Insight to Microbial Fe(III) Reduction Mediated by Redox-Active Humic Acids with Varied Redox Potentials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HA Solution
2.2. Dialysis Experiment
2.3. Electrochemical Analysis with Direct Electrochemical Reduction and Oxidation
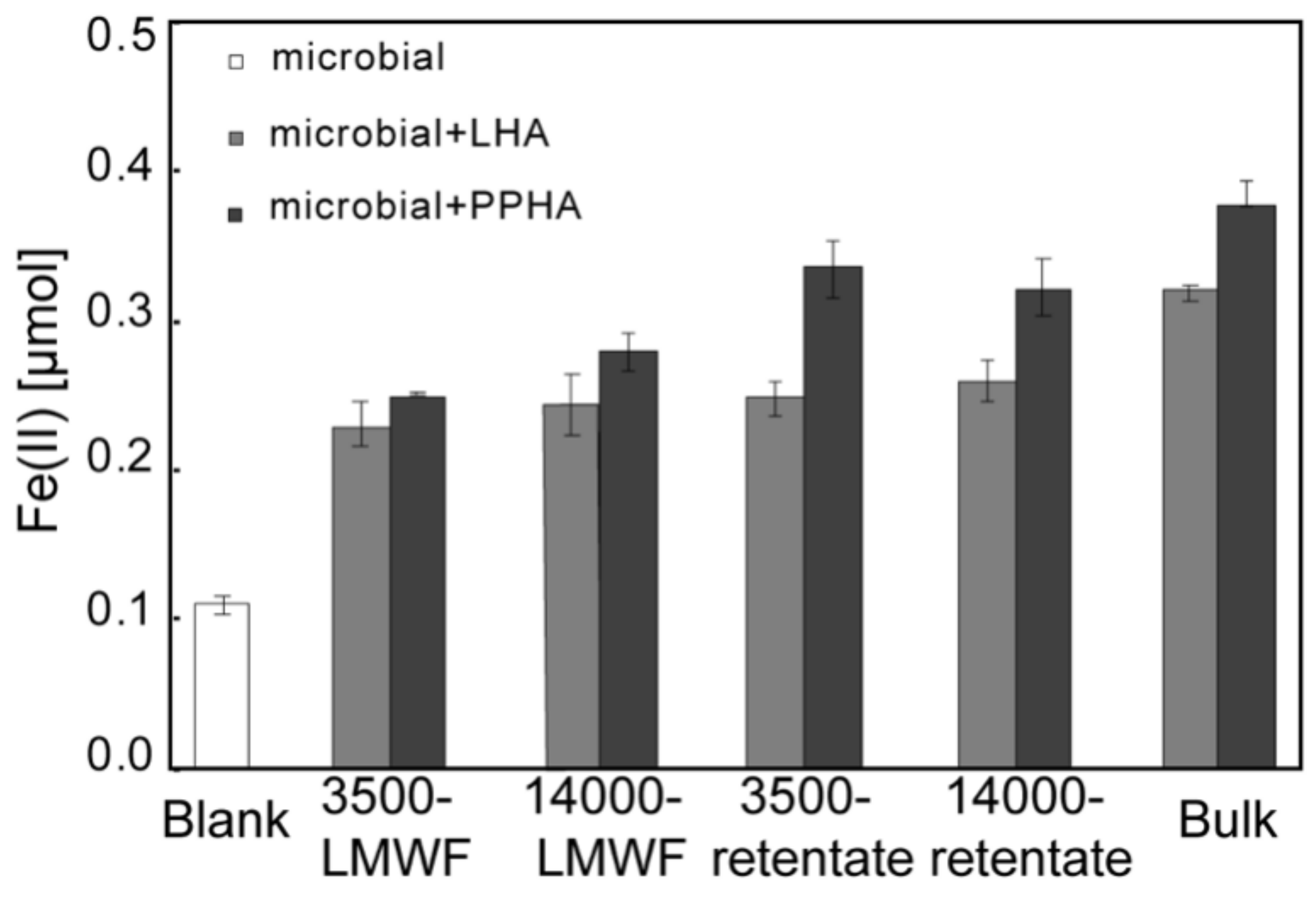
2.4. Microbiological Analysis with Shewanella oneidensis MR-1 Fe(III) Reduction
3. Results and Discussion
3.1. Dialysis Experiment and TOC Contents
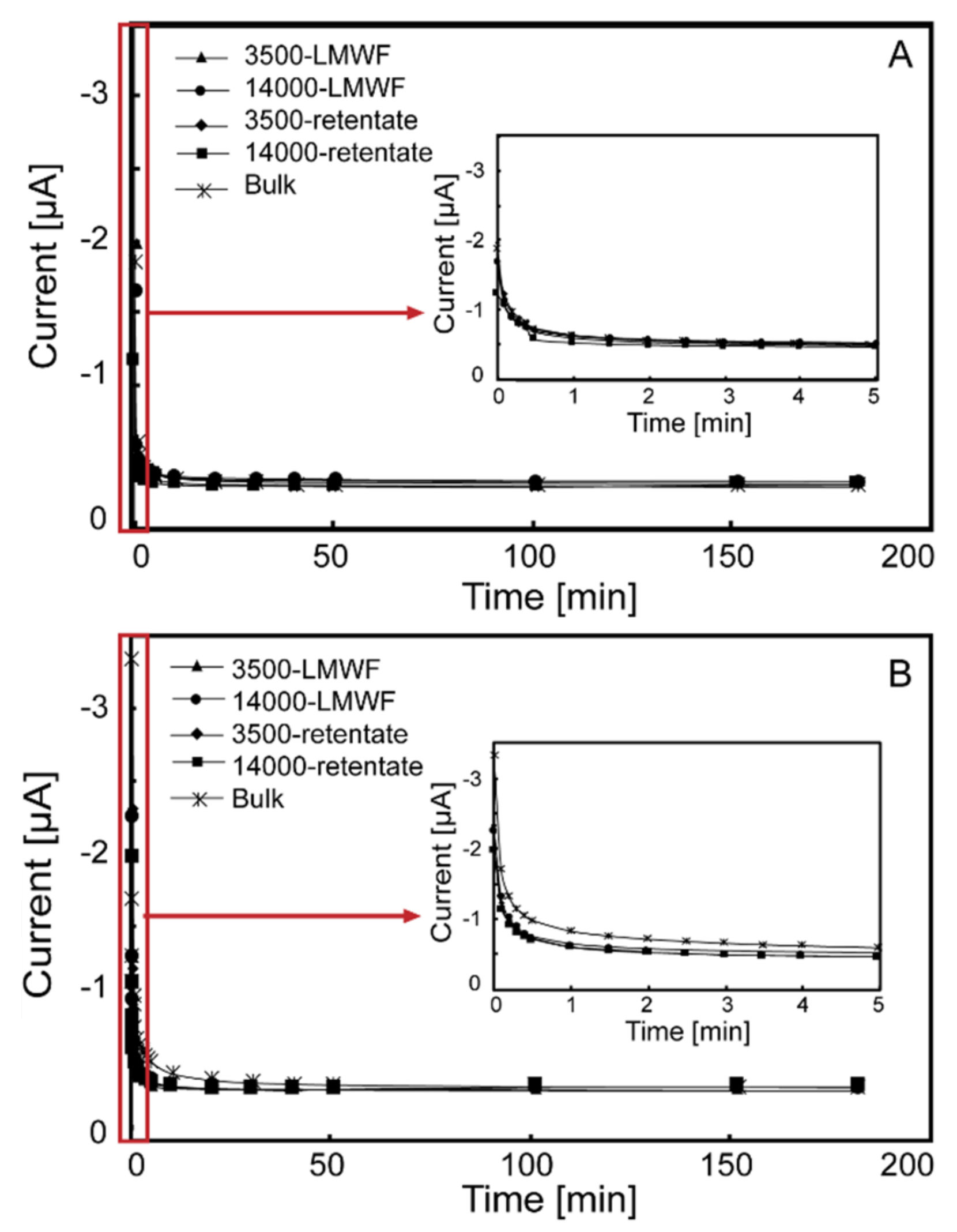
3.2. Direct Electrochemical Reduction/Oxidation of Different Molecular Weight Fractions of HA Samples
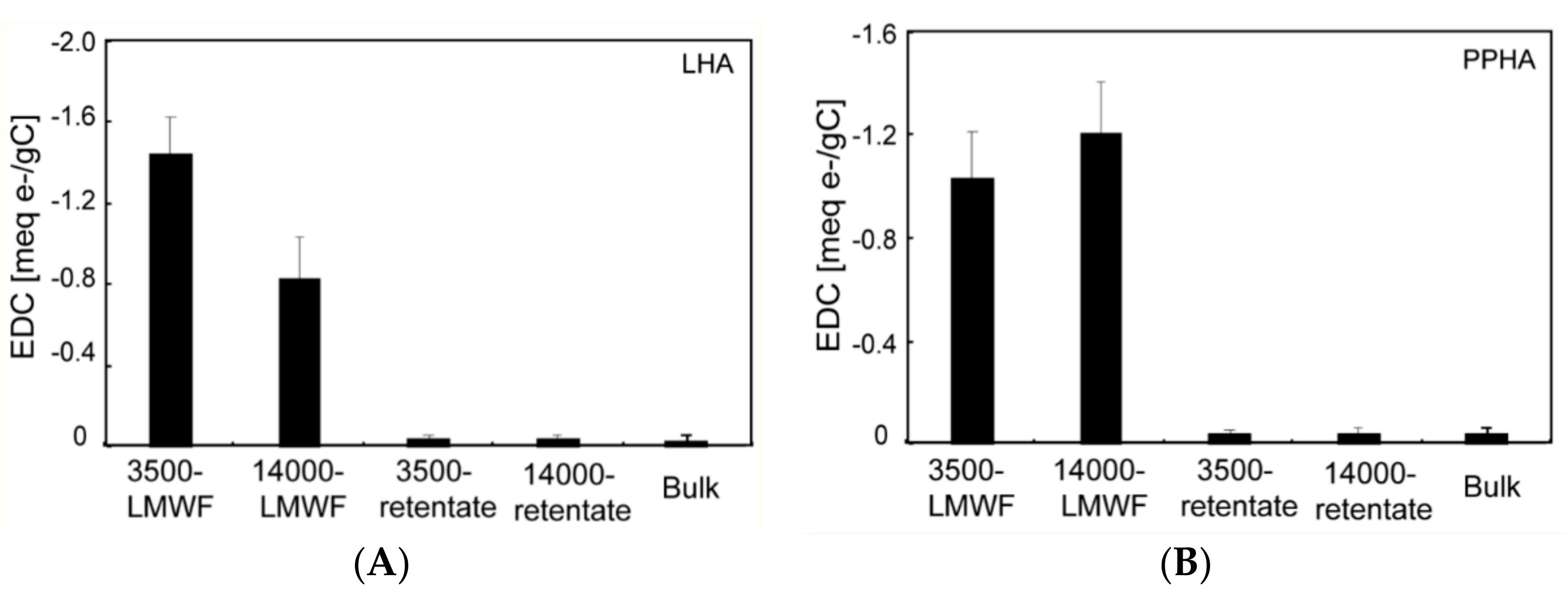
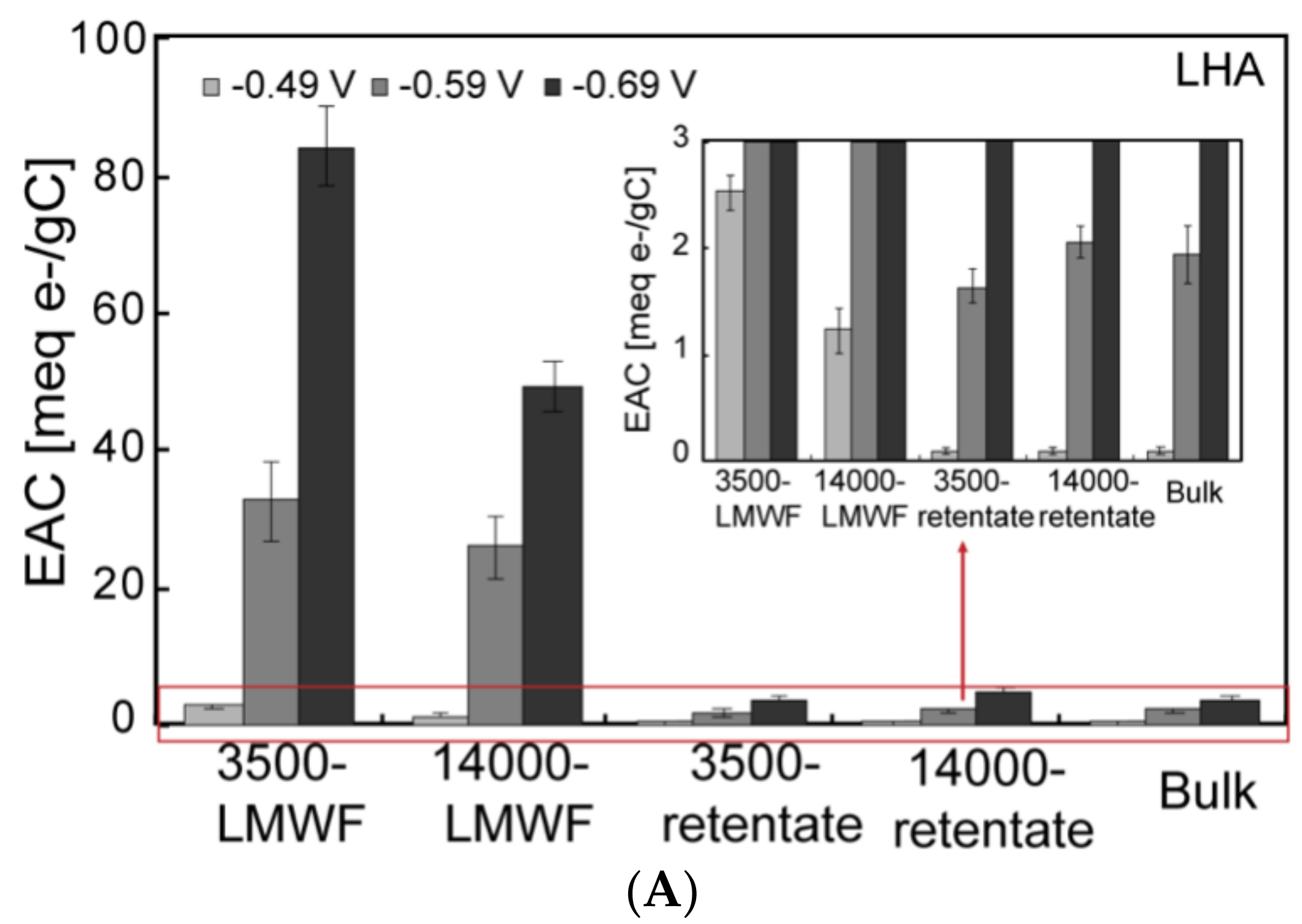
3.3. ESC of Different Molecular Weight Fractions of HA Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel Electrochemical Approach to Assess the Redox Properties of Humic Substances. Environ. Sci. Technol. 2010, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Heitmann, T.; Macalady, D.L.; Blodau, C. Electron Transfer Capacities and Reaction Kinetics of Peat Dissolved Organic Matter. Environ. Sci. Technol. 2007, 41, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved organic matter: Biogeochemistry, dynamics, and environmental significance in soils. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 110, pp. 1–75. [Google Scholar]
- Lv, J.; Han, R.; Huang, Z.; Luo, L.; Cao, D.; Zhang, S. Relationship between Molecular Components and Reducing Capacities of Humic Substances. ACS Earth Space Chem. 2018, 2, 330–339. [Google Scholar] [CrossRef]
- Zou, J.; Huang, J.; Yue, D.; Zhang, H. Roles of oxygen and Mn (IV) oxide in abiotic formation of humic substances by oxidative polymerization of polyphenol and amino acid. Chem. Eng. J. 2020, 393, 124734. [Google Scholar] [CrossRef]
- Jiang, T.; Skyllberg, U.; Wei, S.; Wang, D.; Lu, S.; Jiang, Z.; Flanagan, D.C. Modeling of the structure-specific kinetics of abiotic, dark reduction of Hg(II) complexed by O/N and S functional groups in humic acids while accounting for time-dependent structural rearrangement. Geochim. Cosmochim. Acta 2015, 154, 151–167. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil. Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef] [Green Version]
- Simpson, A.J.; Kingery, W.L.; Hayes, M.H.B.; Spraul, M.; Humpfer, E.; Dvortsak, P.; Kerssebaum, R.; Godejohann, M.; Hofmann, M. Molecular structures and associations of humic substances in the terrestrial environment. Naturwissenschaften 2002, 89, 84–88. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular Structure in Soil Humic Substances: The New View. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Yang, Z.; Kappler, A.; Jiang, J. Reducing Capacities and Distribution of Redox-Active Functional Groups in Low Molecular Weight Fractions of Humic Acids. Environ. Sci. Technol. 2016, 50, 12105–12113. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Xi, B.; Wang, G.; Jiang, J.; He, X.; Mao, X.; Gao, R.; Huang, C.; Zhang, H.; Li, D.; et al. Increased Electron-Accepting and Decreased Electron-Donating Capacities of Soil Humic Substances in Response to Increasing Temperature. Environ. Sci. Technol. 2017, 51, 3176–3186. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Z.; Wang, H.; Jiang, J. Assessing Redox Properties of Natural Organic Matters with regard to Electron Exchange Capacity and Redox-Active Functional Groups. J. Chem. 2020, 2020, 2698213. [Google Scholar] [CrossRef]
- Dryer, D.J.; Korshin, G.V.; Fabbricino, M. In Situ Examination of the Protonation Behavior of Fulvic Acids Using Differential Absorbance Spectroscopy. Environ. Sci. Technol. 2008, 42, 6644–6649. [Google Scholar] [CrossRef]
- Phillips, S.M.; Bellcross, A.D.; Smith, G.D. Light Absorption by Brown Carbon in the Southeastern United States is pH-dependent. Environ. Sci. Technol. 2017, 51, 6782–6790. [Google Scholar] [CrossRef]
- Boyle, E.S.; Guerriero, N.; Thiallet, A.; Del Vecchio, R.; Blough, N.V. Optical Properties of Humic Substances and CDOM: Relation to Structure. Environ. Sci. Technol. 2009, 43, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, R.; Blough, N.V. On the Origin of the Optical Properties of Humic Substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, C.; Blough, N.V. The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties. Environ. Sci. Process. Impacts 2014, 16, 654–671. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, J. Reducing capacities in continuously released low molecular weight fractions from bulk humic acids. J. Environ. Manag. 2019, 244, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Straub, K.L.; Benz, M.; Schink, B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001, 34, 181–186. [Google Scholar] [CrossRef]
- Szilágyi, M. Reduction of Fe3+ ion by humic acid preparations. Soil Sci. 1971, 111, 233–235. [Google Scholar] [CrossRef]
- Struyk, Z.; Sposito, G. Redox properties of standard humic acids. Geoderma 2001, 102, 329–346. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical Redox Processes and their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Klausen, J.; Troeber, S.P.; Haderlein, S.B.; Schwarzenbach, R.P. Reduction of Substituted Nitrobenzenes by Fe(II) in Aqueous Mineral Suspensions. Environ. Sci. Technol. 1995, 29, 2396–2404. [Google Scholar] [CrossRef]
- Charlet, L.; Scheinost, A.; Tournassat, C.; Greneche, J.; Géhin, A.; Fernández-Martínez, A.; Coudert, S.; Tisserand, D.; Brendle, J. Electron transfer at the mineral/water interface: Selenium reduction by ferrous iron sorbed on clay. Geochim. Cosmochim. Acta 2007, 71, 5731–5749. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, Q.; Wu, L.; Zeng, Q.; Dong, H.; Bishop, M.E.; Wang, H. Humic acid-enhanced illite and talc formation associated with microbial reduction of Fe(III) in nontronite. Chem. Geol. 2016, 447, 199–207. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Kappler, A.; Roden, E.E. Iron in microbial metabolisms. Elements 2011, 7, 89–93. [Google Scholar] [CrossRef]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Schmidt, C.; Kappler, A. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat. Rev. Genet. 2014, 12, 797–808. [Google Scholar] [CrossRef]
- Okamoto, A.; Nakamura, R.; Nealson, K.H.; Hashimoto, K. Bound Flavin Model Suggests Similar Electron-Transfer Mechanisms in Shewanella and Geobacter. ChemElectroChem 2014, 1, 1808–1812. [Google Scholar] [CrossRef]
- Benner, S.G.; Hansel, C.M.; Wielinga, B.W.; Barber, T.M.; Fendorf, S. Reductive Dissolution and Biomineralization of Iron Hydroxide under Dynamic Flow Conditions. Environ. Sci. Technol. 2002, 36, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Caccavo, F.; Fendorf, S.; Rosenzweig, R.F. Arsenic Mobilization by the Dissimilatory Fe(III)-Reducing Bacterium Shewanella alga BrY. Environ. Sci. Technol. 1999, 33, 723–729. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Sposito, G. Electron Shuttling by Natural Organic Matter: Twenty Years After. ACS Symp. Ser. 2011, 1071, 113–127. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D. Assessing redox properties of standard humic substances. Int. J. Environ. Sci. Technol. 2017, 14, 1497–1504. [Google Scholar] [CrossRef]
- Han, R.; Liu, T.; Li, F.; Li, X.; Chen, D.; Wu, Y. Dependence of Secondary Mineral Formation on Fe(II) Production from Ferrihydrite Reduction by Shewanella oneidensis MR-1. ACS Earth Space Chem. 2018, 2, 399–409. [Google Scholar] [CrossRef]
- Kumar, A.; Hsu, L.H.H.; Kavanagh, P.; Barrière, F.; Lens, P.N.; Lapinsonnière, L.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 24. [Google Scholar] [CrossRef]
- Pirbadian, S.; Chavez, M.S.; El-Naggar, M.Y. Spatiotemporal mapping of bacterial membrane potential responses to extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2020, 117, 20171–20179. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jangir, Y.; El-Naggar, M.Y. Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochim. Acta 2016, 198, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Cornell, R.M.; Schwertmann, U. The Iron Oxide; Wiley-VCH: New York, NY, USA, 2003; ISBN 3-527-30274-3. [Google Scholar]
- Stookey, L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Fox, L. The removal of dissolved humic acid during estuarine mixing. Estuar. Coast. Shelf Sci. 1983, 16, 431–440. [Google Scholar] [CrossRef]
- Fooken, U.; Liebezeit, G. Distinction of marine and terrestrial origin of humic acids in North Sea surface sediments by absorption spectroscopy. Mar. Geol. 2000, 164, 173–181. [Google Scholar] [CrossRef]
- Mao, J.-D.; Tremblay, L.; Gagné, J.-P.; Kohl, S.; Rice, J.; Schmidt-Rohr, K. Humic acids from particulate organic matter in the Saguenay Fjord and the St. Lawrence Estuary investigated by advanced solid-state NMR. Geochim. Cosmochim. Acta 2007, 71, 5483–5499. [Google Scholar] [CrossRef]
- Mahoney, D.T.; Al Aamery, N.; Fox, J.F.; Riddle, B.; Ford, W.; Wang, Y.T. Equilibrium sediment exchange in the earth’s critical zone: Evidence from sediment fingerprinting with stable isotopes and watershed modeling. J. Soils Sediments 2019, 19, 3332–3356. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.-M.; Kim, G. Significant production of humic fluorescent dissolved organic matter in the continental shelf waters of the northwestern Pacific Ocean. Sci. Rep. 2018, 8, 4887. [Google Scholar] [CrossRef] [PubMed]
- Bravo, C.; Millo, C.; Covelli, S.; Contin, M.; De Nobili, M. Terrestrial-marine continuum of sedimentary natural organic matter in a mid-latitude estuarine system. J. Soils Sediments 2019, 20, 1074–1086. [Google Scholar] [CrossRef]
- Goñi, M.A.; Teixeira, M.J.; Perkey, D.W. Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuar. Coast. Shelf Sci. 2003, 57, 1023–1048. [Google Scholar] [CrossRef]
- Otero, E.; Culp, R.; E Noakes, J.; E Hodson, R. The distribution and δ13C of dissolved organic carbon and its humic fraction in estuaries of southeastern USA. Estuar. Coast. Shelf Sci. 2003, 56, 1187–1194. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant Properties of Humic Substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Roden, E.E.; Kappler, A.; Bauer, I.; Jiang, J.; Paul, A.; Stoesser, R.; Konishi, H.; Xu, H. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat. Geosci. 2010, 3, 417–421. [Google Scholar] [CrossRef]
- Lovley, D.R.; Fraga, J.L.; Blunt-Harris, E.L.; Hayes, L.A.; Phillips, E.J.P.; Coates, J.D. Humic Substances as a Mediator for Microbially Catalyzed Metal Reduction. Acta Hydrochim. Hydrobiol. 1998, 26, 152–157. [Google Scholar] [CrossRef]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Ratasuk, N.; Nanny, M.A. Characterization and Quantification of Reversible Redox Sites in Humic Substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef] [PubMed]


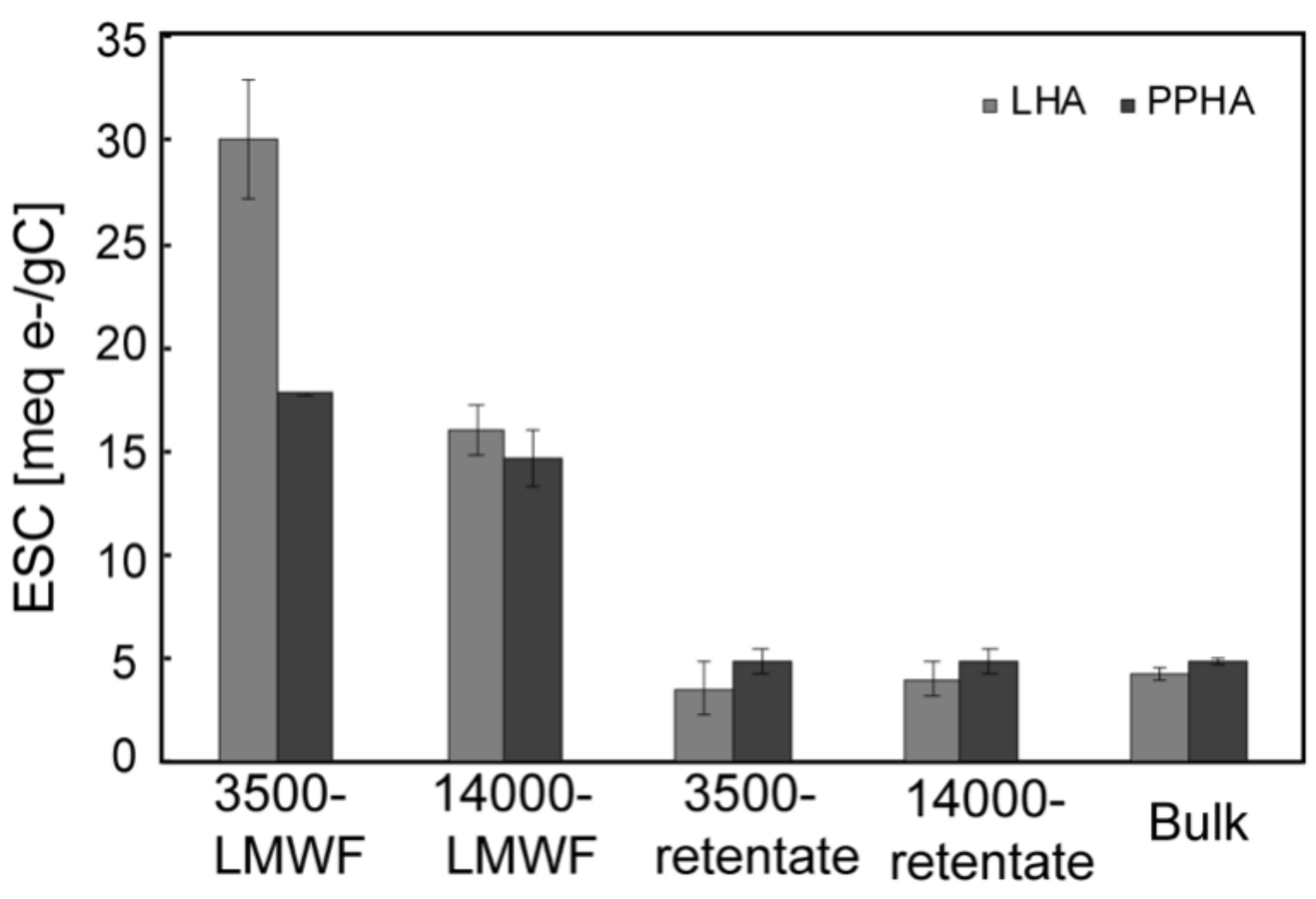
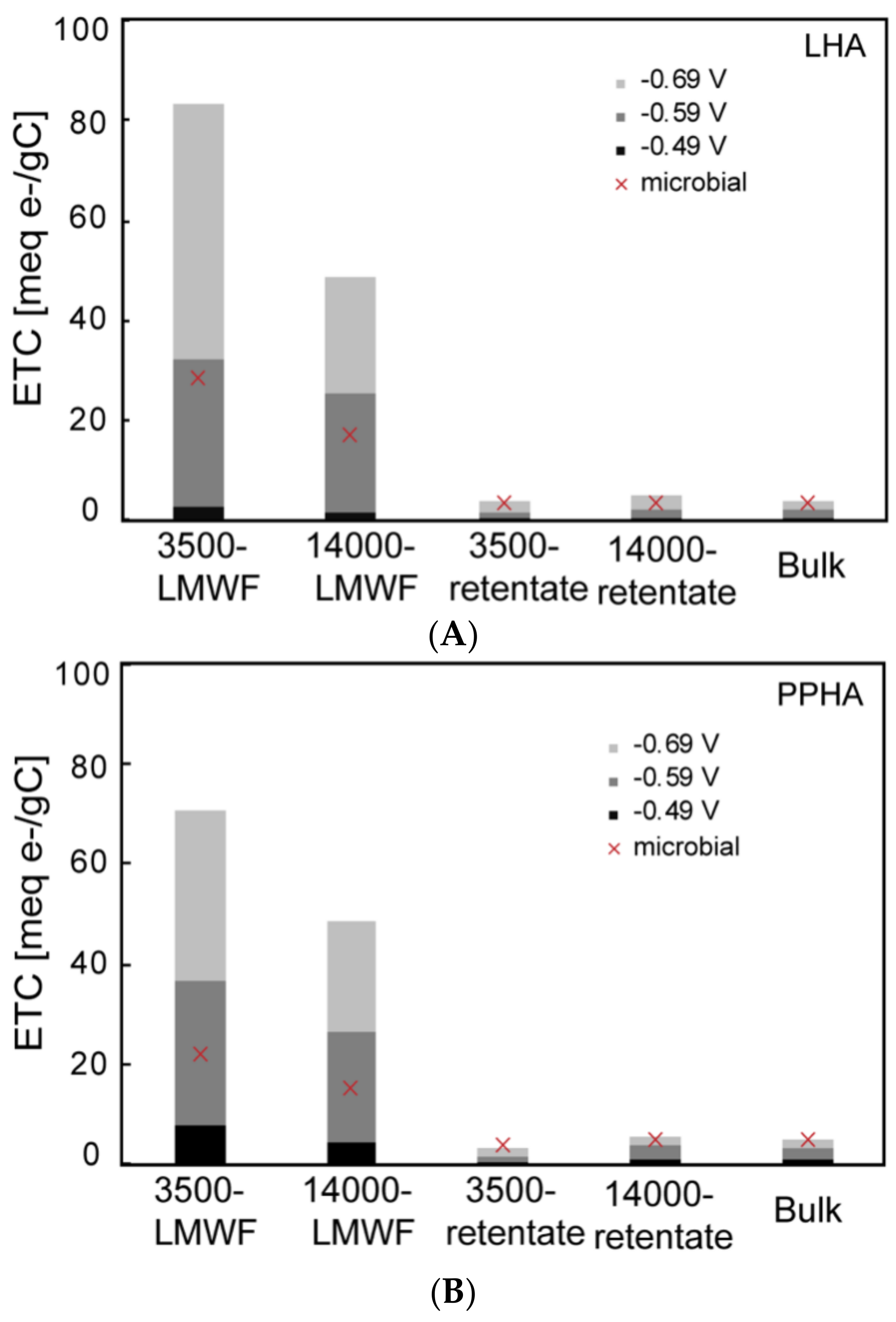
| TOC (mg C/L) | 3500-LMWF | 14,000-LMWF | 3500-Retentate | 14,000-Retentate | Bulk | 3500-LMWF/Bulk | 14,000-LMWF/Bulk |
|---|---|---|---|---|---|---|---|
| PPHA | 8.48 | 11.62 | 210.90 | 198.70 | 234.90 | 3.61% | 4.95% |
| LHA | 4.61 | 9.14 | 217.18 | 195.74 | 228.00 | 2.02% | 4.01% |
| ETC (μmol e-) | Electrochemical Reduced | Microbial Reduced | |||
|---|---|---|---|---|---|
| −0.49 V | −0.59 V | −0.69 V | |||
| PPHA | 3500-LMWF | 0.14 | 0.62 | 1.20 | 0.60 |
| 14,000-LMWF | 0.11 | 0.62 | 1.12 | 0.68 | |
| 3500 retentate | 0.15 | 0.59 | 1.43 | 0.82 | |
| 14,000 retentate | 0.35 | 1.53 | 2.14 | 0.78 | |
| bulk | 0.43 | 1.57 | 2.33 | 0.92 | |
| LHA | 3500-LMWF | 0.03 | 0.40 | 1.04 | 0.56 |
| 14,000-LMWF | 0.03 | 0.54 | 1.03 | 0.58 | |
| 3500 retentate | 0.04 | 0.79 | 1.76 | 0.62 | |
| 14,000 retentate | 0.05 | 0.96 | 2.17 | 0.62 | |
| bulk | 0.06 | 1.14 | 2.16 | 0.78 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Xu, Z.; Yang, Z.; Jiang, J. Insight to Microbial Fe(III) Reduction Mediated by Redox-Active Humic Acids with Varied Redox Potentials. Int. J. Environ. Res. Public Health 2021, 18, 6807. https://doi.org/10.3390/ijerph18136807
Duan J, Xu Z, Yang Z, Jiang J. Insight to Microbial Fe(III) Reduction Mediated by Redox-Active Humic Acids with Varied Redox Potentials. International Journal of Environmental Research and Public Health. 2021; 18(13):6807. https://doi.org/10.3390/ijerph18136807
Chicago/Turabian StyleDuan, Jingtao, Zhiyuan Xu, Zhen Yang, and Jie Jiang. 2021. "Insight to Microbial Fe(III) Reduction Mediated by Redox-Active Humic Acids with Varied Redox Potentials" International Journal of Environmental Research and Public Health 18, no. 13: 6807. https://doi.org/10.3390/ijerph18136807
APA StyleDuan, J., Xu, Z., Yang, Z., & Jiang, J. (2021). Insight to Microbial Fe(III) Reduction Mediated by Redox-Active Humic Acids with Varied Redox Potentials. International Journal of Environmental Research and Public Health, 18(13), 6807. https://doi.org/10.3390/ijerph18136807






