Concentrations of Seven Phthalate Monoesters in Infants and Toddlers Quantified in Urine Extracted from Diapers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Chemicals
2.4. Sample Preparation and Extraction
2.5. Quality Assurance and Quality Control
2.6. Chemical Analytical Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Phthalate Metabolites Concentrations
4.2. Population’s Exposure to Phthalates
4.3. Gender-Specific Phthalate Exposure
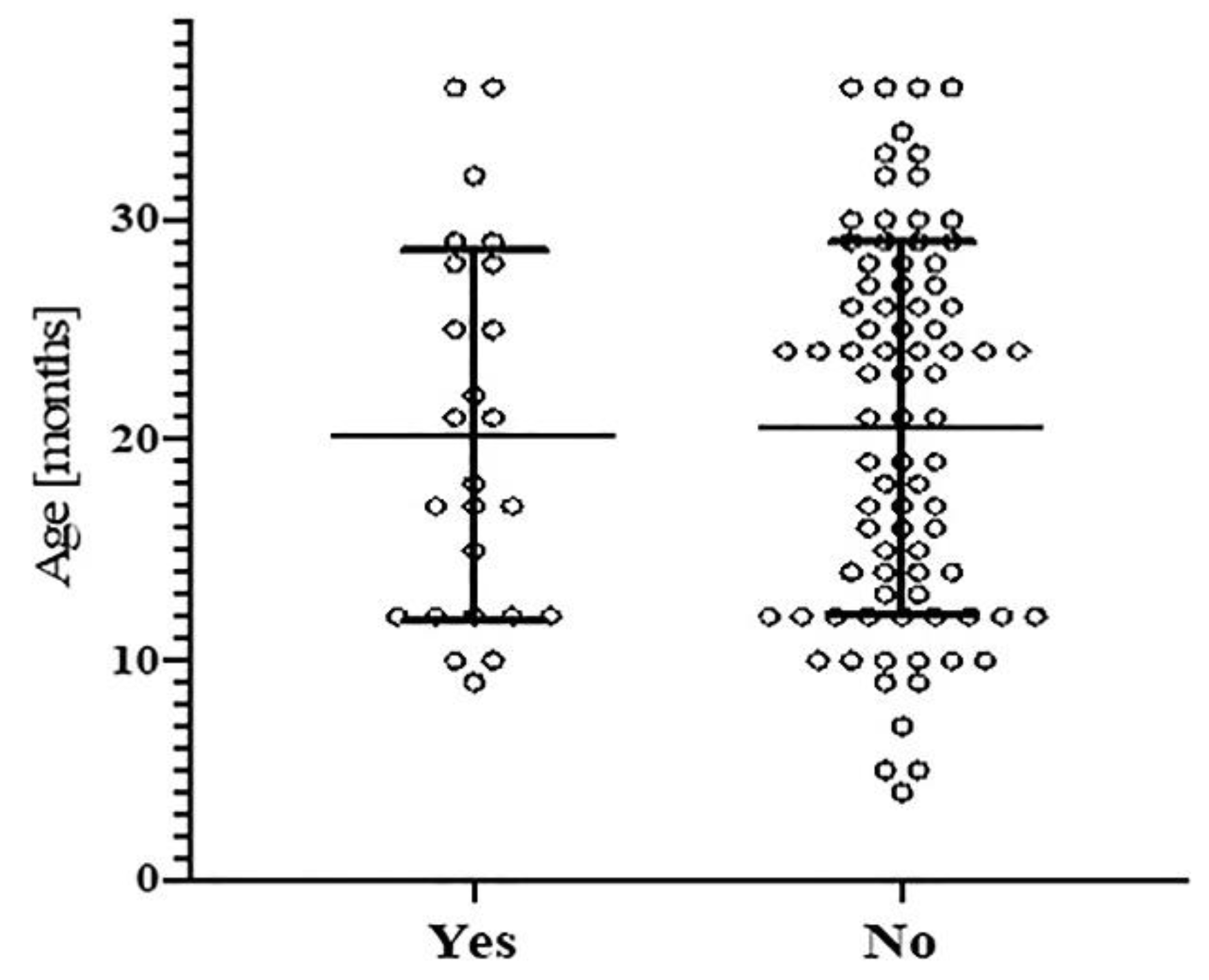
4.4. Age-Specific Phthalate Exposure
4.5. Regulatory Status of Phthalates in Toys and Childcare Articles
4.6. Limitations and Novelty of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuß, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef]
- Chanda, M.; Roy, S.K. Industrial Polymers, Specialty Polymers, and Their Applications. In Plastics Fundamentals, Properties, and Testing; Informa UK Limited: London, UK, 2008. [Google Scholar]
- Radke, E.; Galizia, A.; Thayer, K.A.; Cooper, G.S. Phthalate exposure and metabolic effects: A systematic review of the human epidemiological evidence. Environ. Int. 2019, 132, 104768. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Franken, C.; Lambrechts, N.; Govarts, E.; Koppen, G.; Hond, E.D.; Ooms, D.; Voorspoels, S.; Bruckers, L.; Loots, I.; Nelen, V.; et al. Phthalate-induced oxidative stress and association with asthma-related airway inflammation in adolescents. Int. J. Hyg. Environ. Health 2017, 220, 468–477. [Google Scholar] [CrossRef]
- Qian, X.; Li, J.; Xu, S.; Wan, Y.; Li, Y.; Jiang, Y.; Zhao, H.; Zhou, Y.; Liao, J.; Liu, H.; et al. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ. Int. 2019, 131, 105023. [Google Scholar] [CrossRef]
- Braun, J.M.; Sathyanarayana, S.; Hauser, R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef]
- Radke, E.G.; Glenn, B.S.; Braun, J.M.; Cooper, G.S. Phthalate exposure and female reproductive and developmental outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2019, 130, 104580. [Google Scholar] [CrossRef]
- Radke, E.; Braun, J.M.; Nachman, R.M.; Cooper, G.S. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int. 2020, 137, 105408. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Calafat, A.M. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- EC (European Commission). Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation(EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union L 2006, 396, 1–850. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1907-20140410&from=EN (accessed on 30 March 2021).
- Ordinance on the Reduction of Risks Relating to the Use of Certain Particularly Dangerous Substances, Preparations and Articles (Chemical Risk Reduction Ordinance, ORRChem) of 18 May 2005 (Status as of 1 March 2021). Available online: https://www.fedlex.admin.ch/eli/cc/2005/478/en#app26 (accessed on 1 April 2021).
- ECHA (European Chemicals Agency). Annex XVII to Reach, Entry 52. Conditions of Restrictions on the Manufacture, Placing on the Market and Use of Certain Dangerous Substances, Mixtures and Articles. Available online: https://echa.europa.eu/documents/10162/57096439-2ddd-4f14-b832-85181a09f595 (accessed on 29 March 2021).
- ECHA (European Chemicals Agency). Annex XVII to Reach, Entry 51. Conditions of Restrictions on the Manufacture, Placing on the Market and Use of Certain Dangerous Substances, Mixtures and Articles. Available online: https://echa.europa.eu/documents/10162/aaa92146-a005-1dc2-debe-93c80b57c5ee (accessed on 29 March 2021).
- EC (European Commission). Commission Regulation (EC) No 552/2009 Of 22 June 2009 Amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration,Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annex XVII (Text with EEA Relevance). Off. J. Eur. Union. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R0552 (accessed on 14 October 2020).
- EC (European Commission). Directives Commission Delegated Directive (EU) 2015/863 of 31 March 2015 Amending Annex II to DIRECTIVE 2011/65/EU of the European Parliament and of the Council as Regards the List of Restricted Substances (Text with EEA Relevance). Off. J. Eur. Union. 2015. Available online: https://eur-lex.europa.eu/eli/dir_del/2015/863/oj (accessed on 14 October 2020).
- Health Canada. Industry Guide to Health Canada’s Safety Requirements for Children’s Toys and Related Products; Health Canada: Ottawa, ON, Canada, 2016; Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reportspublications/industry-professionals/industry-guide-safety-requirements-childrentoys-related-products-summary/guidance-document.html (accessed on 19 October 2020).
- CPSC, Consumer Product Safety Commission. Prohibition of Children’s Toys and Child Care Articles Containing Specified Phthalates: Determinations Regarding Certain Plastics. Federal Register, Docket No. CPSC-2016-0017. 2017. Available online: https://www.federalregister.gov/documents/2016/08/17/2016-19464/prohibition-of-childrens-toys-and-child-care-articles-containing-specified-phthalates-determinations (accessed on 21 April 2021).
- EC (European Commission). Commission Regulation (EU) 2018/2005 of 17 December 2018 Amending Annex XVII to REGULATION (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis(2-ethylhexyl) Phthalate (DEHP), Dibutyl Phthalate (DBP), Benzyl Butyl Phthalate (BBP) and Diisobutyl Phthalate (DIBP) (Text with EEA Relevance). Off. J. Eur. Union 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R2005 (accessed on 19 October 2020).
- Oteef, M.D.Y.; Elhassan, M.S. Plastic toys and child care articles as a source of children exposure to phthalates and other plasticisers in Saudi Arabia. Int. J. Environ. Anal. Chem. 2020, 1–15. [Google Scholar] [CrossRef]
- Schwedler, G.; Rucic, E.; Lange, R.; Conrad, A.; Koch, H.M.; Pälmke, C.; Brüning, T.; Schulz, C.; Schmied-Tobies, M.I.; Daniels, A.; et al. Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014–2017. Int. J. Hyg. Environ. Health 2020, 225, 113444. [Google Scholar] [CrossRef]
- Kessler, W.; Numtip, W.; Völkel, W.; Seckin, E.; Csanády, G.A.; Pütz, C.; Klein, D.; Fromme, H.; Filser, J.G. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol. Appl. Pharmacol. 2012, 264, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, A.; Völkel, W.; Fromme, H. Kinetics of the phthalate metabolites mono-2-ethylhexyl phthalate (MEHP) and mono-n-butyl phthalate (MnBP) in male subjects after a single oral dose. Toxicol. Lett. 2016, 252, 22–28. [Google Scholar] [CrossRef]
- Kumar, A.R.; Sivaperumal, P. Analytical methods for the determination of biomarkers of exposure to phthalates in human urine samples. TrAC Trends Anal. Chem. 2016, 75, 151–161. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.-M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Sonawane, B. Incorporating pharmacokinetic differences between children and adults in assessing children’s risks to environmental toxicants. Toxicol. Appl. Pharmacol. 2004, 198, 164–183. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Li, X.; Tian, M.; Huang, Q.; Zhang, J.; Pan, H.; Wen, K.; Huang, Q.; Yan, J.; et al. Infantile phthalate metabolism and toxico/pharmacokinetic implications within the first year of life. Environ. Int. 2020, 144, 106052. [Google Scholar] [CrossRef] [PubMed]
- Navaranjan, G.; Takaro, T.K.; Wheeler, A.J.; Diamond, M.L.; Shu, H.; Azad, M.B.; Becker, A.B.; Dai, R.; Harris, S.A.; Lefebvre, D.L.; et al. Early life exposure to phthalates in the Canadian Healthy Infant Longitudinal Development (CHILD) study: A multi-city birth cohort. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Park, J.; Kim, H.-J.; Cho, G.J.; Kim, G.-H.; Eun, S.-H.; Lee, J.J.; Choi, G.; Suh, E.; et al. Urinary phthalate metabolites over the first 15 months of life and risk assessment – CHECK cohort study. Sci. Total Environ. 2017, 607-608, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Kuiri-Hänninen, T.; Main, K.M.; Dunkel, L.; Sankilampi, U. A Longitudinal Study of Urinary Phthalate Excretion in 58 Full-Term and 67 Preterm Infants from Birth through 14 Months. Environ. Health Perspect. 2014, 122, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Kiranoglu, M.; Schuster, R.; Fromme, H. Phthalate intake by infants calculated from biomonitoring data. Toxicol. Lett. 2014, 225, 222–229. [Google Scholar] [CrossRef]
- Song, N.R.; On, J.-W.; Lee, J.; Park, J.-D.; Kwon, H.-J.; Yoon, H.J.; Pyo, H. Biomonitoring of urinary di(2-ethylhexyl) phthalate metabolites of mother and child pairs in South Korea. Environ. Int. 2013, 54, 65–73. [Google Scholar] [CrossRef]
- Hond, E.D.; Govarts, E.; Willems, H.; Smolders, R.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Seiwert, M.; Fiddicke, U.; Castaño, A.; et al. First Steps toward Harmonized Human Biomonitoring in Europe: Demonstration Project to Perform Human Biomonitoring on a European Scale. Environ. Health Perspect. 2015, 123, 255–263. [Google Scholar] [CrossRef]
- CDC. Centers for Diseas Control and Prevention, 2012. Fourth Annual Report. 2012. Available online: https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 14 October 2020).
- Liu, L.; Xia, T.; Guo, L.; Cao, L.; Zhao, B.; Zhang, J.; Dong, S.; Shen, H. Expressing urine from a gel disposable diaper for biomonitoring using phthalates as an example. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 625–631. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Beach, J.; Raymer, J.; Gardner, M. Disposable diaper to collect urine samples from young children for pyrethroid pesticide studies. J. Expo. Sci. Environ. Epidemiol. 2004, 14, 378–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oya, N.; Ito, Y.; Hioki, K.; Asai, Y.; Aoi, A.; Sugiura, Y.; Ueyama, J.; Oguri, T.; Kato, S.; Ebara, T.; et al. Quantitative analysis of organophosphate insecticide metabolites in urine extracted from disposable diapers of toddlers in Japan. Int. J. Hyg. Environ. Health 2017, 220, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Aoi, A.; Ueda, Y.; Oya, N.; Sugiura, Y.; Ito, Y.; Ebara, T.; Kamijima, M. Biomonitoring method for neonicotinoid insecticides in urine of non-toilet-trained children using LC-MS/MS. Food Addit. Contam. Part A 2020, 37, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ueyama, J.; Kondo, T.; Saito, I.; Shibata, E.; Gotoh, M.; Nomura, H.; Wakusawa, S.; Nakai, K.; Kamijima, M. A non-invasive biomonitoring method for assessing levels of urinary pyrethroid metabolites in diapered children by gas chromatography–mass spectrometry. J. Expo. Sci. Environ. Epidemiol. 2013, 24, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, A.; van Dael, M.; Vermeulen, R.; Russel, F.; Scheepers, P. Urine collection methods for non-toilet-trained children in biological monitoring studies: Validation of a disposable diaper for characterization of tebuconazole exposure. Toxicol. Lett. 2018, 298, 201–206. [Google Scholar] [CrossRef]
- Liu, L.; Xia, T.; Zhang, X.; Barr, D.B.; Alamdar, A.; Zhang, J.; Tian, M.; Huang, Q.; Shen, H. Biomonitoring of infant exposure to phenolic endocrine disruptors using urine expressed from disposable gel diapers. Anal. Bioanal. Chem. 2014, 406, 5049–5054. [Google Scholar] [CrossRef]
- Lucarini, F.; Krasniqi, T.; Rosset, G.B.; Roth, N.; Hopf, N.B.; Broillet, M.-C.; Staedler, D. Exposure to New Emerging Bisphenols Among Young Children in Switzerland. Int. J. Environ. Res. Public Health 2020, 17, 4793. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Samandar, E.; Preau, J.L.; Needham, L.L.; Calafat, A.M. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 2006, 219, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Hubal, E.A.C.; de Wet, T.; Du Toit, L.; Firestone, M.P.; Ruchirawat, M.; van Engelen, J.; Vickers, C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul. Toxicol. Pharmacol. 2014, 69, 113–124. [Google Scholar] [CrossRef]
- Carlstedt, F.; Jönsson, B.; Bornehag, C.-G. PVC flooring is related to human uptake of phthalates in infants. Indoor Air 2012, 23, 32–39. [Google Scholar] [CrossRef]
- McCombie, G.; Biedermann, S.; Suter, G.; Biedermann, M. Survey on plasticizers currently found in PVC toys on the Swiss market: Banned phthalates are only a minor concern. J. Environ. Sci. Health Part A 2017, 52, 1–6. [Google Scholar] [CrossRef]
- Ahbab, M.A.; Barlas, N. Influence of in utero di-n-hexyl phthalate and dicyclohexyl phthalate on fetal testicular development in rats. Toxicol. Lett. 2015, 233, 125–137. [Google Scholar] [CrossRef] [PubMed]
- California Environmental Protection Agency, Office of Environmental Health Hazard Assessment. Evidence of the carcinogenicity of diisononyl phthalate (DINP). 2013. Available online: Oehha.ca.gov/media/downloads/proposition-65/chemicals/dinphid100413.pdf (accessed on 6 June 2021).
- Shun, S.; Shusaku, T.; Hirota, K. Thermoplastic Resin Composition for Tire Inner Liner, Tire Inner Liner, Pneumatic Tire, Manufacturing Method of Tire Inner Liner, and Manufacturing Method of Pneumatic Tire. JP2019182969 (A), Japan Office. Publication Date: 24-10-2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=JP275662608&tab=NATIONALBIBLIO&_cid=P21-KO2M88-10574-1 (accessed on 20 October 2020).
- CPSC, Consumer Product Safety Commission. Toxicity Review of Di-n-Octyl Phthalate (DnOP); Memorandum: Bethesda, MD, USA, 2010. Available online: https://www.cpsc.gov/s3fs-public/ToxicityReviewOfDnOP.pdf (accessed on 10 June 2021).
- Main, K.M.; Mortensen, G.K.; Kaleva, M.M.; Boisen, K.A.; Damgaard, I.N.; Chellakooty, M.; Schmidt, I.M.; Suomi, A.-M.; Virtanen, H.E.; Petersen, D.V.H.; et al. Human Breast Milk Contamination with Phthalates and Alterations of Endogenous Reproductive Hormones in Infants Three Months of Age. Environ. Health Perspect. 2006, 114, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef]
- Auyeung, W.; Canales, R.A.; Beamer, P.; Ferguson, A.C.; Leckie, J.O. Young children’s hand contact activities: An observational study via videotaping in primarily outdoor residential settings. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Beamer, P.; Key, M.E.; Ferguson, A.; Canales, R.A.; Auyeung, W.; Leckie, J.O. Quantified activity pattern data from 6 to 27-month-old farmworker children for use in exposure assessment. Environ. Res. 2008, 108, 239–246. [Google Scholar] [CrossRef]
- Xue, J.; Zartarian, V.; Tulve, N.; Moya, J.; Freeman, N.; Auyeung, W.; Beamer, P. A meta-analysis of children’s object-to-mouth frequency data for estimating non-dietary ingestion exposure. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zartarian, V.; Moya, J.; Freeman, N.; Beamer, P.; Black, K.; Tulve, N.; Shalat, S. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007, 27, 411–420. [Google Scholar] [CrossRef]
- Tulve, N.S.; Suggs, J.C.; McCurdy, T.; Hubal, E.A.C.; Moya, J. Frequency of mouthing behavior in young children. J. Expo. Sci. Environ. Epidemiol. 2002, 12, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.; Hameed, A.; Raza, A. Role of Phthalates as EDCs in Metabolic Disorders, in Endocrine Disrupting Chemicals-Induced Metabolic Disorders and Treatment Strategies; Akash, M.S.H., Rehman, K., Hashmi, M.Z., Eds.; Springer International Publishing: Cham, UK, 2021; pp. 239–250. [Google Scholar]
- Weiss, J.M.; Gustafsson, Å.; Gerde, P.; Bergman, Å.; Lindh, C.H.; Krais, A.M. Daily intake of phthalates, MEHP, and DINCH by ingestion and inhalation. Chemosphere 2018, 208, 40–49. [Google Scholar] [CrossRef]
| Phthalate | CAS | Metabolite | CAS | ||
|---|---|---|---|---|---|
| Diethyl phthalate | DEP | 84-66-2 | Monoethyl phthalate | MEP | 2306-33-4 |
| Benzyl butyl phthalate | BBP | 85-68-7 | Mono-benzyl phthalate | MBzP | 2528-16-7 |
| Dibutyl phthalate | DBP | 84-74-2 | Mono-butyl phthalate | MBP | 131-70-4 |
| Dicyclohexyl phthalate | DCHP | 84-61-7 | Mono-cyclohexyl phthalate | MCHP | 7517-36-4 |
| Di-2-ethylhexyl phthalate | DEHP | 117-81-7 | Mono-2-ethylhexyl phthalate | MEHP | 4376-20-9 |
| Bis(3,5,5-trimethylhexyl) phthalate | DTMHP | 14103-61-8 | Mono(3,5,5-trimethylhexyl) phthalate | MTMHP | 297182-83-3 |
| Di-n-octyl phthalate | DNOP | 117-84-0 | Mono-n-octyl phthalate | MnOP | 5393-19-1 |
| Derivatized Phthalate Metabolite | Diaper Recovery (%) | SPE Recovery (%) | LOD | LOQ |
|---|---|---|---|---|
| MEP | 135% | 98% | 0.20 | 0.61 |
| MBP | 116% | 77% | 0.17 | 0.52 |
| MCHP | 124% | 96% | 0.17 | 0.52 |
| MEHP | 89% | 99% | 0.19 | 0.54 |
| MTMHP | 66% | 94% | 0.12 | 0.38 |
| MnOP | 68% | 89% | 0.26 | 0.80 |
| MBzP | 117% | 115% | 0.18 | 0.56 |
| Derivatized Phthalate Metabolite | Quantifier Ion, Qualifier Ion(s) [m/z] | Retention Time [min] |
|---|---|---|
| MEP | 75.00; 73.00, 223.00 | 8.325 |
| MBP | 75.00; 73.00, 223.00 | 9.400 |
| MCHP | 221.00; 75.00, 221.00 | 10.850 |
| MEHP | 221.00; 75.00, 221.00 | 10.950 |
| MTMHP | 57.00; 73.00, 221.00 | 11.200 |
| MnOP | 221.00; 73.00, 223.00 | 11.400 |
| MBzP | 91.00; 73.00, 179.00 | 11.475 |
| Metabolites | Conc. Range | AM (SD) | GM (GSD) | n of Samples | DF% |
|---|---|---|---|---|---|
| MEP | 3.4–142.9 | 63.2 (51.6) | 36.1 (3.7) | 13 | 11.5 |
| MBP | 10.8–117.4 | 74.7 (46.0) | 54.9 (3.0) | 4 | 3.5 |
| MCHP | 2.3–55.9 | 27.9 (20.3) | 18.2 (3.2) | 8 | 7.1 |
| MEHP | 30.9 | - | - | 2 | 1.8 |
| MTMHP | 16.4–181.5 | 55.0 (70.9) | 34.5 (2.6) | 6 | 5.3 |
| MnOP | 2.0 | - | - | 1 | 0.9 |
| MBzP | 4.4–47.8 | 12.8 (17.2) | 7.9 (2.5) | 6 | 5.3 |
| Country (Collection Year) | n | Months of Age | MEHP | MEP | MBP | MBzP | MCHP | MnOP | MTMHP | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Finland (2006–2009) | 432 | 1–14 | Median Range DF (%) | 0.44 <LOD–849 76.2% | 10.7 0.82–486 100% | 15.2 <LOD–156 99.8% | 24.6 1.85–1985 100% | [32] | |||
| Germany (2008) | 207 | 1–5 | Median Range DF (%) | n.d. <1–19.1 15% | 12.1 <2.5–224.5 78% | 11.5 2.6–88.5 99.5% | 2.5 2.5–40.4 50% | [33] | |||
| Korea (2012–2013) | 268 | 3–15 | Median (IQR) * DF (%) | 2.71 97% | 12.41 99% | [31] | |||||
| Sweden (2009–2012) | 83 | 2–6 | Median Range DF (%) | 19.6 2.7–270.4 100% | 39.1 4.7–315.5 100% | 10.5 0.4–194.9 100% | [47] | ||||
| Canada (2008–2012) | 1072 | 12 | Median GM (GSD) Max Value DF (%) | 1.7 1.8 (2.4) 96 83.8% | 11.4 12.4 (3.3) 5210 95.8% | 22.9 24.3 (2.2) 1002 99.7% | 4.9 5.6 (3.7) 329 87.9% | [30] | |||
| Canada (2008–2012) | 898 | 36 | Median GM (GSD) Max Value DF (%) | 2.2 2.2 (2.3) 143 77.7% | 12.6 13.9 (3.0) 1804 91.1% | 31.7 32.1 (2.2) 1144 98.6% | 7.6 8.5 (3.4) 1071 87.3% | [30] | |||
| China (2012–2014) | 155 | 0–12 | Mean GM Range DF (%) | 5.19 2.51 <LOD–193 98.8% | 16.8 4.01 <LOD–2399 99.9% | 22.2 10.1 <LOD–780.7 99.8% | 0.26 0.12 <LOD–27.2 60.0% | 0.007 0.05 <LOD–0.59 4.99% | [29] | ||
| Switzerland (2019) | 113 | 6–36 | GM Median Range DF (%) | n.d. - <LOD–30.9 1.8% | 36.1 60.3 3.4–142.9 11.5% | 54.9 85.3 10.8–117.4 3.5% | 7.9 4.9 4.4–47.8 5.3% | 18.2 27.3 2.3–55.9 7.1% | n.d. - 2 0.9% | 34.5 27.8 <LOD–181.5 5.3% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, F.; Blanchard, M.; Krasniqi, T.; Duda, N.; Bailat Rosset, G.; Ceschi, A.; Roth, N.; Hopf, N.B.; Broillet, M.-C.; Staedler, D. Concentrations of Seven Phthalate Monoesters in Infants and Toddlers Quantified in Urine Extracted from Diapers. Int. J. Environ. Res. Public Health 2021, 18, 6806. https://doi.org/10.3390/ijerph18136806
Lucarini F, Blanchard M, Krasniqi T, Duda N, Bailat Rosset G, Ceschi A, Roth N, Hopf NB, Broillet M-C, Staedler D. Concentrations of Seven Phthalate Monoesters in Infants and Toddlers Quantified in Urine Extracted from Diapers. International Journal of Environmental Research and Public Health. 2021; 18(13):6806. https://doi.org/10.3390/ijerph18136806
Chicago/Turabian StyleLucarini, Fiorella, Marc Blanchard, Tropoja Krasniqi, Nicolas Duda, Gaëlle Bailat Rosset, Alessandro Ceschi, Nicolas Roth, Nancy B. Hopf, Marie-Christine Broillet, and Davide Staedler. 2021. "Concentrations of Seven Phthalate Monoesters in Infants and Toddlers Quantified in Urine Extracted from Diapers" International Journal of Environmental Research and Public Health 18, no. 13: 6806. https://doi.org/10.3390/ijerph18136806
APA StyleLucarini, F., Blanchard, M., Krasniqi, T., Duda, N., Bailat Rosset, G., Ceschi, A., Roth, N., Hopf, N. B., Broillet, M.-C., & Staedler, D. (2021). Concentrations of Seven Phthalate Monoesters in Infants and Toddlers Quantified in Urine Extracted from Diapers. International Journal of Environmental Research and Public Health, 18(13), 6806. https://doi.org/10.3390/ijerph18136806






