A Descriptive Analysis of Transitions from Smoking to Electronic Nicotine Delivery System (ENDS) Use: A Daily Diary Investigation
Abstract
:1. Introduction
2. Methods
2.1. Smoking-to-Vaping Study Overview
2.2. Measures
2.2.1. Daily Survey—Smoking and ENDS Use
2.2.2. Smoking and ENDS Use, by “Study Day”
2.2.3. Smoking, and ENDS Use, by “Study Week”
2.3. Classification of Smoking and ENDS Use Transitions
2.4. Smoking, ENDS Use and Demographic Covariates
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Transition Classifications and Baseline Characteristics
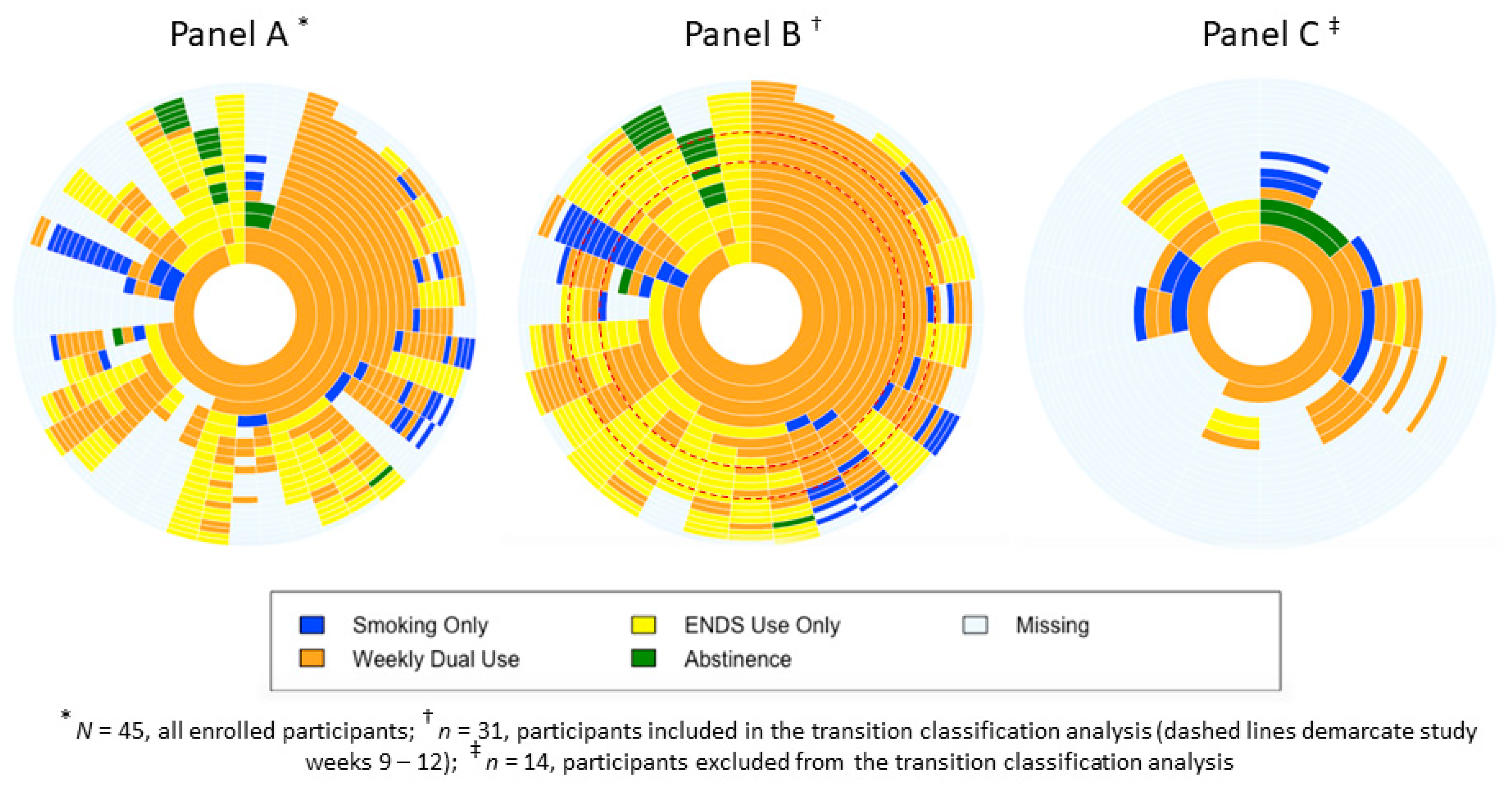
3.3. Sunburst Plots
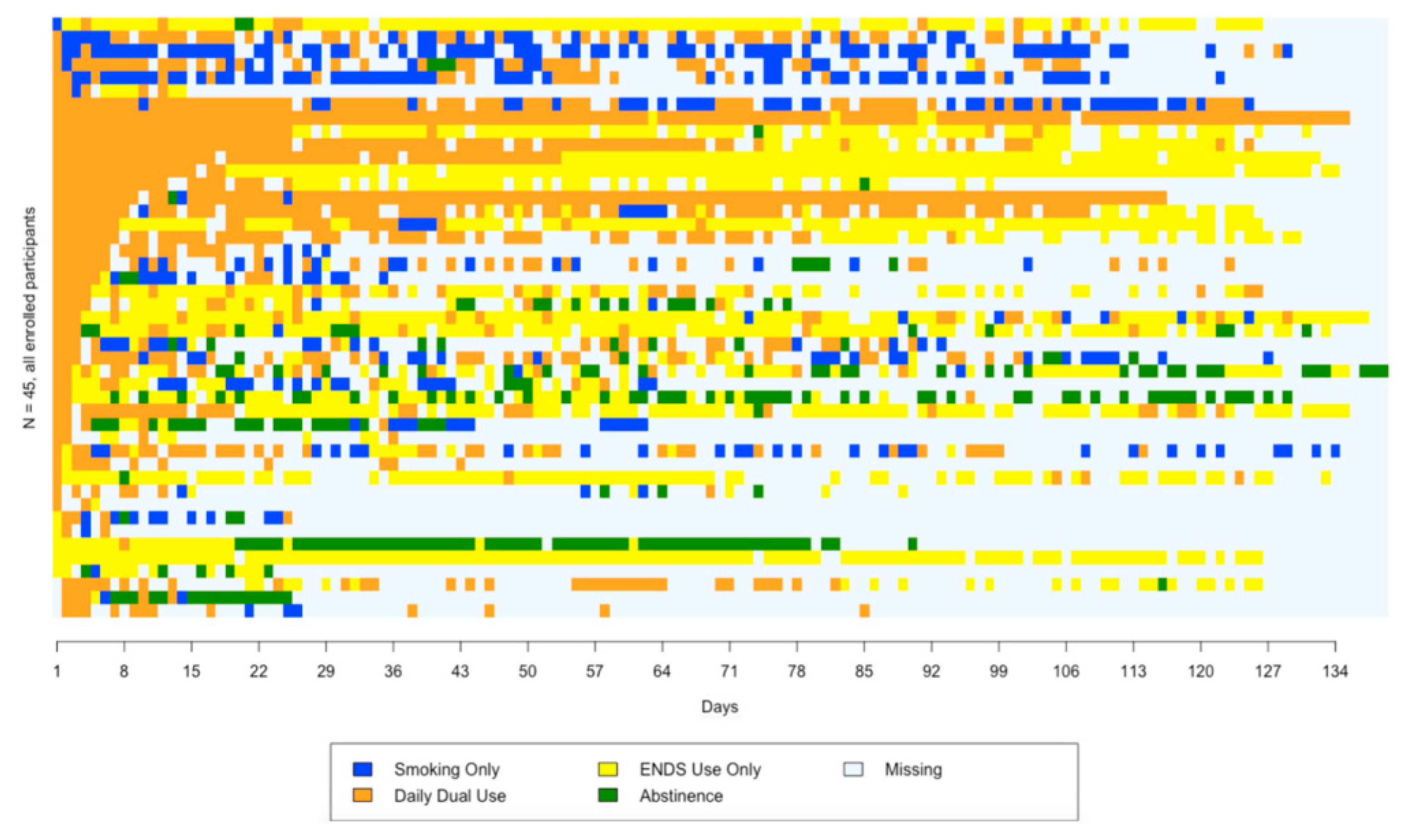
3.4. Sequence Plot
4. Discussion
Strengths and Limitations of This Study
- This study is the first to use an intensive longitudinal approach to examine patterns of smoking and ENDS use among a group of adult smokers.
- The daily diary survey method enabled a more fine-grained description of smoking and ENDS use than those reported in previous studies.
- The small sample of participants was purposively selected and may not be representative of New Zealand smokers.
- Smoking and ENDS use were self-reported, and we lacked a true baseline measure of cigarette consumption before study enrolment.
- High levels of participant withdrawal and loss to follow-up limited the sample used in the transition classification analysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Māori Consultation
Patient and Public Involvement Statement
References
- Abrams, D.B. Promise and peril of e-cigarettes: Can disruptive technology make cigarettes obsolete? JAMA 2014, 311, 135–136. [Google Scholar] [CrossRef]
- Correa, J.B.; Ariel, I.; Menzie, N.S.; Brandon, T.H. Documenting the emergence of electronic nicotine delivery systems as a disruptive technology in nicotine and tobacco science. Addict. Behav. 2017, 65, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkhoran, S.; Glantz, S.A. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir. Med. 2016, 4, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Hartmann-Boyce, J.; McRobbie, H.; Bullen, C.; Begh, R.; Stead, L.F.; Hajek, P. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; McRobbie, H.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; Butler, A.R.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Pasquereau, A.; Guignard, R.; Andler, R.; Nguyen-Thanh, V. Electronic cigarettes, quit attempts and smoking cessation: A 6-month follow-up. Addiction 2017, 112, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.F.; Bullen, C. A longitudinal study of electronic cigarette users. Addict. Behav. 2014, 39, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Gomajee, R.; El-Khoury, F.; Goldberg, M.; Zins, M.; Lemogne, C.; Wiernik, E.; Lequy-Flahault, E.; Romanello, L.; Kousignian, I.; Melchior, M. Association between electronic cigarette use and smoking reduction in France. JAMA Intern. Med. 2019, 179, 1193–1200. [Google Scholar] [CrossRef]
- Brose, L.S.; Hitchman, S.C.; Brown, J.; West, R.; McNeill, A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction 2015, 110, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Berg, C.J.; Barr, D.B.; Stratton, E.; Escoffery, C.; Kegler, M. Attitudes toward e-cigarettes, reasons for initiating e-cigarette use, and changes in smoking behavior after initiation: A pilot longitudinal study of regular cigarette smokers. Open J. Prev. Med. 2014, 4, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Buu, A.; Hu, Y.-H.; Piper, M.E.; Lin, H.-C. The association between e-cigarette use characteristics and combustible cigarette consumption and dependence symptoms: Results from a national longitudinal study. Addict. Behav. 2018, 84, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Biener, L.; Hargraves, J.L. A longitudinal study of electronic cigarette use in a population-based sample of adult smokers: Association with smoking cessation and motivation to quit. Nicotine Tob. Res. 2014, 17, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkhoran, S.; Chang, Y.; Rigotti, N.A. Electronic cigarette use and cigarette abstinence over 2 years among US smokers in the population assessment of tobacco and health study. Nicotine Tob. Res. 2020, 22, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.-L.; Cummins, S.E.; Sun, J.Y.; Zhu, S.-H. Long-term e-cigarette use and smoking cessation: A longitudinal study with US population. Tob. Control 2016, 25 (Suppl. 1), i90–i95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.R.; Smith, D.M.; Goniewicz, M.L. Changes in nicotine product use among dual users of tobacco and electronic cigarettes: Findings from the Population Assessment of Tobacco and Health (PATH) Study, 2013–2015. Subst. Use Misuse 2020, 55, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pierce, J.P.; White, M.; Vijayaraghavan, M.; Compton, W.; Conway, K.; Hartman, A.M.; Messer, K. E-cigarette use and smoking reduction or cessation in the 2010/2011 TUS-CPS longitudinal cohort. BMC Public Health 2016, 16, 1105. [Google Scholar] [CrossRef] [Green Version]
- Beard, E.; Brown, J.; Michie, S.; West, R. Is prevalence of e-cigarette and nicotine replacement therapy use among smokers associated with average cigarette consumption in England? A time-series analysis. BMJ Open 2018, 8, e016046. [Google Scholar] [CrossRef]
- Wu SY-d Wang, M.P.; Li, W.H.; Kwong, A.C.; Lai, V.W.; Lam, T.H. Does electronic cigarette use predict abstinence from conventional cigarettes among smokers in Hong Kong? Int. J. Environ. Res. Public Health 2018, 15, 400. [Google Scholar]
- Weaver, S.R.; Huang, J.; Pechacek, T.F.; Heath, J.W.; Ashley, D.L.; Eriksen, M.P. Are electronic nicotine delivery systems helping cigarette smokers quit? Evidence from a prospective cohort study of US adult smokers, 2015–2016. PLoS ONE 2018, 13, e0198047. [Google Scholar] [CrossRef]
- Maglia, M.; Caponnetto, P.; Di Piazza, J.; La Torre, D.; Polosa, R. Dual use of electronic cigarettes and classic cigarettes: A systematic review. Addict. Res. Theory 2018, 26, 330–338. [Google Scholar] [CrossRef]
- Shi, Y.; Cummins, S.E.; Zhu, S.-H. Use of electronic cigarettes in smoke-free environments. Tob. Control 2017, 26, e19–e22. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.; Hoek, J.; Blank, M.L.; Richards, R.; Ling, P.; Popova, L. Dual use of electronic nicotine delivery systems (ENDS) and smoked tobacco: A qualitative analysis. Tob. Control 2019, 28, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Piper, M.E.; Baker, T.B.; Benowitz, N.L.; Jorenby, D.E. Changes in use patterns over 1 year among smokers and dual users of combustible and electronic cigarettes. Nicotine Tobacco Res. 2020, 22, 672–680. [Google Scholar] [CrossRef]
- Gravely, S.; Meng, G.; Cummings, K.M.; Hyland, A.; Borland, R.; Hammond, D.; O’Connor, R.J.; Goniewicz, M.L.; Kasza, K.A.; McNeill, A.; et al. Changes in smoking and vaping over 18 months among smokers and recent ex-smokers: Longitudinal findings from the 2016 and 2018 ITC Four Country Smoking and Vaping Surveys. Int. J. Environ. Res. Public Health 2020, 17, 7084. [Google Scholar] [CrossRef]
- Osibogun, O.; Bursac, Z.; Mckee, M.; Li, T.; Maziak, W. Cessation outcomes in adult dual users of e-cigarettes and cigarettes: The Population Assessment of Tobacco and Health cohort study, USA, 2013–2016. Int. J. Public Health 2020, 65, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.A.; Giovenco, D.P. Behavioral heterogeneity among cigarette and e-cigarette dual-users and associations with future tobacco use: Findings from the Population Assessment of Tobacco and Health Study. Addict. Behav. 2020, 104, 106263. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- Shiffman, S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychol. 2009, 28, 519. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.R.; Case, K.R.; Hébert, E.T.; Vandewater, E.A.; Raese, K.A.; Perry, C.L.; Businelle, M.S. Characterizing ENDS use in young adults with ecological momentary assessment: Results from a pilot study. Addict. Behav. 2019, 91, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.R.; Laugesen, M.; Bullen, C.; Grace, R.C. Predicting short-term uptake of electronic cigarettes: Effects of nicotine, subjective effects, and simulated demand. Nicotine Tob. Res. 2018, 20, 1265–1271. [Google Scholar] [CrossRef]
- Weaver, S.R.; Kim, H.; Glasser, A.M.; Sutfin, E.L.; Barrington-Trimis, J.; Payne, T.J.; Saddleson, M.; Loukas, A. Establishing consensus on survey measures for electronic nicotine and non-nicotine delivery system use: Current challenges and considerations for researchers. Addict. Behav. 2018, 79, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.-L.; Hoek, J.; George, M.; Gendall, P.; Conner, T.S.; Thrul, J.; Ling, P.M.; Langlotz, T. An exploration of smoking-to-vaping transition attempts using a “smart” electronic nicotine delivery system. Nicotine Tob. Res. 2018, 21, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Haardörfer, R.; Payne, J.B.; Getachew, B.; Vu, M.; Guttentag, A.; Kirchner, T.R. Ecological momentary assessment of various tobacco product use among young adults. Addict. Behav. 2019, 92, 38–46. [Google Scholar] [CrossRef]
- Carpenter, M.J.; Heckman, B.W.; Wahlquist, A.E.; Wagener, T.L.; Goniewicz, M.; Gray, K.M.; Froeliger, B.; Cummings, K.M. A naturalistic, randomized pilot trial of e-cigarettes: Uptake, exposure, and behavioral effects. Cancer Epidemiol. Prev. Biomark. 2017, 26, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoek, J.; Blank, M.L.; Conner, T.; Ferguson, S.; Thompson, L.; Teah, G.; Haggart, K. Smoking-to-Vaping (S2V) Study: Methods Report; ASPIRE 2025: Wellington, New Zealand, 2020. [Google Scholar]
- Tomba, E. Assessment of lifestyle in relation to health. In The Psychosomatic Assessment; Karger Publishers: Basel, Switzerland, 2012; Volume 32, pp. 72–96. [Google Scholar]
- Blank, M.-L.; Hoek, J. Choice and variety-seeking of e-liquids and flavor categories by New Zealand smokers using an electronic cigarette: A longitudinal study. Nicotine Tob. Res. 2021, 23, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. The New Zealand Guidelines for Helping People to Stop Smoking. 2014. Available online: https://www.health.govt.nz/system/files/documents/publications/nz-guidelines-helping-people-stop-smoking-jun14.pdf (accessed on 14 April 2020).
- Ministry of Health. Definitions of Smoking Status. 2015. Available online: https://www.health.govt.nz/our-work/preventative-health-wellness/tobacco-control/tobacco-control-information-practitioners/definitions-smoking-status (accessed on 14 April 2020).
- Pokhrel, P.; Herzog, T.A.; Muranaka, N.; Fagan, P. Young adult e-cigarette users’ reasons for liking and not liking e-cigarettes: A qualitative study. Psychol. Health 2015, 30, 1450–1469. [Google Scholar] [CrossRef] [Green Version]
- Pokhrel, P.; Herzog, T.A.; Muranaka, N.; Regmi, S.; Fagan, P. Contexts of cigarette and e-cigarette use among dual users: A qualitative study. BMC Public Health 2015, 15, 859. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, J.; Hollingworth, W.; Campbell, R. Long-term smoking relapse: A study using the British Household Panel Survey. Nicotine Tob. Res. 2010, 12, 1228–1235. [Google Scholar] [CrossRef]
- Martínez, Ú.; Loredo, V.M.; Simmons, V.N.; Meltzer, L.R.; Drobes, D.J.; O Brandon, K.; Palmer, A.M.; Eissenberg, T.; Bullen, C.R.; Harrell, P.T.; et al. How does smoking and nicotine dependence change after onset of vaping? A retrospective analysis of dual users. Nicotine Tob. Res. 2019, 22, 764–770. [Google Scholar] [CrossRef]
- St Helen, G.; Nardone, N.; Addo, N.; Dempsey, D.; Havel, C.; Jacob, P.; Benowitz, N.L. Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users. Addiction 2020, 115, 757–767. [Google Scholar] [CrossRef]
- National Academies of Science Engineering and Medicine. Public Health Consequences of E-Cigarettes; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Fetterman, J.L.; Keith, R.J.; Palmisano, J.N.; McGlasson, K.L.; Weisbrod, R.M.; Majid, S.; Bastin, R.; Stathos, M.M.; Stokes, A.C.; Robertson, R.M.; et al. alterations in vascular function associated with the use of combustible and electronic cigarettes. J. Am. Heart Assoc. 2020, 9, e014570. [Google Scholar] [CrossRef] [PubMed]
- Franzen, K.F.; Willig, J.; Cayo Talavera, S.; Meusel, M.; Sayk, F.; Reppel, M.; Dalhoff, K.; Mortensen, K.; Droemann, D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc. Med. 2018, 23, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Olgin, J.E.; Nah, G.; Vittinghoff, E.; Cataldo, J.K.; Pletcher, M.J.; Marcus, G.M. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS ONE 2018, 13, e0198681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei, A.D.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Uddin, S.M.I.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; Stokes, A.; Bhatnagar, A.; et al. Association between e-cigarette use and cardiovascular disease among never and current combustible-cigarette smokers. Am. J. Med. 2019, 132, 949–954.e2. [Google Scholar] [CrossRef] [PubMed]
| All Enrolled Participants N = 45 | Included in the Transition Classification Analysis * n = 31 | Excluded from the Transition Classification Analysis n = 14 | |
|---|---|---|---|
| Age, median (Q1 to Q3) † | 32 (23 to 47) | 37 (26 to 50) | 25 (20 to 38) |
| Sex, n (%) | |||
| Male | 19 (42.2) | 15 (48.4) | 4 (28.6) |
| Female | 26 (57.8) | 16 (51.6) | 10 (71.4) |
| Prioritised ethnicity, n (%) | |||
| Māori | 13 (28.9) | 8 (25.8) | 5 (35.7) |
| New Zealand European | 30 (66.7) | 22 (71.0) | 8 (57.2) |
| Other Asian | 2 (4.4) | 1 (3.2) | 1 (7.1) |
| Education, n (%) | |||
| Above high school | 20 (44.4) | 15 (48.4) | 5 (35.7) |
| High school or below | 25 (55.6) | 16 (51.6) | 9 (64.3) |
| Age at first puff of cigarette (years), n (%) | |||
| <13 | 15 (33.3) | 10 (32.3) | 5 (35.7) |
| ≥13 and <19 | 30 (66.7) | 21 (67.7) | 9 (64.3) |
| ≥19 | 0 (0) | 0 (0) | 0 (0) |
| Age at first weekly cigarette smoking (years), n (%) | |||
| <13 | 4 (8.9) | 2 (6.5) | 2 (14.3) |
| ≥13 and <19 | 37 (82.2) | 25 (80.6) | 12 (85.7) |
| ≥19 | 4 (8.9) | 4 (12.9) | 0 (0) |
| Tobacco type, n (%) | |||
| Roll-your-own | 15 (33.3) | 14 (45.2) | 1 (7.1) |
| Tailor-made | 15 (33.3) | 11 (35.5) | 4 (28.6) |
| Both | 15 (33.4) | 6 (19.4) | 9 (64.3) |
| Ever-tried to quit smoking, n (%) | |||
| Yes | 34 (75.6) | 24 (77.4) | 10 (71.4) |
| No | 11 (24.4) | 7 (22.6) | 4 (28.6) |
| Ever-tried ENDS, n (%) | |||
| Yes | 28 (62.2) | 19 (61.3) | 9 (64.3) |
| No | 17 (37.8) | 12 (38.7) | 5 (35.7) |
| Ever-owned ENDS, n (%) | |||
| Yes | 6 (13.3) | 4 (12.9) | 2 (14.3) |
| No | 39 (86.7) | 27 (87.1) | 12 (85.7) |
| Confidence in quitting smoking, median (Q1 to Q3) | 78 (60 to 84) | 79 (60 to 90) | 70.5 (60 to 80) |
| Dual Use n = 21 (67.7%) | ENDS Use Only n = 9 (29.0%) | Smoking Only n = 1 (3.3%) | |
|---|---|---|---|
| Age, median (Q1 to Q3) † | 36 (23 to 49) | 40 (31 to 49) | 37 (NA) |
| Sex, n (row %) | |||
| Male | 11 (73.3) | 4 (26.7) | 0 (0) |
| Female | 10 (62.5) | 5 (31.3) | 1 (6.2) |
| Prioritised ethnicity, n (row %) | |||
| Māori | 6 (75.0) | 2 (25.0) | 0 (0) |
| New Zealand European | 14 (63.6) | 7 (32.8) | 1 (4.6) |
| Other Asian | 1 (100) | 0 (0) | 0 (0) |
| Education, n (row %) | |||
| Above high school | 9 (60.0) | 5 (33.3) | 1 (6.7) |
| High school or below | 12 (75.0) | 4 (25.0) | 0 (0) |
| Age at first puff of cigarette (years), n (row %) | |||
| <13 | 7 (70.0) | 3 (30.0) | 0 (0) |
| ≥13 and <19 | 14 (66.7) | 6 (28.6) | 1 (4.7) |
| ≥19 | 0 (0) | 0 (0) | 0 (0) |
| Age at first weekly cigarette smoking (years), n (row %) | |||
| <13 | 1 (50.0) | 1 (50.0) | 0 (0) |
| ≥13 and <19 | 18 (72.0) | 6 (24.0) | 1 (4.0) |
| ≥19 | 2 (50.0) | 2 (50.0) | 0 (0) |
| Tobacco type, n (row %) | |||
| Roll-your-own | 10 (71.4) | 4 (28.6) | 0 (0) |
| Tailor-made | 7 (63.6) | 3 (27.3) | 1 (9.1) |
| Both | 4 (66.7) | 2 (33.3) | 0 (0) |
| Ever-tried to quit smoking, n (row %) | |||
| Yes | 17 (70.8) | 6 (25.0) | 1 (4.2) |
| No | 4 (57.1) | 3 (42.9) | 0 (0) |
| Ever-tried ENDS, n (row %) | |||
| Yes | 13 (68.4) | 5 (26.3) | 1 (5.3) |
| No | 8 (66.7) | 4 (33.3) | 0 (0) |
| Ever-owned ENDS, n (row %) | |||
| Yes | 2 (50.0) | 1 (25.0) | 1 (25.0) |
| No | 19 (70.4) | 8 (29.6) | 0 (0) |
| Confidence in quitting smoking, median (Q1 to Q3) | 78 (68 to 88) | 84 (73 to 94) | 50 (NA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conner, T.S.; Zeng, J.; Blank, M.-L.; He, V.; Hoek, J. A Descriptive Analysis of Transitions from Smoking to Electronic Nicotine Delivery System (ENDS) Use: A Daily Diary Investigation. Int. J. Environ. Res. Public Health 2021, 18, 6301. https://doi.org/10.3390/ijerph18126301
Conner TS, Zeng J, Blank M-L, He V, Hoek J. A Descriptive Analysis of Transitions from Smoking to Electronic Nicotine Delivery System (ENDS) Use: A Daily Diary Investigation. International Journal of Environmental Research and Public Health. 2021; 18(12):6301. https://doi.org/10.3390/ijerph18126301
Chicago/Turabian StyleConner, Tamlin S., Jiaxu Zeng, Mei-Ling Blank, Vicky He, and Janet Hoek. 2021. "A Descriptive Analysis of Transitions from Smoking to Electronic Nicotine Delivery System (ENDS) Use: A Daily Diary Investigation" International Journal of Environmental Research and Public Health 18, no. 12: 6301. https://doi.org/10.3390/ijerph18126301
APA StyleConner, T. S., Zeng, J., Blank, M.-L., He, V., & Hoek, J. (2021). A Descriptive Analysis of Transitions from Smoking to Electronic Nicotine Delivery System (ENDS) Use: A Daily Diary Investigation. International Journal of Environmental Research and Public Health, 18(12), 6301. https://doi.org/10.3390/ijerph18126301






