Comparing Paclitaxel–Carboplatin with Paclitaxel–Cisplatin as the Front-Line Chemotherapy for Patients with FIGO IIIC Serous-Type Tubo-Ovarian Cancer
Abstract
1. Introduction
1.1. Current Standard of Treatment
1.2. Gap in Knowledge of Current Standard of Therapy
1.3. Rationale of Dose-Dense Therapy
1.4. Previous Studies for Dose-Dense Therapy
2. Materials and Methods
2.1. Patient Population
2.2. Treatment
2.3. Assessments
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Pathological Status
3.2. Adverse Events (AEs)
3.3. Outcomes
3.4. Prognostic Factors
4. Discussion
4.1. Main Findings
4.2. Summary of Studies Addressing Dose-Dense therapy: Survival Outcome (Table 5)
4.3. Dose-Dense Therapy-Related Adverse Events: Prefer the Use of Low-Dose Cisplatin in Place of Carboplatin in Platinum-based Therapy
4.4. The Benefits of Maximal Cytoreductive Surgery and The Consideration of the Location of Residual Tumors
4.5. The Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| Adverse events (AEs) |
| American Society of Clinical Oncology (ASCO) |
| Area under the curve (AUC) |
| Confidence interval (CI) |
| Epithelial tubo-ovarian cancer (ETOC) |
| Estimated glomerular filtration rate (eGFR) |
| Every four weeks (Q4W) |
| Every three weeks (Q3W) |
| Granulocyte colony-stimulating factor (GCSF) |
| Gynecologic Cancer InterGroup (GCIG) |
| Gynecologic Oncology Group (GOG) |
| Hazard ratio (HR) |
| International Collaboration on Ovarian Neoplasms (ICON) |
| International Federation of Gynecology and Obstetrics (FIGO) |
| Interval cytoreductive surgery (ISC) |
| Intravenous (IV) |
| Intraperitoneal (IP) |
| Japanese Gynecologic Oncology Group (JGOG) |
| National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) |
| National comprehensive Cancer Network (NCCN) |
| Neoadjuvant chemotherapy (NACT) |
| Net health benefits (NHBs) |
| Magnetic resonance image (MRI) |
| Multicentre Italian Trials in Ovarian cancer (MITO) |
| Overall survival (OS) |
| Poly(ADP-ribose) polymerase (PARP) inhibitors (PARP inhibitors) |
| Primary peritoneal serous carcinoma (PPSC) |
| Primary cytoreductive surgery (PSC) |
| Primary debulking surgery (PDS) |
| Progression-free survival (PFS) |
| Progressive disease (PD) |
| Quality of life (QOL) |
| Response Evaluation Criteria in Solid Tumors (RECIST) |
References
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 59–78. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.E.; Buas, M.F.; Moysich, K.B. Survival disparities among racial/ethnic groups of women with ovarian cancer: An update on data from the Surveillance, Epidemiology and End Results (SEER) registry. Cancer. Epidemiol. 2019, 62, 101580. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN guidelines insights: Ovarian cancer, version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Hanchette, C.; Zhang, C.H.; Schwartz, G.G. Ovarian cancer incidence in the U.S. and toxic emissions from pulp and paper plants: A geospatial analysis. Int. J. Environ. Res. Public Health 2018, 15, 1619. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, R.; Lin, L.T.; Chern, C.U.; Tsai, H.W.; Wen, Z.H.; Li, Y.H.; Li, C.J.; Tsui, K.H. MicroRNA in ovarian cancer: Biology, pathogenesis, and therapeutic opportunities. Int. J. Environ. Res. Public Health 2019, 16, 1510. [Google Scholar] [CrossRef]
- Hsu, H.C.; Tseng, K.Y.; Wang, H.C.; Sung, F.C.; Ma, W.F. Risk of endometriosis and subsequent ovary and breast cancers in nurses: A population-based cohort study in Taiwan. Int. J. Environ. Res. Public Health 2019, 16, 3469. [Google Scholar] [CrossRef]
- Oda, K.; Hamanishi, J.; Matsuo, K.; Hasegawa, K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol. Oncol. 2018, 151, 381–389. [Google Scholar] [CrossRef]
- Murakami, K.; Kotani, Y.; Shiro, R.; Takaya, H.; Nakai, H.; Matsumura, N. Endometriosis-associated ovarian cancer occurs early during follow-up of endometrial cysts. Int. J. Clin. Oncol. 2020, 25, 51–58. [Google Scholar] [CrossRef]
- Haraguchi, H.; Koga, K.; Takamura, M.; Makabe, T.; Sue, F.; Miyashita, M.; Urata, Y.; Izumi, G.; Harada, M.; Hirata, T.; et al. Development of ovarian cancer after excision of endometrioma. Fertil. Steril. 2016, 106, 1432–1437. [Google Scholar] [CrossRef]
- Su, K.M.; Wang, P.H.; Yu, M.H.; Chang, C.M.; Chang, C.C. The recent progress and therapy in endometriosis-associated ovarian cancer. J. Chin. Med. Assoc. 2020, 83, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, E.Y.; Kim, O.; Schilder, J.M.; Coffey, D.M.; Cho, C.H.; Bast, R.C., Jr. Cell origins of high-grade serous ovarian cancer. Cancers 2018, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Nakamura, M.; Obata, T.; Daikoku, T.; Fujiwara, H. The association and significance of p53 in gynecologic cancers: The potential of targeted therapy. Int. J. Mol. Sci. 2019, 20, 5482. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Bao, L.; Shen, H.; Ji, M.; Yao, L.; Yi, X.; Jiang, W. Unexpected primary fallopian tube carcinoma during gynecological operations: Clinicopathological and prognostic factors analyses of 67 cases. Taiwan J. Obstet. Gynecol. 2019, 58, 626–632. [Google Scholar] [CrossRef]
- Coan, M.; Rampioni Vinciguerra, G.L.; Cesaratto, L.; Gardenal, E.; Bianchet, R.; Dassi, E.; Vecchione, A.; Baldassarre, G.; Spizzo, R.; Nicoloso, M.S. Exploring the role of Fallopian ciliated cells in the pathogenesis of high-grade serous ovarian cancer. Int. J. Mol. Sci. 2018, 19, 2512. [Google Scholar] [CrossRef]
- Chang, C.C.; Su, K.M.; Lu, K.H.; Lin, C.K.; Wang, P.H.; Li, H.Y.; Wang, M.L.; Lin, C.K.; Yu, M.H.; Chang, C.M. Key immunological functions involved in the progression of epithelial ovarian serous carcinoma discovered by the gene ontology-based immunofunctionome analysis. Int. J. Mol. Sci. 2018, 19, 3311. [Google Scholar] [CrossRef]
- Sung, P.L.; Wen, K.C.; Chen, Y.J.; Chao, T.C.; Tsai, Y.F.; Tseng, L.M.; Qiu, J.T.; Chao, K.C.; Wu, H.H.; Chuang, C.M.; et al. The frequency of cancer predisposition gene mutations in hereditary breast and ovarian cancer patients in Taiwan: From BRCA1/2 to multi-gene panels. PLoS ONE 2017, 12, e0185615. [Google Scholar] [CrossRef]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Piccart, M.J.; Bertelsen, K.; Stuart, G.; Cassidy, J.; Mangioni, C.; Simonsen, E.; James, K.; Kaye, S.; Vergote, I.; Blom, R.; et al. Long-term follow-up confirms a survival advantage of the paclitaxel–cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int. J. Gynecol. Cancer 2003, 13, 144–148. [Google Scholar]
- Piccart, M.J.; Bertelsen, K.; James, K.; Cassidy, J.; Mangioni, C.; Simonsen, E.; Stuart, G.; Kaye, S.; Vergote, I.; Blom, R.; et al. Randomized intergroup trial of cisplatin–paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: Three-year results. J. Natl. Cancer Inst. 2000, 92, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S. Carboplatin versus cisplatin in ovarian cancer. Semin. Oncol. 1995, 22, 88–90. [Google Scholar] [PubMed]
- Neijt, J.P.; Engelholm, S.A.; Tuxen, M.K.; Sorensen, P.G.; Hansen, M.; Sessa, C.; de Swart, C.A.; Hirsch, F.R.; Lund, B.; van Houwelingen, H.C. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J. Clin. Oncol. 2000, 18, 3084–3092. [Google Scholar] [CrossRef]
- du Bois, A.; Lück, H.J.; Meier, W.; Adams, H.P.; Mobus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schröder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baerge, R.; et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guideline in Oncology, Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1.2020—March 11, 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. (accessed on 18 March 2020).
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Chelariu-Raicu, A.; Coleman, R.L.; Sood, A.K. Anti-angiogenesis therapy in ovarian cancer: Which patient is it most likely to benefit? Oncology 2019, 33, 629378. [Google Scholar]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. ICON7 trial investigators. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Clamp, A.R.; James, E.C.; McNeish, I.A.; Dean, A.; Kim, J.W.; O’Donnell, D.M.; Hook, J.; Coyle, C.; Blagden, S.; Brenton, J.D.; et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019, 394, 2084–2095. [Google Scholar] [CrossRef]
- Vasey, P.A.; Jayson, G.C.; Gordon, A.; Gabra, H.; Coleman, R.; Atkinson, R.; Parkin, D.; Paul, J.; Hay, A.; Kaye, S.B.; et al. Phase III randomized trial of docetaxel–carboplatin versus paclitaxel–carboplatin as first-line chemotherapy for ovarian carcinoma. J. Natl. Cancer Inst. 2004, 96, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Hosokawa, K.; Kinoshita, Y.; Watanabe, A.; Yamaguchi, T.; Kuroboshi, H.; Kato, Y.; Yasuda, J.; Fujita, H.; Nakata, Y.; et al. A pilot study of docetaxel–carboplatin versus paclitaxel–carboplatin in Japanese patients with epithelial ovarian cancer. Int. J. Clin. Oncol. 2007, 12, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Scambia, G.; Ferrandina, G.; Savarese, A.; Sorio, R.; Breda, E.; Gebbia, V.; Musso, P.; Frigerio, L.; Del Medico, P.; et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: The MITO-2 randomized phase III trial. J. Clin. Oncol. 2011, 29, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Brady, M.F.; McGuire, W.P.; Harper, P.G.; Alberts, D.S.; Friedlander, M.; Colombo, N.; Fowler, J.M.; Argenta, P.A.; De Geest, K.; et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A Phase III Trial of the Gynecologic Cancer Intergroup. J. Clin. Oncol. 2009, 27, 1419–1425. [Google Scholar] [CrossRef]
- Staropoli, N.; Ciliberto, D.; Botta, C.; Fiorillo, L.; Grimaldi, A.; Lama, S.; Caraglia, M.; Salvino, A.; Tassone, P.; Tagliaferri, P. Pegylated liposomal doxorubicin in the management of ovarian cancer: A systematic review and metaanalysis of randomized trials. Cancer. Biol. Ther. 2014, 15, 707–720. [Google Scholar] [CrossRef]
- Katsumata, N.; Yasuda, M.; Takahashi, F.; Isonishi, S.; Jobo, T.; Aoki, D.; Tsuda, H.; Sugiyama, T.; Kodama, S.; Kimura, E.; et al. Japanese Gynecologic Oncology Group. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009, 374, 1331–1338. [Google Scholar] [CrossRef]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef]
- Fujiwara, K.; Hasegawa, K.; Nagao, S. Landscape of systemic therapy for ovarian cancer in 2019: Primary therapy. Cancer 2019, 125 (Suppl. 24), 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar] [PubMed]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Di Pinto, A.; D’Oria, O.; Aleksa, N.; Musella, A. Dose-dense weekly chemotherapy in advanced ovarian cancer: An updated meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2018, 125, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Reimer, D.; Zeimet, A.G. Front-line therapy of advanced epithelial ovarian cancer: Standard treatment. Ann. Oncol. 2017, 28, viii36–viii39. [Google Scholar] [CrossRef]
- Kemp, Z.; Ledermann, J. Update on first-line treatment of advanced ovarian carcinoma. Int. J. Womens Health 2013, 5, 45–51. [Google Scholar]
- Bookman, M.A. First-line chemotherapy in epithelial ovarian cancer. Clin. Obstet. Gynecol. 2012, 55, 96–113. [Google Scholar] [CrossRef]
- Su, M.H.; Chen, G.Y.; Lin, J.H.; Lee, H.H.; Chung, K.C.; Wang, P.H. Paclitaxel-related dermatological problems: Not only alopecia occurs. Taiwan. J. Obstet. Gynecol. 2019, 58, 877–879. [Google Scholar] [CrossRef]
- Liu, C.H.; Horng, H.C.; Wang, P.H. A case of ovarian cancer present with acute respiratory distress: Spontaneous rupture of diaphragm. Taiwan J. Obstet. Gynecol. 2019, 58, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Su, M.H.; Cho, S.W.; Kung, Y.S.; Lin, J.H.; Lee, W.L.; Wang, P.H. Update on the differential diagnosis of gynecologic organ-related diseases in women presenting with ascites. Taiwan J. Obstet. Gynecol. 2019, 58, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.F.; Lau, H.Y.; Wu, H.H.; Hsu, H.C.; Twu, N.F.; Cheng, W.F. Prognostic factors of early stage epithelial ovarian carcinoma. Int. J. Environ. Res. Public Health 2019, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Chiu, M.L.; Wang, T.Y.; Chen, T.C.; Chang, C.L.; Su, T.H.; Wang, K.G.; Wang, K.L.; Yang, Y.C.; Chen, J.R. Effect of chemotherapy, laparoscopy, and cytology on stage IC ovarian clear cell carcinoma: A long-term, single-center study. Int. J. Environ. Res. Public Health 2020, 17, 491. [Google Scholar] [CrossRef]
- Lee, J.M.; Minasian, L.; Kohn, E.C. New strategies in ovarian cancer treatment. Cancer. 2019, 125 (Suppl. 24), 4623–4629. [Google Scholar] [CrossRef]
- Tsibulak, I.; Zeimet, A.G.; Marth, C. Hopes and failures in front-line ovarian cancer therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 14–19. [Google Scholar] [CrossRef]
- Wendel Naumann, R.; Coleman, R.L.; Brown, J.; Moore, K.N. Phase III trials in ovarian cancer: The evolving landscape of front line therapy. Gynecol. Oncol. 2019, 153, 436–444. [Google Scholar] [CrossRef]
- Liu, J.; Matulonis, U.A. New advances in ovarian cancer. Oncology. (Williston Park) 2010, 24, 721–728. [Google Scholar]
- Markman, M. Chemotherapy: Limited use of the intraperitoneal route for ovarian cancer-why? Nat. Rev. Clin. Oncol. 2015, 12, 628–630. [Google Scholar] [CrossRef]
- van der Burg, M.E.; van Lent, M.; Buyse, M.; Kobierska, A.; Colombo, N.; Favalli, G.; Lacave, A.J.; Nardi, M.; Renard, J.; Pecorelli, S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 1995, 332, 629–634. [Google Scholar] [CrossRef]
- Vergote, I.; Trope, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Ehlen, T.; Reed, N.S.; Casado, A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J. Clin. Oncol. 2011, 29, 4076–4078. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, S.; Terao, Y.; Hirayama, T.; Fujino, K.; Ujihira, T.; Ota, T.; Takeda, S. Safety and efficacy of neoadjuvant chemotherapy with bevacizumab in advanced-stage peritoneal/ovarian cancer patients. Taiwan J. Obstet. Gynecol. 2018, 57, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H. Neoadjuvant chemotherapy before definite operative approach for women with advanced-stage epithelial ovarian cancer. Taiwan J. Obstet. Gynecol. 2018, 57, 623–624. [Google Scholar] [CrossRef]
- Bartels, H.C.; Rogers, A.C.; McSharry, V.; McVey, R.; Walsh, T.; O’Brien, D.; Boyd, W.D.; Brennan, D.J. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol. Oncol. 2019, 154, 622–630. [Google Scholar] [CrossRef]
- Shibutani, T.; Nagao, S.; Suzuki, K.; Kaneda, M.; Yamamoto, K.; Jimi, T.; Yano, H.; Kitai, M.; Shiozaki, T.; Matsuoka, K.; et al. Dose-dense paclitaxel and carboplatin vs. conventional paclitaxel and carboplatin as neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: A retrospective study. Int. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Kim, Y.N.; Lee, Y.J.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Comparison between weekly versus 3-weekly paclitaxel in combination with carboplatin as neoadjuvant chemotherapy in advanced ovarian cancer. J. Gynecol. Oncol. 2020, 31, e23. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Lyons, T.J.; Goodall, R.J.; Kehoe, S.; Morrison, J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane. Database. Syst. Rev. 2019, 10, CD005343. [Google Scholar] [CrossRef]
- Hasegawa, K.; Shimada, M.; Takeuchi, S.; Fujiwara, H.; Imai, Y.; Iwasa, N.; Wada, S.; Eguchi, H.; Oishi, T.; Sugiyama, T.; et al. A phase 2 study of intraperitoneal carboplatin plus intravenous dose-dense paclitaxel in front-line treatment of suboptimal residual ovarian cancer. Br. J. Cancer. 2020. [Google Scholar] [CrossRef]
- Shi, T.; Jiang, R.; Pu, H.; Yang, H.; Tu, D.; Dai, Z.; Cai, Y.; Zhang, Y.; Cheng, X.; Jia, H.; et al. Survival benefits of dose-dense early postoperative intraperitoneal chemotherapy in front-line therapy for advanced ovarian cancer: A randomised controlled study. Br. J. Cancer 2019, 121, 425–428. [Google Scholar] [CrossRef]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; De Felice, F.; Perniola, G.; Palaia, I.; Musella, A.; Di Donato, V.; Cascialli, G.; Cascialli, G.; Muzii, L.; Tombolini, V.; et al. Role of intraperitoneal chemotherapy in ovarian cancer in the platimum-taxane-based era: A meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 136, 64–69. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer. 2020, 127, 76–95. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Floquet, A.; Kim, J.W.; Rau, J.; del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Vergote, I.; Colombo, N.; et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014, 32, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Kristensen, G.; Ray-Coquard, I.; Reuss, A.; Pignata, S.; Colombo, N.; Denison, U.; Vergote, I.; Del Campo, J.M.; Ottevanger, P.; et al. AGO Study Group led Gynecologic Cancer Intergroup/European Network of Gynaecologic Oncology Trials Groups Intergroup Consortium. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016, 17, 78–89. [Google Scholar]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, Y.; Wang, P.H. Poly(ADP-ribose) polymerase (PARP) inhibitors and ovarian cancer. Taiwan J. Obstet. Gynecol. 2017, 56, 713–714. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Vergote, I.; du Bois, A.; Floquet, A.; Rau, J.; Kim, J.W.; Del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Colombo, N.; et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2019, 155, 186–191. [Google Scholar] [CrossRef]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Combined strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer: A literature review. Diagnostics 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, H.; Wan, T.; Zhang, C.; Tong, C.; Liu, J. Comparison of PARPis with angiogenesis inhibitors and chemotherapy for maintenance in ovarian cancer: A network meta-analysis. Adv. Ther. 2019, 36, 3368–3380. [Google Scholar] [CrossRef]
- Vergote, I.; Scambia, G.; O’Malley, D.M.; Van Calster, B.; Park, S.Y.; Del Campo, J.M.; Meier, W.; Bamias, A.; Colombo, N.; Wenham, R.M.; et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 862–876. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.A.; Coleman, R.L.; Arend, R.C.; Armstrong, D.K.; Bala, S.; Mills, G.B.; Sood, A.K.; Herzog, T.J. Advancing drug development in gynecologic malignancies. Clin. Cancer Res. 2019, 25, 4874–4880. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Wang, P.H. Aberrant sialylation in ovarian cancers. J. Chin. Med. Assoc. 2020, 83, 337–344. [Google Scholar] [CrossRef]
- Matanes, E.; Gotlieb, W.H. Immunotherapy of gynecological cancers. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 97–110. [Google Scholar] [CrossRef]
- Lee, W.L.; Wang, P.H. Immunology and ovarian cancers. J. Chin. Med. Assoc. 2020, 83, 425–432. [Google Scholar] [CrossRef]
- Suidan, R.S.; He, W.; Sun, C.C.; Zhao, H.; Rauh-Hain, J.A.; Fleming, N.D.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am. J. Obstet. Gynecol. 2019, 221, 136.e1–136.e9. [Google Scholar] [CrossRef]
- Markman, M.; Liu, P.Y.; Wilczynski, S.; Monk, B.; Copeland, L.J.; Alvarez, R.D.; Jiang, C.; Alberts, D.; Southwest Oncology Group; Gynecologic Oncology Group. Southwest Oncology, Oncology G. Gynecologic, Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial. J. Clin. Oncol. 2003, 21, 2460–2465. [Google Scholar]
- de Jongh, F.E.; de Wit, R.; Verweij, J.; Sparreboom, A.; van den Bent, M.J.; Stoter, G.; van der Burg, M.E. Dose-dense cisplatin/paclitaxel. a well-tolerated and highly effective chemotherapeutic regimen in patients with advanced ovarian cancer. Eur. J. Cancer. 2002, 38, 2005–2013. [Google Scholar] [CrossRef]
- Viret, F.; Bertucci, F.; Genre, D.; Gravis, G.; Chabannon, C.; Conte, M.; Houvenaeghel, G.; Maraninchi, D.; Viens, P. Intensive sequential dose dense chemotherapy with stem cell support as first-line treatment in advanced ovarian carcinoma: A phase II study. Bone, Marrow, Transplant. 2002, 30, 879–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchetti, P.; Urien, S.; Cappellini, G.A.; Ronzino, G.; Ficorella, C. Weekly administration of paclitaxel: Theoretical and clinical basis. Crit. Rev. Oncol. Hematol. 2002, 44, S3–S13. [Google Scholar] [CrossRef]
- Fizazi, K.; Zelek, L. Is one cycle every three or four week’s obsolete? A critical review of dose-dense chemotherapy in solid neoplasms. Ann. Oncol. 2000, 11, 133–149. [Google Scholar] [CrossRef]
- Goldie, J.H.; Coldman, A.J. The genetic origin of drug resistance in neoplasms: Implication for systemic therapy. Cancer. Res. 1984, 44, 3643–3653. [Google Scholar]
- Norton, L.; Simon, R. The Norton-Simon hypothesis revisited. Cancer. Treat. Rep. 1986, 70, 163–169. [Google Scholar]
- Simon, R.; Norton, L. The Norton-Simon hypothesis: Designing more effective and less toxic chemotherapeutic regimens. Nat. Clin. Pract. Oncol. 2006, 3, 406–407. [Google Scholar] [CrossRef]
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671. [Google Scholar] [CrossRef]
- Milani, A.; Kristeleit, R.; McCormack, M.; Raja, F.; Luvero, D.; Widschwendter, M.; MacDonald, N.; Mould, T.; Olatain, A.; Hackshaw, A.; et al. Switching from standard to dose-dense chemotherapy in front-line treatment of advanced ovarian cancer: A retrospective study of feasibility and efficacy. ESMO Open 2017, 1, e000117. [Google Scholar] [CrossRef]
- Rettenmaier, M.A.; Micha, J.P.; Bohart, R.; Goldstein, B.H. A retrospective study comparing the efficacy of dose-dense chemotherapy, intraperitoneal chemotherapy and dose-dense chemotherapy with hyperthermic intraperitoneal chemotherapy in the treatment of advanced stage ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 44, 101–105. [Google Scholar] [CrossRef]
- Murphy, M.; Martin, G.; Mahmoudjafari, Z.; Bivona, C.; Grauer, D.; Henry, D. Intraperitoneal paclitaxel and cisplatin compared with dose-dense paclitaxel and carboplatin for patients with stage III ovarian cancer. J. Oncol. Pharm. Pract. 2020, 1078155219899460. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Hsu, Y.T.; Wu, C.C.; Lai, Y.Z.; Wang, C.; Yang, Y.C.; Wu, T.C.; Hung, C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer. Res. 2013, 73, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Stasenko, M.; Reynolds, R.K.; Johnston, C.; Brackman, M.; McLean, K.; Uppal, S. Adherence to hematologic hold parameters in carboplatin and dose-dense paclitaxel chemotherapy for ovarian malignancies: A survey of NCCN member institutions. J. Natl. Compr. Cancer Netw. 2016, 14, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Boraska Jelavić, T.; Boban, T.; Brčić, L.; Vrdoljak, E. Is macrocytosis a potential biomarker of the efficacy of dose-dense paclitaxel–carboplatin combination therapy in patients with epithelial ovarian cancer? Anticancer Drugs 2017, 28, 922–927. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yang, Y.C.; Wang, K.L.; Chen, T.C.; Chen, J.R.; Weng, C.S.; Chien, H.J.; Chang, C.L. Possible surrogate marker for an effective dose-dense chemotherapy in treating ovarian cancer. Taiwan J Obstet. Gynecol. 2016, 55, 405–409. [Google Scholar] [CrossRef][Green Version]
- Lee, W.L.; Chan, I.S.; Wang, P.H. Does a simple hematological examination predict the response and side effects in patients undergoing induction chemotherapy and/or neoadjuvant chemotherapy? J. Chin. Med. Assoc. 2020, 83, 107–108. [Google Scholar] [CrossRef]
- Vasey, P.A. “Dose dense” chemotherapy in ovarian cancer. Int. J. Gynecol. Cancer. 2005, 15 (Suppl. 3), 226–232. [Google Scholar] [CrossRef]
- Muggia, F.M. Sequential single agents as first-line chemotherapy for ovarian cancer: A strategy derived from the results of GOG-132. Int. J. Gynecol. Cancer. 2003, 13 (Suppl. 2), 156–162. [Google Scholar] [CrossRef]
- Muggia, F.M. Relevance of chemotherapy dose and schedule to outcomes in ovarian cancer. Semin. Oncol. 2004, 31 (Suppl. 15), 19–24. [Google Scholar] [CrossRef]
- van der Burg, M.E.; van der Gaast, A.; Vergote, I.; Burger, C.W.; van Doorn, H.C.; de Wit, R.; Stoter, G.; Verweij, J. What is the role of dose-dense therapy? Int. J. Gynecol. Cancer. 2005, 15 (Suppl. 3), 233–240. [Google Scholar] [CrossRef]
- van den Bent, M.J.; van Putten, W.L.; Hilkens, P.H.; de Wit, R.; van der Burg, M.E. Retreatment with dose-dense weekly cisplatin after previous cisplatin chemotherapy is not complicated by significant neuro-toxicity. Eur. J. Cancer. 2002, 38, 387–391. [Google Scholar] [CrossRef]
- Vasey, P.A.; Kaye, S.B. Dose intensity in ovarian cancer. In Ovarian Cancer Controversies in Management; Gershenson, D.M., McGuire, W.P., Eds.; Churchill Livingstone: New York, NY, USA, 1997; pp. 139–169. [Google Scholar]
- Fruscio, R.; Garbi, A.; Parma, G.; Lissoni, A.A.; Garavaglia, D.; Bonazzi, C.M.; Dell’anna, T.; Mangioni, C.; Milani, R.; Colombo, N. Randomized phase III clinical trial evaluating weekly cisplatin for advanced epithelial ovarian cancer. J. Natl. Cancer. Inst. 2011, 103, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Boere, I.A.; van der Burg, M.E. Review of dose-intense platinum and/or paclitaxel containing chemotherapy in advanced and recurrent epithelial ovarian cancer. Curr. Pharm. Des. 2012, 18, 3741–3753. [Google Scholar] [CrossRef] [PubMed]
- Tiersten, A.D.; Sill, M.W.; Knight, D.; Muggia, F.; Garcia, A.A.; Swensen, R.; Warshal, D.P.; Mannel, R.S.; Fracasso, P.M. A phase I trial of dose-dense (biweekly) carboplatin combined with paclitaxel and pegfilgrastim: A feasibility study in patients with untreated Stage III and IV ovarian, tubal or primary peritoneal cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2010, 118, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, E.; Karavasilis, V.; Tzamakou, E.; Haidou, C.; Piperidou, C.; Pavlidis, N. Pharmacodynamics of non-break weekly paclitaxel (Taxol) and pharmacokinetics of Cremophor-EL vehicle: Results of a dose-escalation study. Anticancer Drugs 2002, 13, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Rosenberg, P. Role of weekly paclitaxel in the treatment of advanced ovarian cancer. Crit. Rev. Oncol. Hematol. 2002, 44, S43–S51. [Google Scholar] [CrossRef]
- Mori, T.; Kinoshita, Y.; Watanabe, A.; Yamaguchi, T.; Hosokawa, K.; Honjo, H. Retention of paclitaxel in cancer cells for 1 week in vivo and in vitro. Cancer. Chemother. Pharmacol. 2006, 58, 665–672. [Google Scholar] [CrossRef]
- Kumar, A.; Hoskins, P.J.; Tinker, A.V. Dose-dense paclitaxel in advanced ovarian cancer. Clin. Oncol. (R. Coll. Radiol) 2015, 27, 40–47. [Google Scholar] [CrossRef]
- Kaye, S.B. First-line chemotherapy for ovarian cancer—the controversy continues. Br. J. Cancer. 2002, 87, 813–814. [Google Scholar] [CrossRef][Green Version]
- Fiseha, T.; Mengesha, T.; Girma, R.; Kebede, E.; Gebreweld, A. Estimation of renal function in adult outpatients with normal serum creatinine. BMC Res. Notes 2019, 12, 462. [Google Scholar] [CrossRef]
- Chang, W.H.; Horng, H.C.; Yeh, C.C.; Guo, C.Y.; Chou, Y.J.; Huang, N.; Lee, W.L.; Wang, P.H. Risks of female genital tract related cancers (gynecological cancers) or breast cancer in women with and without chronic kidney disease: A population-based cohort study in Taiwan. Medicine 2018, 97, e0157. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.S.; Chang, W.H.; Wang, K.C.; Huang, N.; Guo, C.Y.; Chou, Y.J.; Lee, W.L.; Wang, P.H. Endometriosis might be inversely associated with developing chronic kidney disease: A population-based cohort study in Taiwan. Int. J. Mol. Sci. 2016, 17, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.E.; Shoenbill, K.; Mitchell, S.A.; Dueck, A.C.; Schrag, D.; Bruner, D.W.; Minasian, L.M.; St Germain, D.; O’Mara, A.M.; Baumgartner, P.; et al. Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J. Am. Med. Inform. Assoc. 2019, 26, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.W.; Basch, E.; Hudson, L.L.; Chung, A.E.; Mendoza, T.R.; Mitchell, S.A.; St Germain, D.; Baumgartner, P.; Sit, L.; Rogak, L.J.; et al. Software for administering the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events: Usability Study. JMIR Hum. Factors 2018, 5, e10070. [Google Scholar] [CrossRef]
- Raimondi, A.; Randon, G.; Sepe, P.; Claps, M.; Verzoni, E.; de Braud, F.; Procopio, G. The evaluation of response to immunotherapy in metastatic renal cell carcinoma: Open challenges in the clinical practice. Int. J. Mol. Sci. 2019, 20, 4263. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet. Oncol 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Beaumont, H.; Bertrand, A.S.; Klifa, C.; Patriti, S.; Cippolini, S.; Lovera, C.; Iannessi, A. Radiology workflow for RECIST assessment in clinical trials: Can we reconcile time-efficiency and quality? Eur. J. Radiol. 2019, 118, 257–263. [Google Scholar] [CrossRef]
- Cheng, M.; Lee, H.H.; Chang, W.H.; Lee, N.R.; Huang, H.Y.; Chen, Y.J.; Horng, H.C.; Lee, W.L.; Wang, P.H. Weekly dose-dense paclitaxel and triweekly low-dose cisplatin: A well-tolerated and effective chemotherapeutic regimen for first-line treatment of advanced ovarian, fallopian tube, and primary peritoneal cancer. Int. J. Environ. Res. Public Health 2019, 16, 4794. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhao, B.B.; Li, L. The significance of the change pattern of serum CA125 level for judging prognosis and diagnosing recurrences of epithelial ovarian cancer. J. Ovarian. Res. 2016, 9, 57. [Google Scholar] [CrossRef]
- Abaid, L.N.; Micha, J.P.; Rettenmaier, M.A.; Brown, J.V.; Mendivil, A.A.; Lopez, K.L.; Goldstein, B.H. A phase II study of modified dose-dense paclitaxel and every 4-week carboplatin for the treatment of advanced-stage primary epithelial ovarian, fallopian tube, or peritoneal carcinoma. Cancer. Chemother. Pharmacol. 2013, 72, 101–107. [Google Scholar] [CrossRef]
- Fleming, N.D.; Coleman, R.L.; Tung, C.; Westin, S.N.; Hu, W.; Sun, Y.; Bhosale, P.; Munsell, M.F.; Sood, A.K. Phase II trial of bevacizumab with dose-dense paclitaxel as first-line treatment in patients with advanced ovarian cancer. Gynecol. Oncol. 2017, 147, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bun, S.; Yunokawa, M.; Ebata, T.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yonemori, K.; Shimizu, C.; Fujiwara, Y.; Kato, T.; et al. Feasibility of dose-dense paclitaxel/carboplatin therapy in elderly patients with ovarian, fallopian tube, or peritoneal cancer. Cancer. Chemother. Pharmacol. 2016, 78, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Bun, S.; Yunokawa, M.; Ebata, T.; Kobayashi Kato, M.; Shimoi, T.; Kato, T.; Tamura, K. Feasibility of initial treatment in elderly patients with ovarian cancer in Japan: A retrospective study. Int. J. Clin. Oncol. 2019, 24, 1111–1118. [Google Scholar] [CrossRef]
- Minagawa, Y.; Kigawa, J.; Kanamori, Y.; Itamochi, H.; Terakawa, N.; Okada, M.; Kitada, F. Feasibility study comparing docetaxel–cisplatin versus docetaxel–carboplatin as first-line chemotherapy for ovarian cancer. Gynecol. Oncol. 2006, 101, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.M.; George, J.N. Microangiopathic hemolytic anemia and thrombocytopenia in patients with cancer. J. Oncol. Pract. 2016, 12, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Mones, J.V.; Soff, G. Management of thrombocytopenia in cancer patients. Cancer. Treat. Res. 2019, 179, 139–150. [Google Scholar]
- Al-Samkari, H.; Connors, J.M. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019, 3, 3770–3779. [Google Scholar] [CrossRef]
- Boyd, L.R.; Muggia, F.M. Carboplatin/paclitaxel induction in ovarian cancer: The finer points. Oncol. (Williston Park) 2018, 32, 418–420. [Google Scholar]
- Egorin, M.J.; Van Echo, D.A.; Tipping, S.J.; Olman, E.A.; Whitacre, M.Y.; Thompson, B.W.; Aisner, J. Pharmacokinetics and dosage reduction of cis-diammine(1,1-cyclobutanedicarboxylato)platinum in patients with impaired renal function. Cancer. Res. 1984, 44, 5432–5438. [Google Scholar]
- Wang, P.H.; Yuan, C.C.; Shyong, W.Y.; Chiang, S.C.; Chao, J.Y.; Yen, M.S.; Ng, H.T. Optimal debulking surgery is an independent prognostic factor in patients with FIGO IIIC primary epithelial ovarian carcinoma. Zhonghua Yi Xue Za Zhi (Taipei) 2000, 63, 220–225. [Google Scholar]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, CD007565. [Google Scholar] [CrossRef] [PubMed]
- Javellana, M.; Hoppenot, C.; Lengyel, E. The road to long-term survival: Surgical approach and longitudinal treatments of long-term survivors of advanced-stage serous ovarian cancer. Gynecol. Oncol. 2019, 152, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Manning-Geist, B.L.; Hicks-Courant, K.; Gockley, A.A.; Clark, R.M.; Del Carmen, M.G.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley, M.J., Jr. Moving beyond “complete surgical resection” and “optimal”: Is low-volume residual disease another option for primary debulking surgery? Gynecol. Oncol. 2018, 150, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sioulas, V.D.; Schiavone, M.B.; Kadouri, D.; Zivanovic, O.; Roche, K.L.; O’Cearbhaill, R.; Abu-Rustum, N.R.; Levine, D.A.; Sonoda, Y.; Gardner, G.J.; et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol. Oncol. 2017, 145, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, Y.; Qian, M.; Lv, L.; Ying, X. The improved effects of a multidisciplinary team on the survival of breast cancer patients: Experiences from China. Int. J. Environ. Res. Public Health 2020, 17, 277. [Google Scholar] [CrossRef]
- Natarajan, R.; Aljaber, D.; Au, D.; Thai, C.; Sanchez, A.; Nunez, A.; Resto, C.; Chavez, T.; Jankowska, M.M.; Benmarhnia, T.; et al. Environmental exposures during puberty: Window of breast cancer risk and epigenetic damage. Int. J. Environ. Res. Public Health 2020, 17, 493. [Google Scholar] [CrossRef]
- Atlan, M.; Neman, J. Targeted transdermal delivery of curcumin for breast cancer prevention. Int. J. Environ. Res. Public Health 2019, 16, 4949. [Google Scholar] [CrossRef]
- Paulauskiene, J.; Stelemekas, M.; Ivanauskiene, R.; Petkeviciene, J. The cost-effectiveness analysis of cervical cancer screening using a systematic invitation system in Lithuania. Int. J. Environ. Res. Public Health 2019, 16, 5035. [Google Scholar] [CrossRef]
- Lipscomb, J.; Escoffery, C.; Gillespie, T.W.; Henley, S.J.; Smith, R.A.; Chociemski, T.; Almon, L.; Jiang, R.; Sheng, X.; Goodman, M.; et al. Improving screening uptake among breast cancer survivors and their first-degree relatives at elevated risk to breast cancer: Results and implications of a randomized study in the state of Georgia. Int. J. Environ. Res. Public Health 2020, 17, 977. [Google Scholar] [CrossRef]
- Harano, K.; Terauchi, F.; Katsumata, N.; Takahashi, F.; Yasuda, M.; Takakura, S.; Takano, M.; Yamamoto, Y.; Sugiyama, T. Quality-of-life outcomes from a randomized phase III trial of dose-dense weekly paclitaxel and carboplatin compared with conventional paclitaxel and carboplatin as a first-line treatment for stage II-IV ovarian cancer: Japanese Gynecologic Oncology Group Trial (JGOG3016). Ann. Oncol. 2014, 25, 251–257. [Google Scholar]
- Dalton, H.J.; Yu, X.; Hu, L.; Kapp, D.S.; Benjamin, I.; Monk, B.J.; Chan, J.K. An economic analysis of dose dense weekly paclitaxel plus carboplatin versus every-3-week paclitaxel plus carboplatin in the treatment of advanced ovarian cancer. Gynecol. Oncol. 2012, 124, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.L.; Shahabi, S. Cost-effectiveness analysis of dose-dense versus standard intravenous chemotherapy for ovarian cancer: An economic analysis of results from the Gynecologic Oncology Group protocol 262 randomized controlled trial. Gynecol. Oncol. 2017, 145, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Herzog, T.J.; Cohn, D.E. Dose dense chemotherapy for front-line ovarian cancer treatment: The price is right? Gynecol. Oncol. 2017, 145, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Foote, J.; Secord, A.A.; Liang, M.; Cohn, D.E.; Jewell, E.; Havrilesky, L.J. ASCO value framework highlights the relative value of treatment options in ovarian cancer. J. Oncol. Pract. 2017, 13, e1030–e1039. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Hou, J.Y.; Burke, W.M.; Tergas, A.I.; Chen, L.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Utilization and toxicity of alternative delivery methods of adjuvant chemotherapy for ovarian cancer. Obstet. Gynecol. 2016, 127, 985–991. [Google Scholar] [CrossRef] [PubMed]
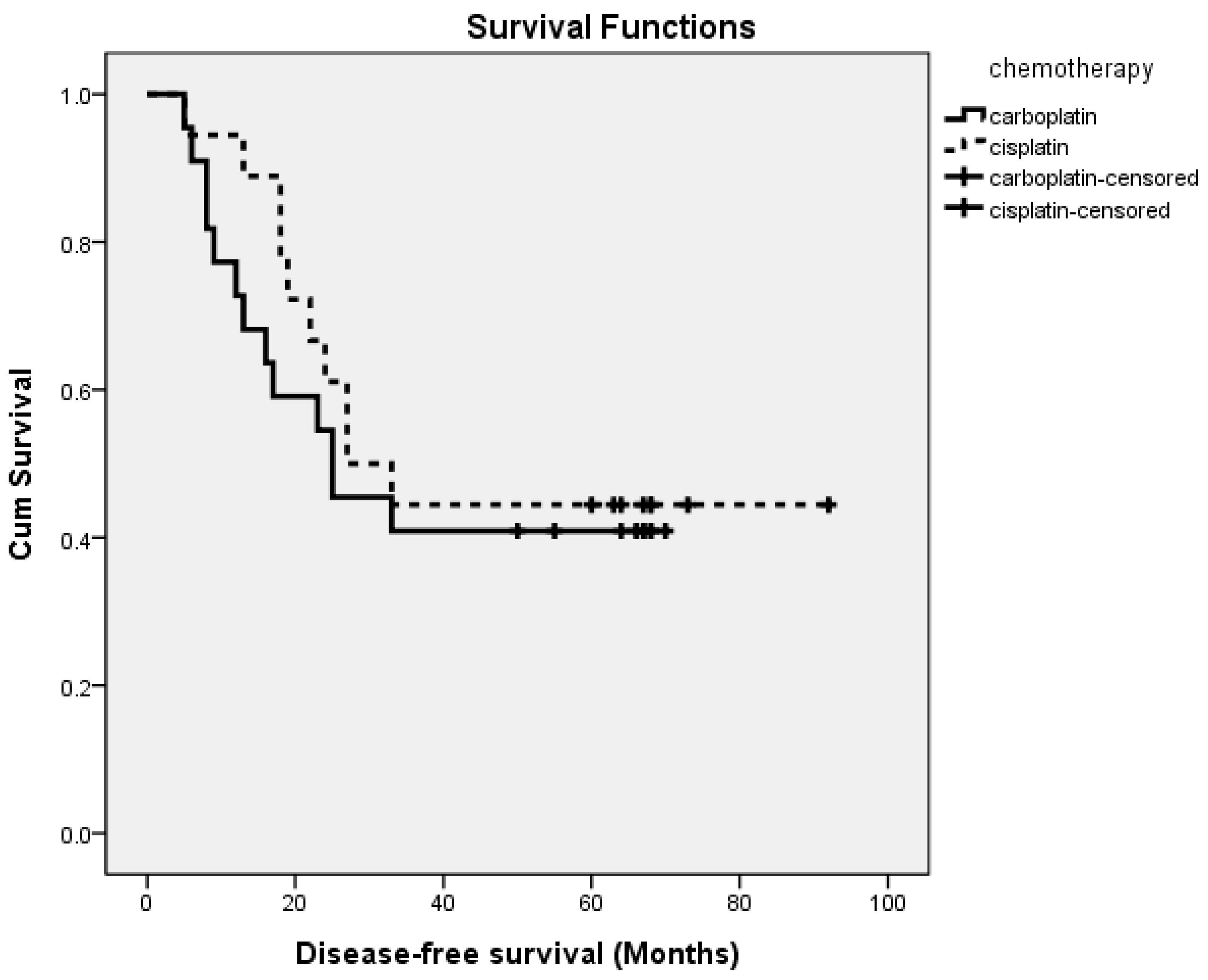
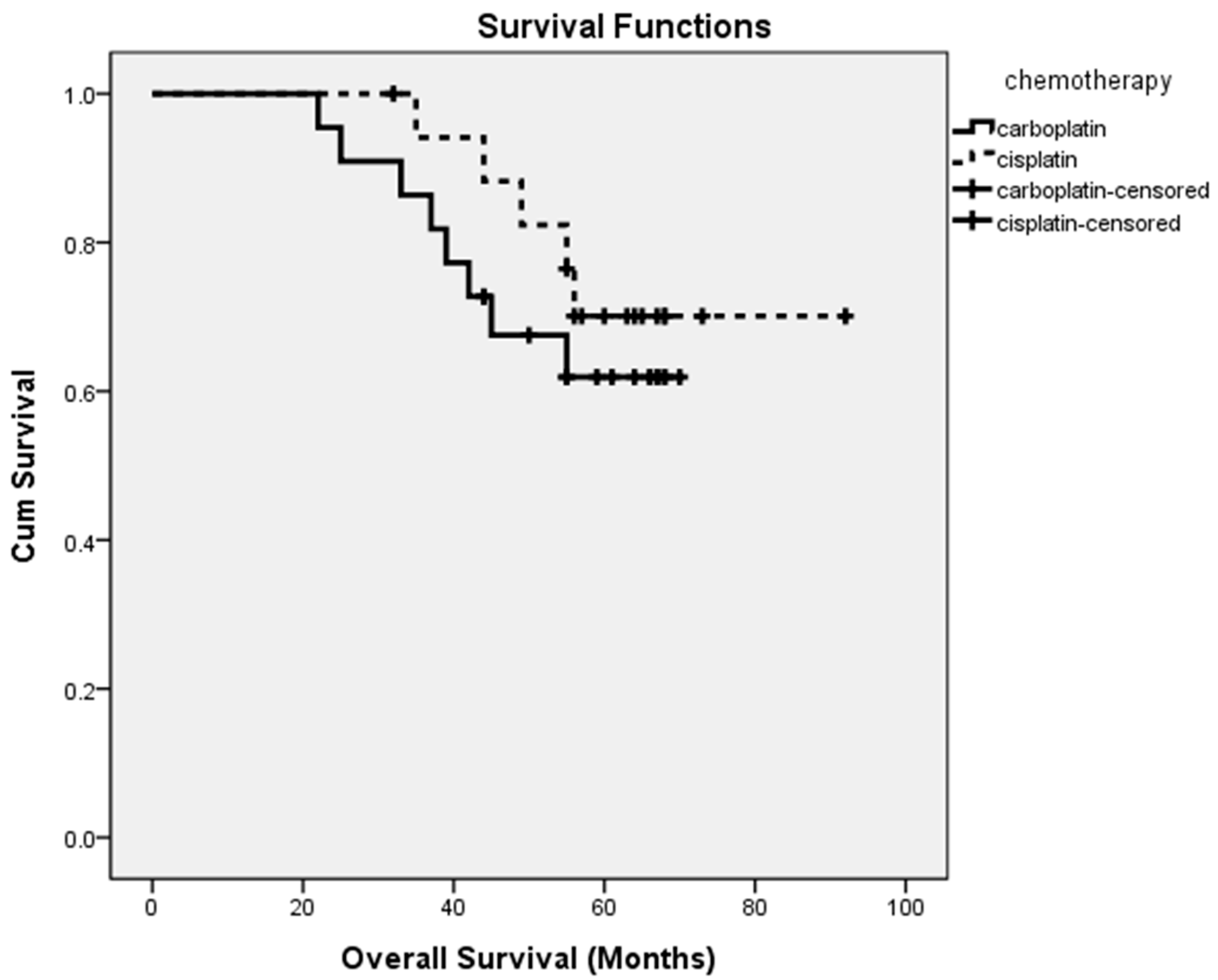
| Carboplatin | Cisplatin | p | |
|---|---|---|---|
| Number of patients | 22 | 18 | |
| Age (years) | 58.5 ± 9.4 | 59.4 ± 9.4 | 0.768 |
| Size of residual tumors | 0.949 | ||
| ≤1cm | 12 (54.5%) | 10 (55.6%) | |
| >1cm | 10 (45.5%) | 8 (44.4%) | |
| Site of residual tumor | 0.676 | ||
| Localized | 12 (54.5%) | 11 (61.1%) | |
| Whole abdominal cavity | 10 (45.5%) | 7 (38.9%) | |
| Period to complete the front-line chemotherapy | 0.018 | ||
| ≤21 weeks | 12 (54.5%) | 16 (88.9%) | |
| >21 weeks | 10 (45.5%) | 2 (11.1%) | |
| ECOG | 0.884 | ||
| 0-1 | 21 (95.5%) | 17 (94.4%) | |
| 2-3 | 1 (4.5%) | 1 (5.6%) |
| Events | Any grade, n (%) | Grade 3/4, n (%) | ||||
|---|---|---|---|---|---|---|
| CARBO | CIS | p | CARBO | CIS | p | |
| Neutropenia | 20 (90.9) | 7 (38.9) | < 0.0001 | 17 (77.3) | 5 (27.8) | 0.002 |
| Anemia | 21 (95.5) | 18 (100) | 0.360 | 5 (22.7) | 1 (5.6) | 0.130 |
| Thrombocytopenia | 5 (22.7) | 3 (16.7) | 0.634 | 3 (13.6) | 0 | 0.103 |
| Renal toxicity | 1 (4.5) | 3 (16.7) | 0.204 | 0 | 0 | |
| Proteinuria | 5 (22.7) | 1 (5.6) | 0.130 | 0 | 0 | |
| Peripheral neuropathy | 8 (36.4) | 7 (38.9) | 0.870 | 0 | 0 | |
| Nausea | 9 (40.9) | 9 (50.0) | 0.565 | 0 | 0 | |
| Parameters | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | |
| Size | |||||
| ≤1cm | 22 | 1(Ref) | 1(Ref) | ||
| >1cm | 18 | 8.68 (3.23–23.35) | <0.0001 | 14.38 (4.18–49.46) | <0.0001 |
| Site | |||||
| Localized | 23 | 1 (Ref) | 1 (Ref) | ||
| WAC | 17 | 1.38 (0.61–3.14) | 0.440 | 0.71 (0.28–1.82) | 0.470 |
| Period | |||||
| ≤21 weeks | 28 | 1 (Ref) | 1 (Ref) | ||
| >21 weeks | 12 | 28.49 (8.36–97.06) | <0.0001 | 81.24 (14.03–470.31) | <0.0001 |
| Parameters | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | |
| Size | |||||
| ≤1cm | 22 | 1(Ref) | 1(Ref) | ||
| >1cm | 18 | 22.37 (2.88–173.86) | 0.003 | 11.83 (1.48–94.72) | 0.020 |
| Site | |||||
| Localized | 23 | 1 (Ref) | 1 (Ref) | ||
| WAC | 17 | 2.41 (0.79–7.39) | 0.122 | 2.54 (0.76–8.53) | 0.131 |
| Period | |||||
| ≤21 weeks | 28 | 1 (Ref) | 1 (Ref) | ||
| >21 weeks | 12 | 15.31 (4.13–56.78) | <0.0001 | 9.57 (2.34–39.18) | 0.002 |
| Authors | Stage | n | Regimen | PFS | OS |
|---|---|---|---|---|---|
| Prospective randomized trials | |||||
| Katsumata [40] | II–IV | 312 | P 80 mg/m2 (D1,8,15), C 6 (D1) | 28.2 (M) | 100.5 (M) |
| Pignata [32] | IC-IV | 406 | P 60 mg/m2 (D1,8,15), C 2 (D1,8,15) | 18.3 (M) | |
| Chan [41] | II–IV | 340 | P 80 mg/m2 (D1,8,15), C 6 ±BEV (D1) | 14.7 (M) | - |
| 55 | P 80mg/m2 (D1,8,15), C 6 (D1) | 14.2 (M) | - | ||
| Clamp [33] | I-IV | P 80 mg/m2 (D1,8,15), C 5,6 ± BEV (D1) | 20.8 (M) | ||
| P 80 mg/m2 (D1,8,15), C 2 (D1,8,15) ± BEV (D1) | 21.0 (M) | ||||
| Walker [72] | II–IV | 521 | P 80 mg/m2 (D1,8,15), C 6 +BEV (D1) | 24.9 (M) | 75.5 (M) |
| Retrospective study, including phase II study | |||||
| Abaid [132] | III-IV | 88 | P 80 mg/m2 (D1,8,15), C 5 (D1), stop one week | 22.5 (M) | 31.5 (M) |
| Fleming [133] | III-IV | 33 | P 80 mg/m2 (D1,8,15), C 5 + BEV (D1) | 16.9-22.4 (M) | |
| Murphy [102] | III | 38 | P 80 mg/m2 (D1,8,15), C 5 (D1) | 31.3 (m) | 54.5 (m) |
| Boraska Jelavić [105] | I-IV | 43 | P 80 mg/m2 (D1,8,15), C 5 (D1) | 20-24 (M) | |
| Rettenmaier [78] | I-IV | 100 | P 80 mg/m2 (D1,8,15), C 5 (D1) | 27.6 (M) | |
| Cheng [101] | IIIC-IV | 32 | P 80 mg/m2 (D1,8,15), Cisplatin 20 mg/m2 (D1) | 27.0 (M) | 56 (m) |
| Current study | IIIC | 18 | P 80 mg/m2 (D1,8,15), Cisplatin 20 mg/m2 (D1) | 30.0 (M) | 58.5 (M) |
| 22 | P 80 mg/m2 (D1,8,15), C 5 (D1) | 25.0 (M) | 55.0 (M) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Cheng, M.; Lee, N.-R.; Huang, H.-Y.; Lee, W.-L.; Chang, W.-H.; Wang, P.-H. Comparing Paclitaxel–Carboplatin with Paclitaxel–Cisplatin as the Front-Line Chemotherapy for Patients with FIGO IIIC Serous-Type Tubo-Ovarian Cancer. Int. J. Environ. Res. Public Health 2020, 17, 2213. https://doi.org/10.3390/ijerph17072213
Huang C-Y, Cheng M, Lee N-R, Huang H-Y, Lee W-L, Chang W-H, Wang P-H. Comparing Paclitaxel–Carboplatin with Paclitaxel–Cisplatin as the Front-Line Chemotherapy for Patients with FIGO IIIC Serous-Type Tubo-Ovarian Cancer. International Journal of Environmental Research and Public Health. 2020; 17(7):2213. https://doi.org/10.3390/ijerph17072213
Chicago/Turabian StyleHuang, Chen-Yu, Min Cheng, Na-Rong Lee, Hsin-Yi Huang, Wen-Ling Lee, Wen-Hsun Chang, and Peng-Hui Wang. 2020. "Comparing Paclitaxel–Carboplatin with Paclitaxel–Cisplatin as the Front-Line Chemotherapy for Patients with FIGO IIIC Serous-Type Tubo-Ovarian Cancer" International Journal of Environmental Research and Public Health 17, no. 7: 2213. https://doi.org/10.3390/ijerph17072213
APA StyleHuang, C.-Y., Cheng, M., Lee, N.-R., Huang, H.-Y., Lee, W.-L., Chang, W.-H., & Wang, P.-H. (2020). Comparing Paclitaxel–Carboplatin with Paclitaxel–Cisplatin as the Front-Line Chemotherapy for Patients with FIGO IIIC Serous-Type Tubo-Ovarian Cancer. International Journal of Environmental Research and Public Health, 17(7), 2213. https://doi.org/10.3390/ijerph17072213






