Accuracy of a Smartphone Application Measuring Snoring in Adults—How Smart Is It Actually?
Abstract
:1. Introduction
2. Materials and Methods
- starting and ending time of monitoring
- time in bed
- monitoring time
- duration and ratio of quiet sleep, light, loud and epic snoring
- total time of snoring
- Snore Score, which is a specific score given by the SnoreLab app.
- starting and ending of monitoring
- time in bed
- total time of snoring
- snore index (SI, number of snoring events per hour)
- snore index obstructive (SI obstructive, number of snoring events per hour associated with obtrusive events)
- Sensitivity = TP/(TP + FN)
- Specificity = TN/(FP + TN)
- Positive predictive value = TP/(TP + FP)
- Negative predictive value = TN/(FN + TN)
- Prevalence = TP/Total study population
- Accuracy = (TP + TN)/Total study population.
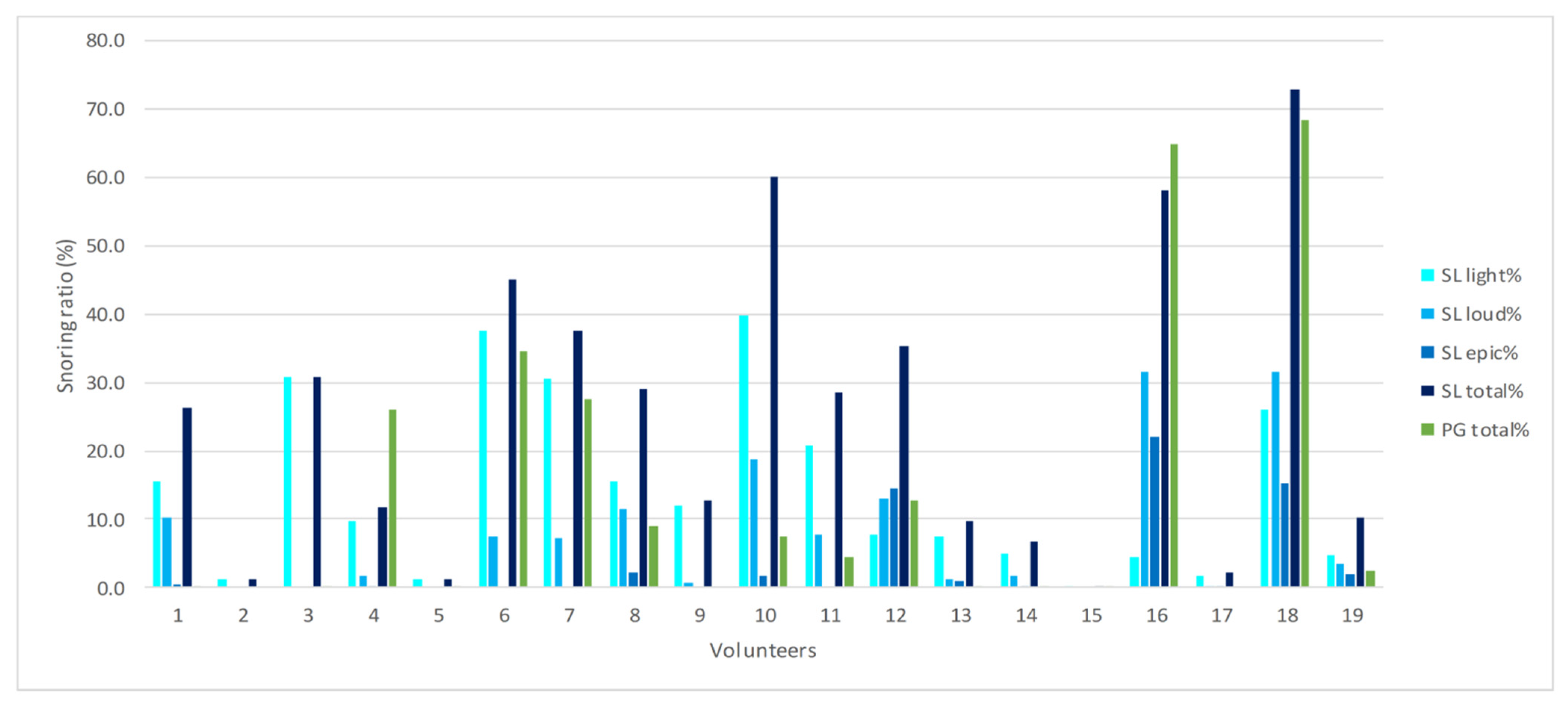
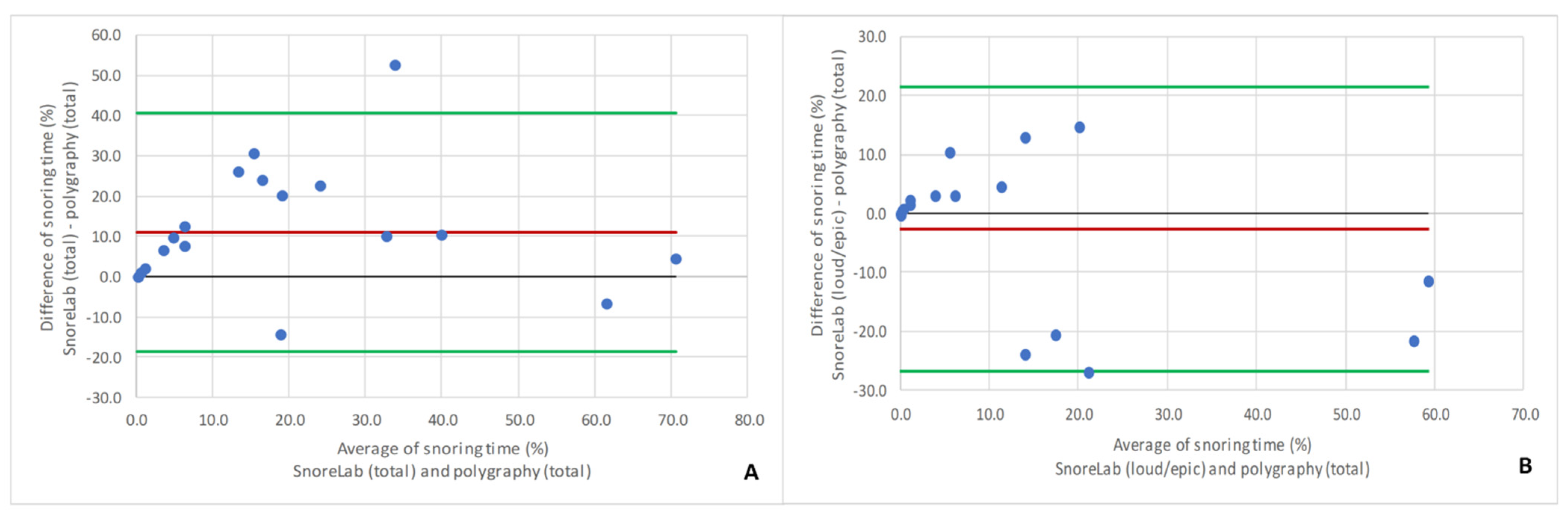
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, C.H.; Wong, B.M.; Tang, J.L.; Ng, D.K. Gender difference in snoring and how it changes with age: Systematic review and meta-regression. Sleep Breath. 2012, 16, 977–986. [Google Scholar] [CrossRef]
- Li, C.; Hoffstein, V. Snoring. In Principles and Practice of Sleep Medicine, 5th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; Chapter 102; pp. 1172–1176. [Google Scholar]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Myers, K.A.; Mrkobrada, M.; Simel, D.L. Does this patient have obstructive sleep apnea? The Rational Clinical Examination systematic review. JAMA 2013, 310, 731–741. [Google Scholar] [CrossRef]
- Patel, S.J. Obstructive Sleep Apnea. Ann. Intern. Med. 2019, 171, ITC81–ITC96. [Google Scholar] [CrossRef]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Semelka, M.; Wilson, J.; Floyd, R. Diagnosis and treatment of obstructive sleep apnea in adults. Am. Fam. Physician 2016, 94, 355–360. [Google Scholar]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Hillman, D.; Mitchell, S.; Streatfield, J.; Burns, C.; Bruck, D.; Pezzullo, L. The economic cost of inadequate sleep. Sleep 2018, 41, 1–13. [Google Scholar] [CrossRef]
- Kapur, V.; Strohl, K.P.; Redline, S.; Iber, C.; O’Connor, G.; Nieto, J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002, 6, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Mold, J.W.; Quattlebaum, C.; Schinnerer, E.; Boekman, L.; Orr, W.; Hollabaugh, K. Identification by primary care clinicians of patients with obstructive sleep apnea: A practice-based research network (PBRN) study. J. Am. Board Fam. Med. 2011, 24, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuck, B.A.; Braumann, B.; Heiser, C.; Herzog, M.; Maurer, J.T.; Plößl, S.; Steffen, A.; Sommer, J.U.; Verse, T.; Hofauer, B. S3-Leitlinie “Diagnostik und Therapie des Schnarchens des Erwachsenen”. Somnologie 2019, 23, 178–208. [Google Scholar] [CrossRef]
- Mayer, G.; Arzt, M.; Braumann, B.; Ficker, J.H.; Fietze, I.; Galetke, W.; Maurer, T.; Orth, M.; Penzel, T.; Pistner, H.P.; et al. S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen–Kapitel “Schlafbezogene Atmungsstörungen”. Somnologie 2017, 20, S97–S180. [Google Scholar]
- Stuck, B.A.; Arzt, M.; Fietze, I.; Galetke, W.; Hein, H.; Heiser, C.; Herkenrath, S.D.; Hofauer, B.; Maurer, J.T.; Mayer, G.; et al. Teil-Aktualisierung S3-Leitlinie Schlafbezogene Atmungsstörungen bei Erwachsenen. Somnologie 2020, 24, 176–208. [Google Scholar] [CrossRef]
- Kusheida, C.A.; Litter, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J., Jr.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice parameters for the indications for polysomnography and related procedures. An update for 2005. Sleep 2005, 28, 499–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Behar, J.; Roebuck, A.; Domingos, J.A.; Gederi, E.; Clifford, G.D. A review of current sleep screening applications for smartphones. Physiol. Meas. 2013, 34, R29. [Google Scholar] [CrossRef]
- Lindemann, J.; Augenstein, B.; Stupp, F.; Saul, B.; Reichert, M.; Riepl, R.; Sommer, F.; Grossi, A.S. Diagnostische Genauigkeit ambulanter Polygrafiegeräte. HNO 2017, 65, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://research2guidance.com/mhealth-app-developer-economics/ (accessed on 15 October 2020).
- Baron, K.G.; Duffecy, J.; Berendsen, M.A.; Cheung, I.N.; Lattie, E.; Manalo, N.C. Feeling validated yet? A scoping review of the use of consumer-targeted wearable and mobile technology to measure and improve sleep. Sleep Med. Rev. 2018, 40, 151–159. [Google Scholar] [CrossRef]
- Fino, E.; Mazzetti, M. Monitoring healthy and disturbed sleep through smartphone applications: A review of experimental evidence. Sleep Breath. 2019, 23, 13–24. [Google Scholar] [CrossRef]
- Camacho, M.; Robertson, M.; Abdullatif, J.; Certal, V.; Kram, Y.A.; Ruoff, C.M.; Brietzke, S.E.; Capasso, R. Smartphone apps for snoring. J. Laryngol. Otol. 2015, 129, 974–979. [Google Scholar] [CrossRef]
- Stippig, A.; Hübers, U.; Emerich, M. Apps in sleep medicine. Sleep Breath. 2015, 19, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.K.; Lin, Y.C.; Lin, C.W.; Ting, C.S.; Chiang, Y.Y.; Kao, Y.H. Validation of snoring detection using a smartphone app. Sleep Breath. 2021, 1–7. [Google Scholar] [CrossRef]
- Nakano, H.; Hirayama, K.; Sadamitsu, Y.; Toshimitsu, A.; Fujita, H.; Hin, S.; Tanigawa, T. Monitoring sound to quantify snoring and sleep apnea severity using a smartphone: Proof of concept. J. Clin. Sleep Med. 2014, 10, 73–78. [Google Scholar] [CrossRef]
- Tiron, R.; Lyon, G.; Kilroy, H.; Osman, A.; Kelly, N.; O’Mahony, N.; Lopes, C.; Coffey, S.; McMahon, S.; Wren, M.; et al. Screening for obstructive sleep apnea with novel hybrid acoustic smartphone app technology. J. Thorac. Dis. 2020, 12, 4476–4495. [Google Scholar] [CrossRef] [PubMed]
- The Federal Health Monitoring System, Germany. Available online: https://www.gbe-bund.de/gbe/pkg_isgbe5.prc_menu_olap?p_uid=gast&p_aid=52123572&p_sprache=D&p_help=2&p_indnr=434&p_version=2&p_ansnr=72674692 (accessed on 22 June 2021).
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE Publications: London, UK, 2013. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. Analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analysis using G*Power 3.1: Test for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Behar, J.; Roebuck, A.; Shadid, M.; Daly, J.; Hallack, A.; Palmuis, N.; Stradling, J.; Clifford, G.D. SleepAp: An automated obstructive sleep apnoea screening application for smartphones. IEEE J. Biomed. Health Inform. 2015, 19, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgdorf, A.; Güthe, I.; Jovanovic, M.; Kutafina, E.; Kohlschein, C.; Bitsch, J.A.; Jonas, S.M. The mobile sleep lab app: An open-source framework for mobile sleep assessment based on consumer-grad wearable devices. Comput. Biol. Med. 2018, 103, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.P.; Williams, A.J. Sleep apps: What role do they play in clinical medicine? Curr. Opin. Pulm. Med. 2017, 23, 512–516. [Google Scholar] [CrossRef] [PubMed]
- SnoreLab-Homepage. Available online: https://www.snorelab.com/faqs/ (accessed on 26 April 2021).
- Figueras-Alvarez, O.; Cantó-Navés, O.; Cabratosa-Termes, J.; Roig-Cayón, M.; Felipe-Spada, N.; Tomàs-Aliberas, J. Snoring intensity assessment with three different smartphones using the SnoreLab application in one participant. J. Clin. Sleep Med. 2020, 16, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaugh, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; Version 2.2; American Academy of Sleep Medicine: Darien, IL, USA, 2015. [Google Scholar]
| Polygraphy (Gold Standard) | |||
|---|---|---|---|
| Snoring beyond threshold | Snoring below threshold | ||
| SnoreLab app | Snoring beyond threshold | True positive (TP) | False positive (FP) |
| Snoring below threshold | False negative (FN) | True negative (TN) | |
| Median | Min | Max | Mean | SD | |
|---|---|---|---|---|---|
| SnoreLab app | |||||
| Monitoring time (hh:mm:ss) | 07:23:00 | 05:13:00 | 11:44:00 | 07:27:44 | 01:21:30 |
| Total snoring (%) | 26.3 | 0.2 | 72.8 | 25.3 | 22.0 |
| Light snoring (%) | 9.7 | 0.2 | 39.8 | 14.3 | 12.9 |
| Loud snoring (%) | 3.5 | 0.0 | 31.6 | 7.8 | 9.9 |
| Epic snoring (%) | 0.2 | 0.0 | 22.1 | 3.2 | 6.5 |
| Loud + epic snoring (%) | 5.4 | 0.0 | 53.6 | 10.9 | 15.8 |
| Snore Score | 15.0 | 1.0 | 117.0 | 29.1 | 35.2 |
| Polygraphy | |||||
| Monitoring time (hh:mm:ss) | 07:29:58 | 05:16:02 | 10:00:00 | 07:26:57 | 01:00:58 |
| Total snoring (%) | 2.4 | 0.0 | 68.4 | 13.1 | 21.5 |
| Snore index | 7.9 | 0.0 | 450.1 | 70.6 | 131.6 |
| Snore index obstructive | 0.1 | 0.0 | 38.4 | 4.4 | 9.6 |
| Apnea-hypopnea-index | 9.7 | 0.9 | 38.7 | 12.2 | 10.4 |
| Thresholds of Percentage of Total Snoring per Night | ||||
|---|---|---|---|---|
| >50% | >25% | >10% | >5% | |
| Prevalence | 10.5 | 21.1 | 31.5 | 42.1 |
| Sensitivity | 100.0 | 80.0 | 100.0 | 100.0 |
| Specificity | 94.1 | 57.1 | 53.8 | 54.5 |
| Positive predictive value | 66.6 | 40.0 | 50.0 | 61.5 |
| Negative predictive value | 100.0 | 88.8 | 100.0 | 100.0 |
| Accuracy | 94.7 | 63.2 | 68.4 | 73.6 |
| Variables | r |
|---|---|
| Snore Score (SL)/Snore index (PG) | 0.813 |
| Snore index (PG)/Snore index obstructive (PG) | 0.809 |
| AHI (PG)/Snore Score (SL) | 0.495 |
| AHI (PG)/Snore index (PG) | 0.576 |
| AHI (PG)/Snore index obstructive (PG) | 0.645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaus, K.; Stummer, A.-L.; Ruf, S. Accuracy of a Smartphone Application Measuring Snoring in Adults—How Smart Is It Actually? Int. J. Environ. Res. Public Health 2021, 18, 7326. https://doi.org/10.3390/ijerph18147326
Klaus K, Stummer A-L, Ruf S. Accuracy of a Smartphone Application Measuring Snoring in Adults—How Smart Is It Actually? International Journal of Environmental Research and Public Health. 2021; 18(14):7326. https://doi.org/10.3390/ijerph18147326
Chicago/Turabian StyleKlaus, Katharina, Anna-Lena Stummer, and Sabine Ruf. 2021. "Accuracy of a Smartphone Application Measuring Snoring in Adults—How Smart Is It Actually?" International Journal of Environmental Research and Public Health 18, no. 14: 7326. https://doi.org/10.3390/ijerph18147326
APA StyleKlaus, K., Stummer, A.-L., & Ruf, S. (2021). Accuracy of a Smartphone Application Measuring Snoring in Adults—How Smart Is It Actually? International Journal of Environmental Research and Public Health, 18(14), 7326. https://doi.org/10.3390/ijerph18147326






