Malaria Infection and Risk for Endemic Burkitt Lymphoma: A Systematic Review and Meta-Analysis
Abstract
1. Background
2. Methods
2.1. Protocol
2.2. Search Strategy and Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk-of-Bias Assessment
2.6. Outcomes
2.7. Data Analysis
3. Results
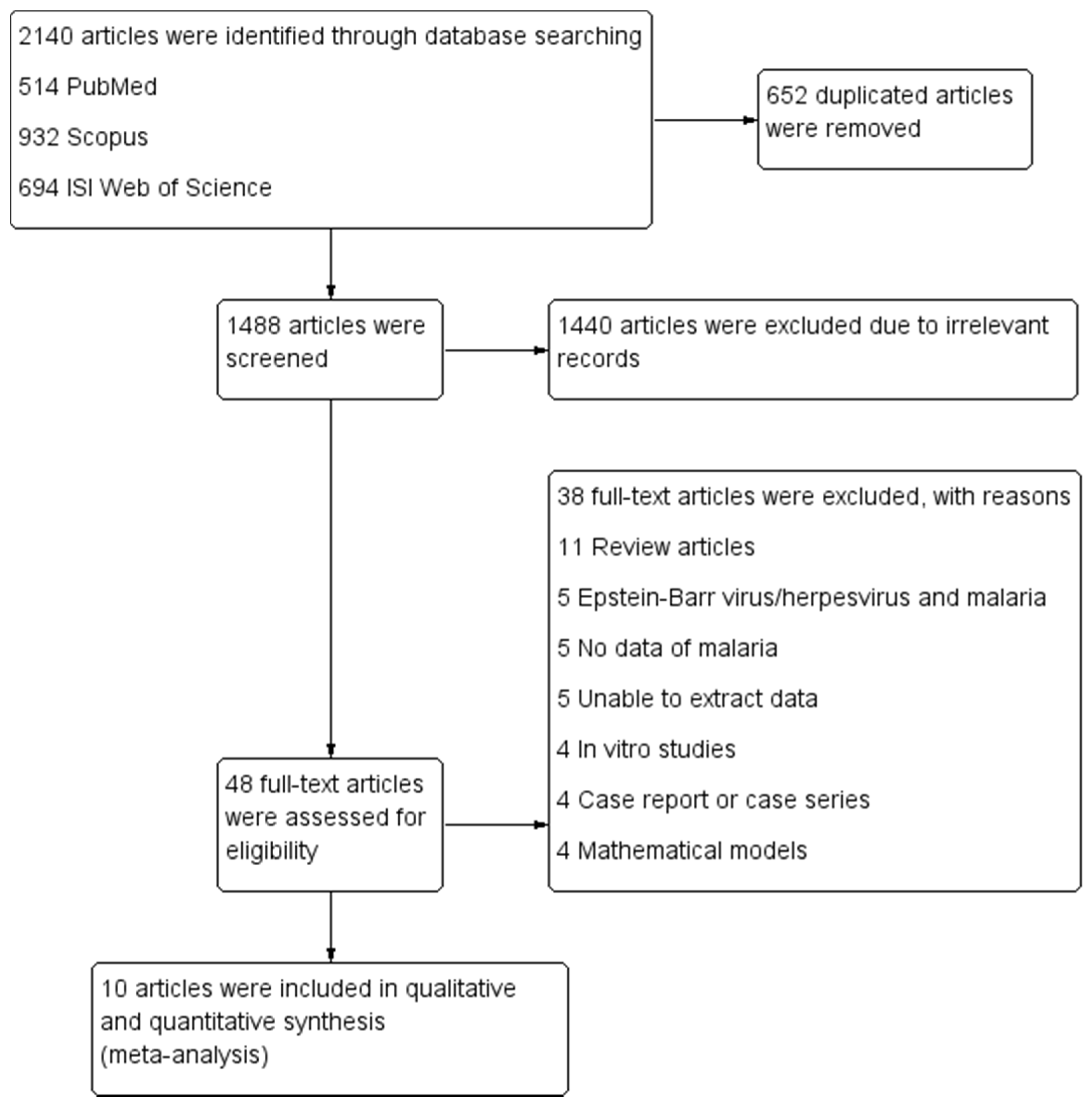
3.1. Search Results
3.2. Characteristics and Quality of the Included Studies
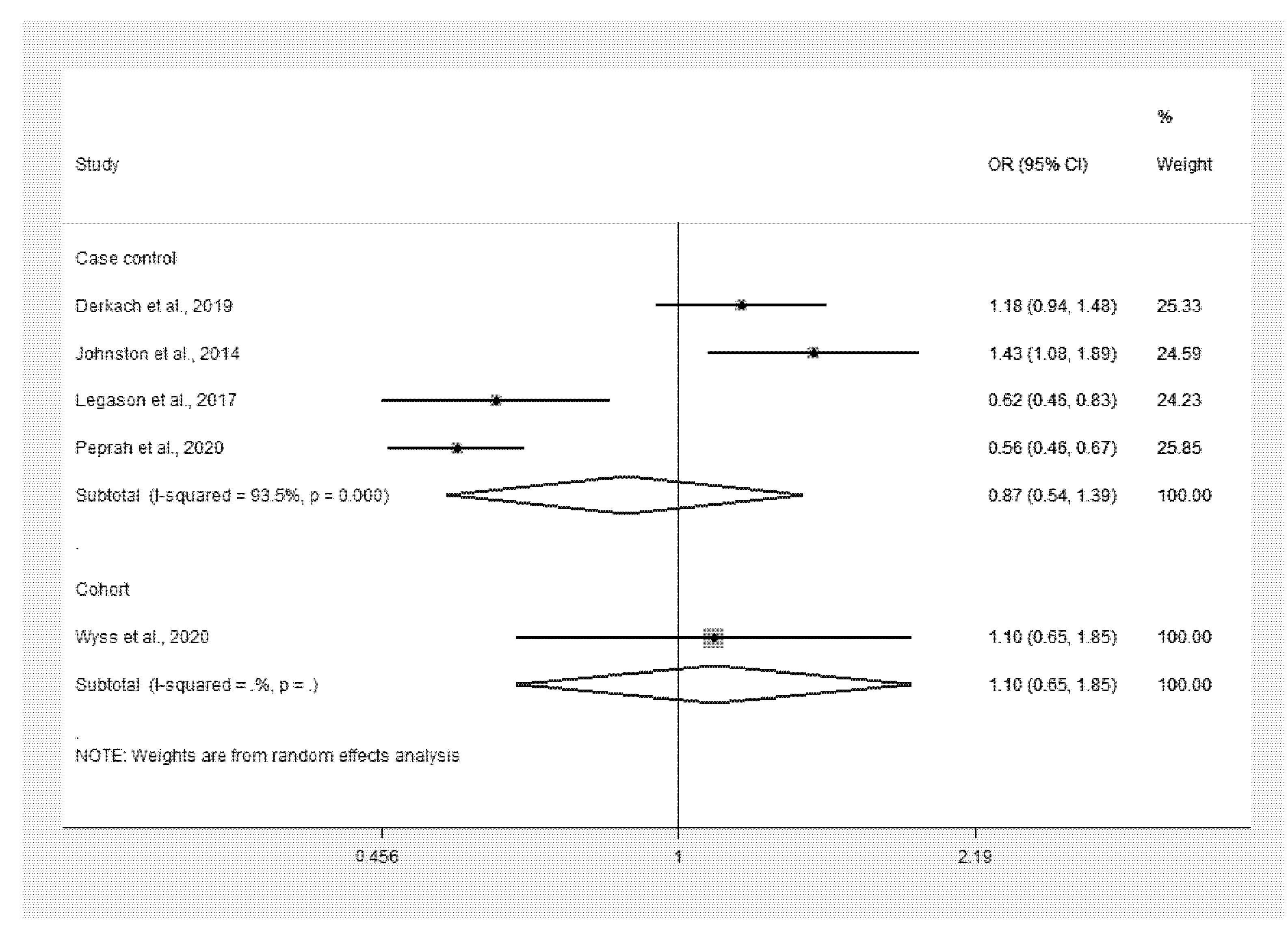
3.3. Malaria Infection and Odds of eBL
3.4. Increased Titer of IgGs to Malarial Antigens and Odds of eBL
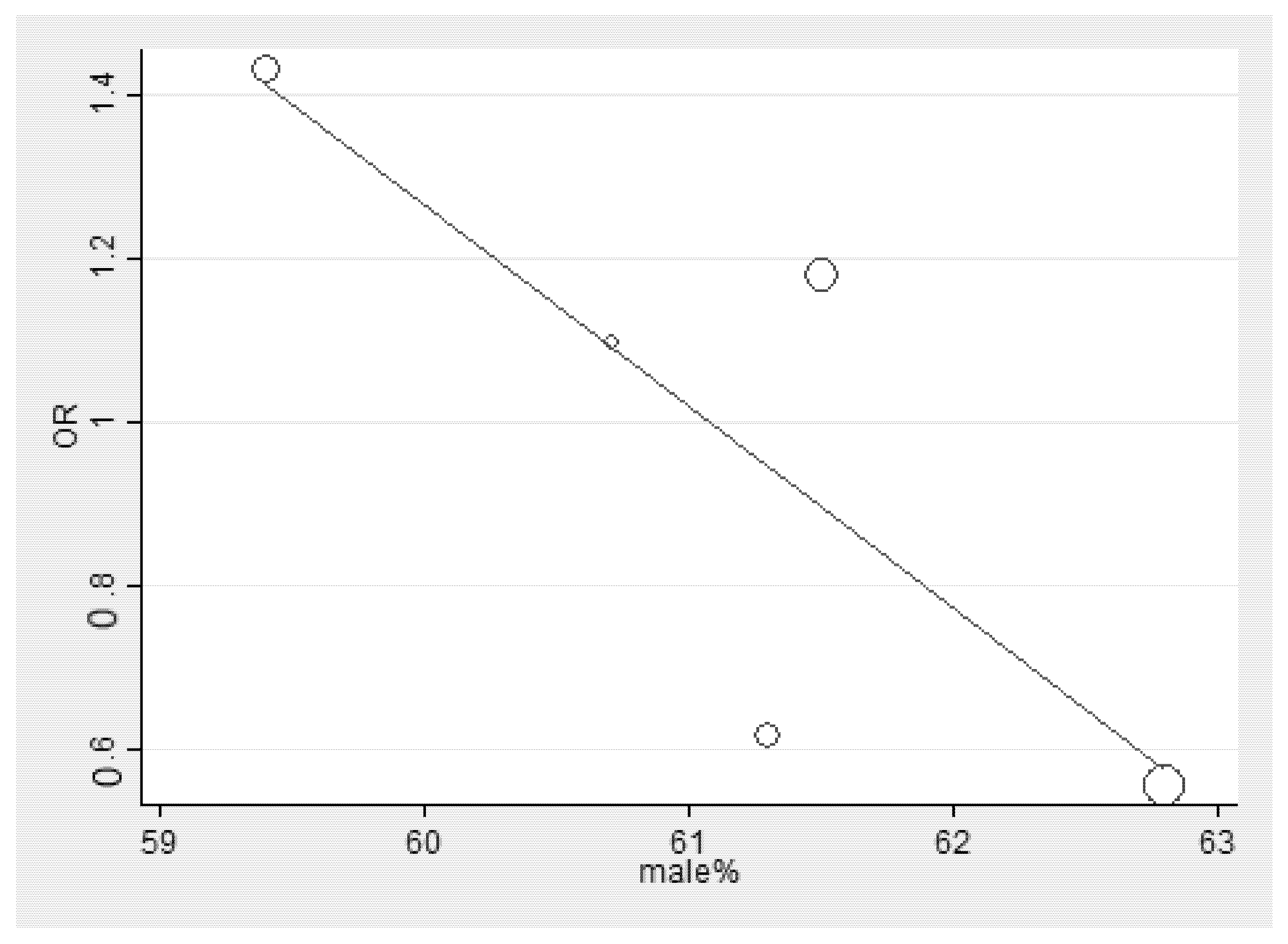
3.5. Meta-Regression Analysis
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahittikorn, A.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.J.; Kotepui, M. Comparison of Plasmodium ovale curtisi and Plasmodium ovale wallikeri infections by a meta-analysis approach. Sci. Rep. 2021, 11, 6409. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.J.; Masangkay, F.R. Prevalence and risk factors related to poor outcome of patients with severe Plasmodium vivax infection: A systematic review, meta-analysis, and analysis of case reports. BMC Infect. Dis. 2020, 20, 363. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Severity and mortality of severe Plasmodium ovale infection: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0235014. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Prevalence of severe Plasmodium knowlesi infection and risk factors related to severe complications compared with non-severe P. knowlesi and severe P. falciparum malaria: A systematic review and meta-analysis. Infect. Dis. Poverty 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Global prevalence and mortality of severe Plasmodium malariae infection: A systematic review and meta-analysis. Malar. J. 2020, 19, 274. [Google Scholar] [CrossRef]
- Sapkota, S.; Shaikh, H. Non-Hodgkin Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- O’Conor, G.T.; Davies, J.N. Malignant tumors in African children. With special reference to malignant lymphoma. J. Pediatr. 1960, 56, 526–535. [Google Scholar] [CrossRef]
- Derkach, A.; Otim, I.; Pfeiffer, R.M.; Onabajo, O.O.; Legason, I.D.; Nabalende, H.; Ogwang, M.D.; Kerchan, P.; Talisuna, A.O.; Ayers, L.W.; et al. Associations between IgG reactivity to Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) antigens and Burkitt lymphoma in Ghana and Uganda case-control studies. EBioMedicine 2019, 39, 358–368. [Google Scholar] [CrossRef]
- Johnston, W.T.; Mutalima, N.; Sun, D.; Emmanuel, B.; Bhatia, K.; Aka, P.; Wu, X.; Borgstein, E.; Liomba, G.N.; Kamiza, S.; et al. Relationship between Plasmodium falciparum malaria prevalence, genetic diversity and endemic Burkitt lymphoma in Malawi. Sci. Rep. 2014, 4, 3741. [Google Scholar] [CrossRef]
- Legason, I.D.; Pfeiffer, R.M.; Udquim, K.I.; Bergen, A.W.; Gouveia, M.H.; Kirimunda, S.; Otim, I.; Karlins, E.; Kerchan, P.; Nabalende, H.; et al. Evaluating the causal link between malaria infection and endemic Burkitt lymphoma in Northern Uganda: A mendelian randomization study. EBioMedicine 2017, 25, 58–65. [Google Scholar] [CrossRef]
- Peprah, S.; Ogwang, M.D.; Kerchan, P.; Reynolds, S.J.; Tenge, C.N.; Were, P.A.; Kuremu, R.T.; Wekesa, W.N.; Sumba, P.O.; Masalu, N.; et al. Risk factors for Burkitt lymphoma in East African children and minors: A case-control study in malaria-endemic regions in Uganda, Tanzania and Kenya. Int. J. Cancer 2020, 146, 953–969. [Google Scholar] [CrossRef]
- Quintana, M.D.P.; Smith-Togobo, C.; Moormann, A.; Hviid, L. Endemic Burkitt lymphoma—An aggressive childhood cancer linked to Plasmodium falciparum exposure, but not to exposure to other malaria parasites. APMIS 2020, 128, 129–135. [Google Scholar] [CrossRef]
- Wyss, K.; Granath, F.; Wångdahl, A.; Djärv, T.; Fored, M.; Naucler, P.; Färnert, A. Malaria and risk of lymphoid neoplasms and other cancer: A nationwide population-based cohort study. BMC Med. 2020, 18, 296. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- JBI. Checklist for Case Control Studies. Available online: https://jbi.global/critical-appraisal-tools (accessed on 1 May 2021).
- Aguilar, R.; Casabonne, D.; O’Callaghan-Gordo, C.; Vidal, M.; Campo, J.J.; Mutalima, N.; Angov, E.; Dutta, S.; Gaur, D.; Chitnis, C.E.; et al. Assessment of the combined effect of Epstein-Barr virus and Plasmodium falciparum infections on endemic Burkitt lymphoma using a multiplex serological approach. Front. Immunol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Aka, P.; Vila, M.C.; Jariwala, A.; Nkrumah, F.; Emmanuel, B.; Yagi, M.; Palacpac, N.M.; Periago, M.V.; Neequaye, J.; Kiruthu, C.; et al. Endemic Burkitt lymphoma is associated with strength and diversity of Plasmodium falciparum malaria stage-specific antigen antibody response. Blood 2013, 122, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.M.; Newton, R.; Casabonne, D.; Ziegler, J.; Mbulaiteye, S.; Mbidde, E.; Wabinga, H.; Jaffe, H.; Beral, V. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: A case-control study in Uganda. Int. J. Cancer 2008, 122, 1319–1323. [Google Scholar] [CrossRef]
- Guech-Ongey, M.; Yagi, M.; Palacpac, N.M.; Emmanuel, B.; Talisuna, A.O.; Bhatia, K.; Stefan, D.C.; Biggar, R.J.; Nkrumah, F.; Neequaye, J.; et al. Antibodies reactive to Plasmodium falciparum serine repeat antigen in children with Burkitt lymphoma from Ghana. Int. J. Cancer 2012, 130, 1908–1914. [Google Scholar] [CrossRef]
- Mutalima, N.; Molyneux, E.; Jaffe, H.; Kamiza, S.; Borgstein, E.; Mkandawire, N.; Liomba, G.; Batumba, M.; Lagos, D.; Gratrix, F.; et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: Results from a case-control study. PLoS ONE 2008, 3, e2505. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.2; Cochrane: Cochrane, AB, Canada, 2021; updated February 2021. [Google Scholar]
- Rainey, J.J.; Mwanda, W.O.; Wairiumu, P.; Moormann, A.M.; Wilson, M.L.; Rochford, R. Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. Trop. Med. Int. Health TMIH 2007, 12, 936–943. [Google Scholar] [CrossRef]
- Iversen, U.; Iversen, O.H.; Bluming, A.Z.; Ziegler, J.L.; Kyalwasi, S. Cell kinetics of African cases of Burkitt lymphoma. A preliminary report. Eur. J. Cancer 1972, 8, 305–308. [Google Scholar] [CrossRef]
- Peprah, S.; Tenge, C.; Genga, I.O.; Mumia, M.; Were, P.A.; Kuremu, R.T.; Wekesa, W.N.; Sumba, P.O.; Kinyera, T.; Otim, I.; et al. A cross-sectional population study of geographic, age-specific, and household risk factors for asymptomatic Plasmodium falciparum malaria infection in Western Kenya. Am. J. Trop. Med. Hyg. 2019, 100, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. Burkitt lymphoma—A stalking horse for cancer research? Semin. Cancer Biol. 2009, 19, 347–350. [Google Scholar] [CrossRef]
- Rochford, R.; Cannon, M.J.; Moormann, A.M. Endemic Burkitt’s lymphoma: A polymicrobial disease? Nat. Rev. Microbiol. 2005, 3, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Moormann, A.M.; Chelimo, K.; Sumba, O.P.; Lutzke, M.L.; Ploutz-Snyder, R.; Newton, D.; Kazura, J.; Rochford, R. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J. Infect. Dis. 2005, 191, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.M.; Syed, N.; Whittle, H.; Crawford, D.H. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet 1991, 337, 876–878. [Google Scholar] [CrossRef]
- Chene, A.; Donati, D.; Orem, J.; Mbidde, E.R.; Kironde, F.; Wahlgren, M.; Bejarano, M.T. Endemic Burkitt’s lymphoma as a polymicrobial disease: New insights on the interaction between Plasmodium falciparum and Epstein-Barr virus. Semin. Cancer Biol. 2009, 19, 411–420. [Google Scholar] [CrossRef]


| No. | Author, Year | Study Area (Years of Survey) | Study Design | Age (mean ± SD or Median [Range]) | Sex (male [%]) | Participants | Percentage of Malaria Cases in Case/Control Groups |
|---|---|---|---|---|---|---|---|
| 1. | Derkach et al., 2019 | Ghana (1965–1994) Uganda (2010–2015) | Case-control study | 0–15 years All cases Cases Range (n): 0–2 (9), 3–5 (56), 6–8 (110), 9–11 (96), 12–14 (62), ≥15 (11) Controls Range (n): 0–2 (38), 3–5 (99), 6–8 (216), 9–11 (234), 12–14 (142), ≥15 (20) Ghana Cases Range (n): 0–2 (2), 3–5 (29), 6–8 (62), 9–11 (36), 12–14 (13), ≥15 (18) Controls Range (n): 0–2 (5), 3–5 26), 6–8 (54), 9–11 (43), 12–14 (13), ≥15 (8) Uganda Cases Range (n): 0–2 (7), 3–5 27), 6–8 (48), 9–11 (60), 12–14 (49), ≥15 (3) Controls Range (n): 0–2 (33), 3–5 (73), 6–8 (162), 9–11 (191), 12–14 (129), ≥15 (12) | All Cases (341): male (61.5%) Controls (749): male (56.6%) Ghana Cases (150): male (63.3%) Controls (149): male (63.8%) Uganda: Cases (191): male (60.2%) Controls (600): male (54.8%) | Cases: Burkitt lymphoma Controls: healthy children in the same age range | All: cases (49.9%), controls (42.3%) Ghana: cases (69.1%), controls (31.3%) Uganda: cases (35.1%), controls (45%) |
| 2. | Johnston et al., 2014 | Malawi (2005–2010) | Case-control study | 0–15 years Cases: 7.7 (se 0.2) Controls: 6.5 (se 0.3) | Cases (303): male (59.4%) Controls (274): male (55.8%) | Cases: Burkitt lymphoma Controls: children admitted to the same hospital with a wide range of both malignant and nonmalignant conditions | Cases (64.7%), controls (45.3%) |
| 3. | Legason et al., 2017 | Uganda (2011–2015) | Case-control study | 0–15 years Cases: 7.7 (3–28) Controls: 7.34 (3–40) | Cases (199): male (61.3%) Controls (624): male (55.1%) | Cases: Burkitt lymphoma Controls: healthy children in the same age range | Cases (34.7%), controls (56.3%) |
| 4. | Peprah et al., 2020 | Uganda, Tanzania, and Kenya (2010–2016) | Case-control study | 0–15 years All cases Cases (689): 7.3 ± 3.7 Controls (2934): 7.5 ± 3.5 Uganda Cases (316): 8.0 ± 3.4 Controls (1150): 7.7 ± 3.3 Tanzania: Cases (71): 6.8 ± 3.8 Controls (819): 7.4 ± 3.3 Kenya: Cases (246): 6.6 ± 3.8 Case controls (965): 7.4 ± 3.7 | All cases Cases (689): male (62.8%) Controls (2934): male (53.1) Uganda Cases (316): male (62%) Controls (1150): male (53%) Tanzania: Cases (127): male (55.9%) Controls (819): male (52.7%) Kenya: Cases (246): male (67.5%) Controls (965): male (53.6%) | Cases: Burkitt lymphoma Controls: health facility, school, or neighborhood controls | All: cases (25.4%), controls (45.7%) Uganda; cases (34.6%), controls (51%) Tanzania: cases (19.5%), controls (41.3%) Kenya: cases (15.8%), controls (43%) |
| 5. | Wyss et al., 2020 | Sweden (1987–2015) | Cohort study | Incidence of lymphoid neoplasm (4125): 34.7 ± 18.5 Matched comparator (66,997): NA | Incidence of lymphoid neoplasm (4125): male (60.7%) Matched comparator (66,997): male (59.7%) | Cases: Lymphoid neoplasm Controls: matched comparator cohort from the general population | Incidence of lymphoid neoplasm (0.36%), matched comparator (0.33%) |
| No. | Author, Year | Study Area (Years of the Survey) | Study Design | Age Range (Years) | Sex (Male [%]) | Participants | Burden of IgGs to Malarial Antigens |
|---|---|---|---|---|---|---|---|
| 1. | Aguilar et al., 2017 | Malawi (2005–2006) | Case-control study | Cases: 7.8 ± 2.9 Controls: 6.3 ± 3.7 | Cases (271): male (59.4%) Controls (174): male (56.9%) | Cases: Burkitt lymphoma Controls: histologically diagnosed with other conditions | Cases (271): lower (15.5%), medium (46.1%), higher (38.4%) Controls (171): lower (33.9%), medium (33.9%), higher (32.2%) |
| 2. | Aka et al., 2013 | Ghana (1965–1994) | Case-control study | 0–15 years | Cases (354): male (59%) Controls (384): male (55.7%) | Cases: Burkitt lymphoma Controls: healthy location-matched controls | Cases (354): positive ≥ 1 of 4 antigens (95.5%), negative to all antigens (4.52%) Controls (384): positive ≥ 1 of 4 antigens (94.8%), negative to all antigens (5.21%) |
| 3. | Carpenter et al., 2008 | Uganda (1994–1999) | Case-control study | 0–15 years | Cases (325): male (60.9%) Controls (579): male (39.1%) | Cases: Burkitt lymphoma Controls: HIV-negative children aged 15 years or less with benign, noninfectious, surgical, or orthopedic conditions | Cases (325): high (12.9%), low (22.2%), negative (3.69%) Controls (579): high (2.76%), low (6.56%), negative (2.76%) |
| 4. | Guech-Ongey et al., 2012 | Ghana (1965–1994) | Case-control study | 0–14 years | Cases (657): male (61.9%) Controls (498): male (54.4%) | Cases: Burkitt lymphoma Controls: healthy children from the same community where the case arose | Cases (657): lower (41.2%), medium (33%), higher (25.6%) Controls (498): lower (33.1%), medium (33.7%), higher (33.1%) |
| 5. | Mutalima et al., 2008 | Malawi (2005–2006) | Case-control study | 0–15 years | Cases (148): male (60.1%) Controls (104): male (54.8%) | Cases: Burkitt lymphoma Controls: histologically diagnosed with other conditions | Cases (129): high/medium (50.4%), low/negative (8.5%) Controls (90): high/medium (22.2%), low/negative (27.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotepui, K.U.; Kotepui, M. Malaria Infection and Risk for Endemic Burkitt Lymphoma: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5886. https://doi.org/10.3390/ijerph18115886
Kotepui KU, Kotepui M. Malaria Infection and Risk for Endemic Burkitt Lymphoma: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(11):5886. https://doi.org/10.3390/ijerph18115886
Chicago/Turabian StyleKotepui, Kwuntida Uthaisar, and Manas Kotepui. 2021. "Malaria Infection and Risk for Endemic Burkitt Lymphoma: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 11: 5886. https://doi.org/10.3390/ijerph18115886
APA StyleKotepui, K. U., & Kotepui, M. (2021). Malaria Infection and Risk for Endemic Burkitt Lymphoma: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(11), 5886. https://doi.org/10.3390/ijerph18115886





