco, M.; Palomeras, E.; et al. Age- and sex-specific risk profiles and in-hospital mortality in 13,932 Spanish stroke patients. Cerebrovasc. Dis. 2019, 47, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Vahlberg, B.; Bring, A.; Hellström, K.; Zetterberg, L. Level of physical activity in men and women with chronic stroke. Physiother. Theory Pract. 2019, 35, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Morton, S.; Fitzsimons, C.F.; Hall, J.F.; Corepal, R.; English, C.; Forster, A.; Lawton, R.; Patel, A.; Mead, G.; et al. Factors influencing sedentary behaviours after stroke: Findings from qualitative observations and interviews with stroke survivors and their caregivers. BMC Public Health 2020, 20, 967. [Google Scholar] [CrossRef] [PubMed]
| Category | Criteria * |
|---|---|
| Very active | Option 1: ≥5 days/week and ≥30 min/session of VPA OR Option 2: ≥3 days/week and ≥20 min/session of VPA + ≥5 days/week and ≥30 min/session of MPA or walking |
| Active | Option 1: ≥3 days/week and ≥20 min/session of VPA OR Option 2: ≥5 days/week and ≥30 min/session of MPA or walking OR Option 3: ≥5 days/week and ≥ 150 min/week of any combination (VPA + MPA+ walking) |
| Irregularly active: This category is divided into two subgroups: | |
| Irregularly active a | ≥5 days/week OR ≥150 min/week of any combination (VPA + MPA+ walking) |
| Irregularly active b | Not meeting any of the recommendation criteria (frequency OR duration) of type A |
| Sedentary | Not engage in at least 10 min of continuous activity during the week |
| Variable | Value |
|---|---|
| Sex, male/female, n (%) | 37 (66.1)/19 (33.9) |
| Age (years) | 58.1 (11.1) |
| Body mass index (kg/m2) | 28.7 (4.6) |
| Type of stroke: ischemic/hemorrhagic, n (%) | 34 (60.7)/22 (39.3) |
| Side of hemiparesis: Left/Right, n (%) | 33 (58.9)/23 (41.1) |
| Time since stroke (months), median (IQR) | 63.0 (37.8–105.8) |
| MOCA, median (IQR) | 23.5 (20.0–25.8) |
| FACHS, n (%) | |
| (i) 2 | 10 (17.8) |
| (ii) 3 | 12 (21.4) |
| (iii) 4 | 27 (48.2) |
| (iv) 5 | 7 (12.5) |
| Gait aid, n (%) | |
| (i) Single cane | 19 (33.9) |
| (ii) Tripod cane | 3 (5.4) |
| (iii) Walker | 1 (1.8) |
| (iv) Ankle-foot orthosis | 18 (32.1) |
| TUG test (seconds), median (IQR) | 13.2 (10.7–20.8) |
| 5 × STS (seconds), median (IQR) | 14.6 (12.2–17.7) |
| 10 MWT, comfortable walking speed, (m/s) | 0.8 (0.4) |
| SIS-16, median (IQR) | 86.7 (18.36) |
| MRS, n (%) | |
| (i) 0 | 2 (3.6) |
| (ii) 1 | 9 (16.1) |
| (iii) 2 | 24 (42.9) |
| (iv) 3 | 14 (25.0) |
| (v) 4 | 7 (12.5) |
| Variable | IPAQ Long-Form | Accelerometer | Wilcoxon Z (p-Value) | Spearman’s Rho (p-Value) |
|---|---|---|---|---|
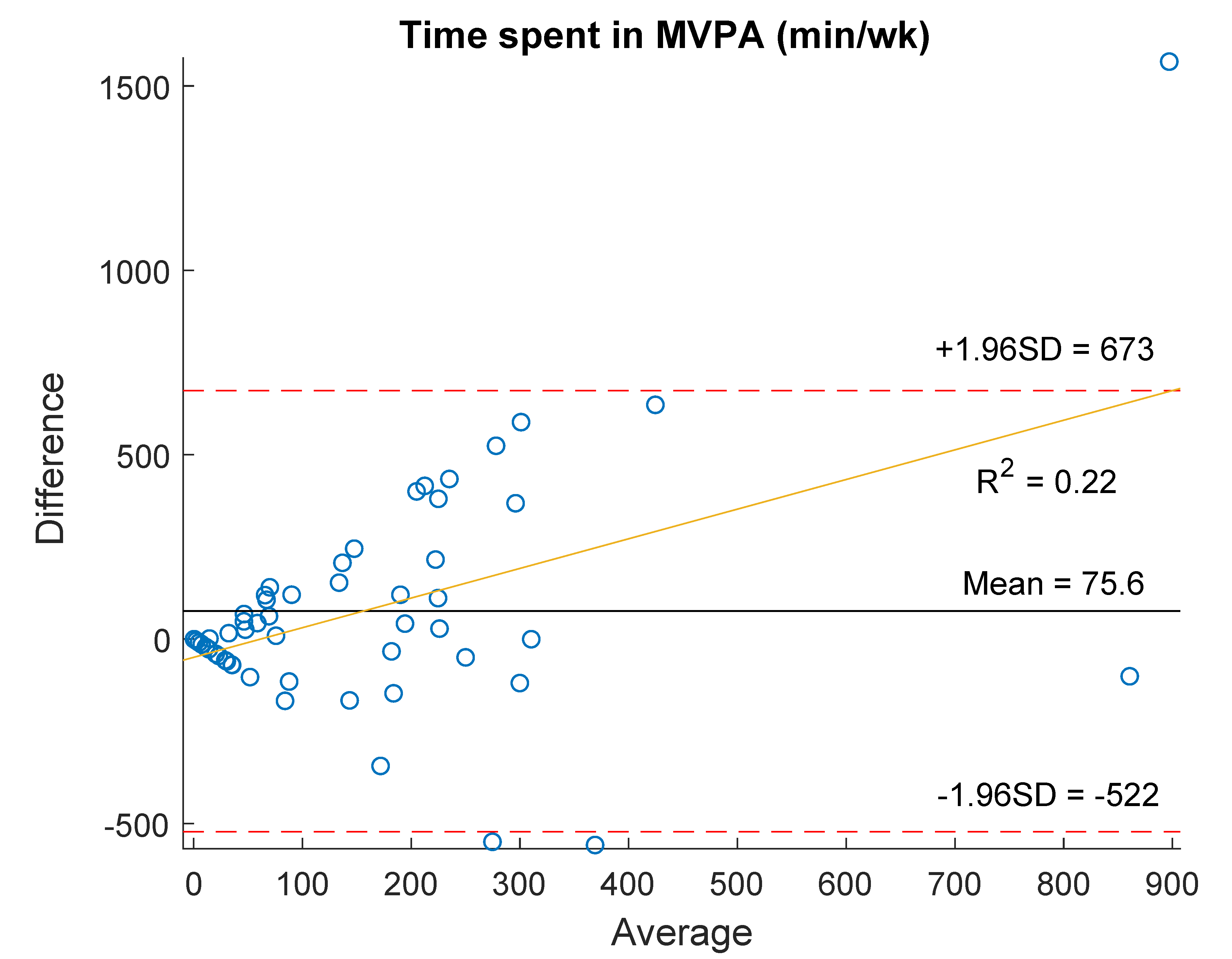
| Time spent in MVPA (min/wk) | 95.0 (0.0–265.0) | 43.4 (19.3–162.4) | −1.39 (0.16) | 0.10 (0.45) |
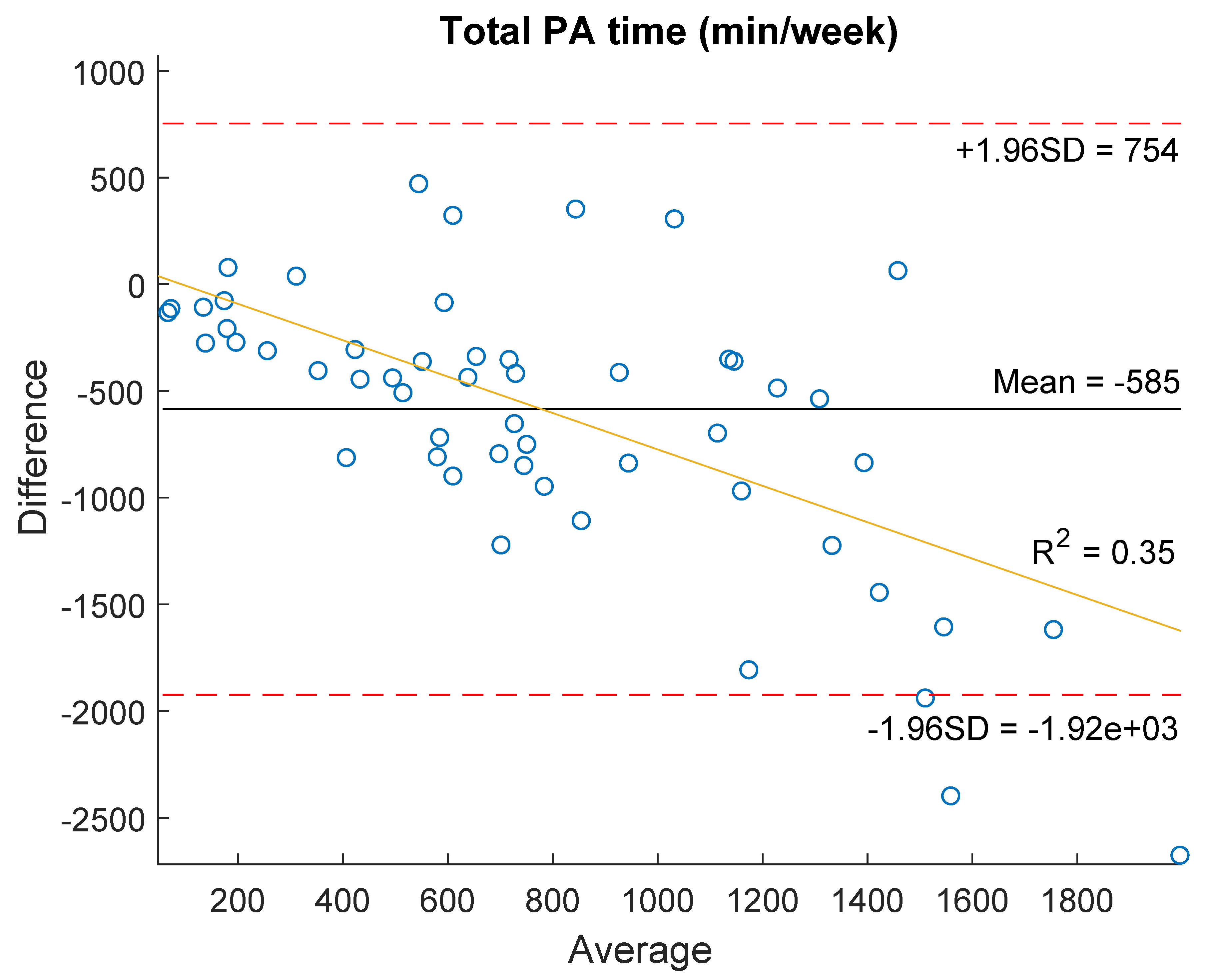
| Total PA time (min/wk) | 372.5 (183.8–736.5) | 940.8 (560.4–1421.5) | −5.44 (<0.001) * | 0.55 (<0.001) * |
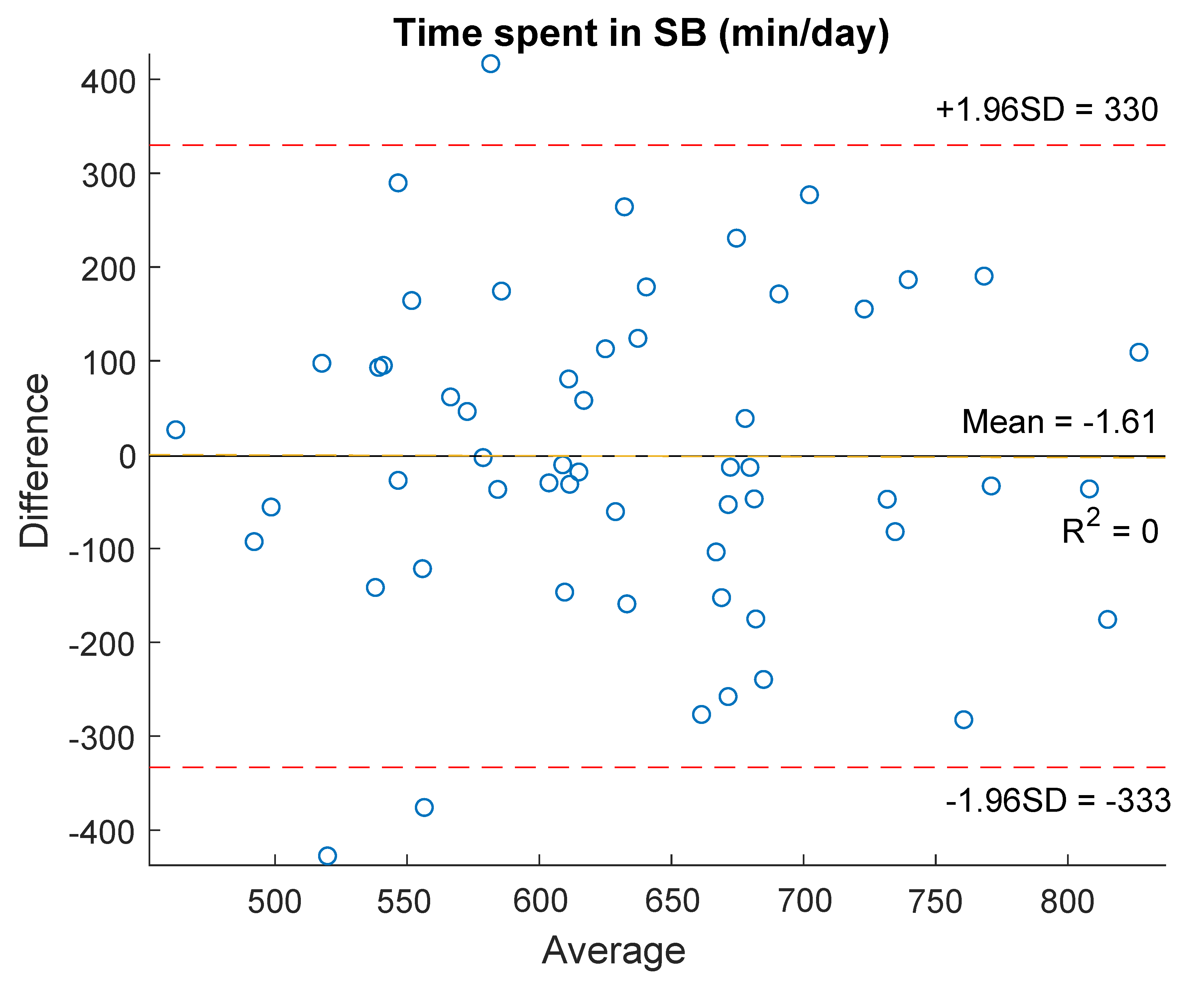
| Time spent in SB (min/d) | 617.1 (565.9–705.7) | 621.5 (553.1–729.9) | −0.03 (0.97) | 0.04 (0.79) |
| IPAQ Long-Form Data | TUG Test (s) | 5 × STS (s) | 10 MWT (m/s) | SIS-16 | MRS |
|---|---|---|---|---|---|
| Time spent in MVPA (min/wk) | −0.38; 0.004 * | −0.29; 0.047 * | 0.41; 0.002 * | 0.34; 0.011 * | −0.47; <0.001 * |
| Total PA time (min/wk) | −0.52; <0.001 * | −0.31; 0.030 * | 0.47; <0.001 * | 0.38; 0.004 * | −0.60; <0.001 * |
| Sedentary time (min/d) | 0.23; 0.09 | 0.12; 0.43 | −0.23; 0.09 | −0.29; 0.029 * | 0.31; 0.021 * |
| Accelerometer Category | Total | |||
|---|---|---|---|---|
| Compliant | Non-Compliant | |||
| IPAQ-LF category | Sufficiently active | 21 | 5 | 26 |
| Insufficiently active | 21 | 9 | 30 | |
| Total | 42 | 14 | 56 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruescas-Nicolau, M.-A.; Sánchez-Sánchez, M.L.; Cortés-Amador, S.; Pérez-Alenda, S.; Arnal-Gómez, A.; Climent-Toledo, A.; Carrasco, J.J. Validity of the International Physical Activity Questionnaire Long Form for Assessing Physical Activity and Sedentary Behavior in Subjects with Chronic Stroke. Int. J. Environ. Res. Public Health 2021, 18, 4729. https://doi.org/10.3390/ijerph18094729
Ruescas-Nicolau M-A, Sánchez-Sánchez ML, Cortés-Amador S, Pérez-Alenda S, Arnal-Gómez A, Climent-Toledo A, Carrasco JJ. Validity of the International Physical Activity Questionnaire Long Form for Assessing Physical Activity and Sedentary Behavior in Subjects with Chronic Stroke. International Journal of Environmental Research and Public Health. 2021; 18(9):4729. https://doi.org/10.3390/ijerph18094729
Chicago/Turabian StyleRuescas-Nicolau, Maria-Arantzazu, María Luz Sánchez-Sánchez, Sara Cortés-Amador, Sofía Pérez-Alenda, Anna Arnal-Gómez, Assumpta Climent-Toledo, and Juan J. Carrasco. 2021. "Validity of the International Physical Activity Questionnaire Long Form for Assessing Physical Activity and Sedentary Behavior in Subjects with Chronic Stroke" International Journal of Environmental Research and Public Health 18, no. 9: 4729. https://doi.org/10.3390/ijerph18094729
APA StyleRuescas-Nicolau, M.-A., Sánchez-Sánchez, M. L., Cortés-Amador, S., Pérez-Alenda, S., Arnal-Gómez, A., Climent-Toledo, A., & Carrasco, J. J. (2021). Validity of the International Physical Activity Questionnaire Long Form for Assessing Physical Activity and Sedentary Behavior in Subjects with Chronic Stroke. International Journal of Environmental Research and Public Health, 18(9), 4729. https://doi.org/10.3390/ijerph18094729










