Environmental Lead Exposure and Influenza and Respiratory Syncytial Virus Diagnoses in Young Children: A Test-Negative Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
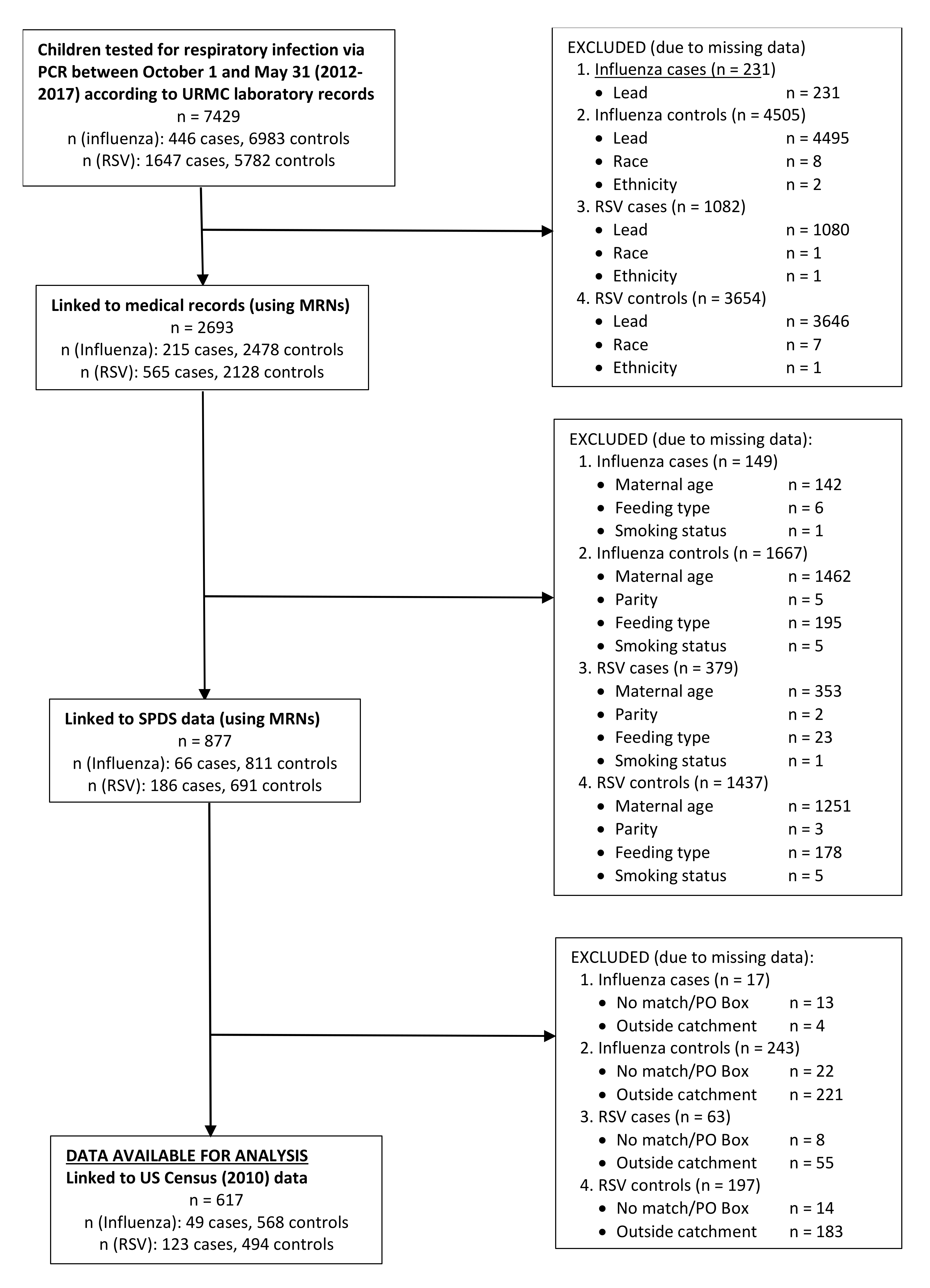
2.1. Case and Control Selection
2.2. Blood Lead Determination
2.3. Collection of All Other Covariables
2.4. Statistical Analyses
2.4.1. Descriptive Analyses
2.4.2. Analyses of Lead and RSV/Influenza
2.4.3. Secondary Analyses
2.4.4. Sensitivity Analyses
2.5. Vaccination Information
3. Results
3.1. Sample Characteristics
3.2. Blood Lead Distribution
3.3. Lead and RSV
3.4. Lead and Influenza
3.5. Secondary Analyses
3.6. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Children, the Flu, and the Flu Vaccine; CDCP: Atlanta, GA, USA, 2016. Available online: https://www.cdc.gov/flu/protect/children.htm (accessed on 5 January 2017).
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced Antibody Responses to Vaccinations in Children Exposed to Polychlorinated Biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Jusko, T.A.; De Roos, A.J.; Schwartz, S.M.; Lawrence, B.P.; Palkovicova, L.; Nemessanyi, T.; Drobna, B.; Fabisikova, A.; Kočan, A.; Sonneborn, D.; et al. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months of age. Environ. Res. 2010, 110, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Stølevik, S.B.; Nygaard, U.C.; Namork, E.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Knutsen, H.K.; Aaberge, I.; Vainio, K.; Van Loveren, H.; et al. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 2013, 51, 165–172. [Google Scholar] [CrossRef]
- Weisglas-Kuperus, N. Immunologic Effects of Background Prenatal and Postnatal Exposure to Dioxins and Polychlorinated Biphenyls in Dutch Infants. Pediatr. Res. 1995, 38, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Looker, C.; Luster, M.I.; Calafat, A.M.; Johnson, V.J.; Burleson, G.R.; Burleson, F.G.; Fletcher, T. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci. 2013, 138, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef]
- Cao, J.; Xu, X.; Hylkema, M.N.; Zeng, E.Y.; Sly, P.D.; Suk, W.A.; Bergman, Å.; Huo, X. Early-life Exposure to Widespread Environmental Toxicants and Health Risk: A Focus on the Immune and Respiratory Systems. Ann. Glob. Health 2016, 82, 119–131. [Google Scholar] [CrossRef]
- Li, S.; Zhengyan, Z.; Rong, L.; Hanyun, C. Decrease of CD4+ T-Lymphocytes in Children Exposed to Environmental Lead. Biol. Trace Element Res. 2005, 105, 19–26. [Google Scholar] [CrossRef]
- Lutz, P.M.; Wilson, T.J.; Ireland, J.; Jones, A.L.; Gorman, J.S.; Gale, N.L.; Johnson, J.C.; Hewett, J.E.; Reeves, A. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology 1999, 134, 63–78. [Google Scholar] [CrossRef]
- Sarasua, S.M.; Vogt, R.F.; Henderson, L.O.; Jones, P.A.; Lybarger, J.A. Serum immunoglobulins and lymphocyte subset distributions in children and adults living in communities assessed for lead and cadmium exposure. J. Toxicol. Environ. Health Part A 2000, 60, 1–15. [Google Scholar] [CrossRef]
- Sun, L.; Hu, J.; Zhao, Z.; Li, L.; Cheng, H. Influence of exposure to environmental lead on serum immunoglobulin in preschool children. Environ. Res. 2003, 92, 124–128. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Li, R.; Sun, L.; Li, Z.-Y.; Yang, R.-L. Effect of lead exposure on the immune function of lymphocytes and erythrocytes in preschool children. J. Zhejiang Univ. Sci. A 2004, 5, 1001–1004. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. How the Flu Virus Can Change: “Drift” and “Shift”. Available online: https://www.cdc.gov/flu/protect/children.htm (accessed on 5 January 2017).
- Dietert, R.; Piepenbrink, M.S. Lead and Immune Function. Crit. Rev. Toxicol. 2006, 36, 359–385. [Google Scholar] [CrossRef]
- Hurt, A.C.; Alexander, R.; Hibbert, J.; Deed, N.; Barr, I.G. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J. Clin. Virol. 2007, 39, 132–135. [Google Scholar] [CrossRef]
- New York State Department of Health. 67-1.2 Lead Screening and Follow-up of Children by Health Care Providers. In NYS Regulations for Lead Poisoning and Controls; 2009. Available online: https://www.health.ny.gov/regulations/nycrr/title_10/part_67/#sec67-1-2 (accessed on 4 October 2018).
- Allison, M.A.; Daley, M.F.; Crane, L.A.; Barrow, J.; Beaty, B.L.; Allred, N.; Berman, S.; Kempe, A. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003–2004 season. J. Pediatr. 2006, 149, 755–762. [Google Scholar] [CrossRef]
- Ampofo, K.; Gesteland, P.H.; Bender, J.; Mills, M.; Daly, J.; Samore, M.; Byington, C.L.; Pavia, A.T.; Srivastava, R. Epidemiology, Complications, and Cost of Hospitalization in Children with Laboratory-Confirmed Influenza Infection. Pediatrics 2006, 118, 2409–2417. [Google Scholar] [CrossRef]
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M. Live Attenuated versus Inactivated Influenza Vaccine in Infants and Young Children. N. Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef]
- Belshe, R.B.; Gruber, W.C.; Mendelman, P.M.; Mehta, H.B.; Mahmood, K.; Reisinger, K.; Treanor, J.; Zangwill, K.; Hayden, F.G.; Bernstein, D.I.; et al. Correlates of Immune Protection Induced by Live, Attenuated, Cold-Adapted, Trivalent, Intranasal Influenza Virus Vaccine. J. Infect. Dis. 2000, 181, 1133–1137. [Google Scholar] [CrossRef]
- Durani, Y.; Friedman, M.J.; Attia, M.W. Clinical predictors of respiratory syncytial virus infection in children. Pediatr. Int. 2008, 50, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, E.S.; Haber, M.; Chang, A.; Shope, T.; Teo, S.; Ginsberg, M.; Waecker, N.; Cox, N.J. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA 2000, 284, 1677–1682. [Google Scholar] [CrossRef]
- Ritzwoller, D.P.; Bridges, C.B.; Shetterly, S.; Yamasaki, K.; Kolczak, M.; France, E.K. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics 2005, 116, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A.F. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J. Pediatr. 2003, 143 (Suppl. S5), S118–S126. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Biomonitoring Summary. 2016. Available online: https://www.cdc.gov/biomonitoring/Lead_BiomonitoringSummary.html (accessed on 4 October 2018).
- Karmaus, W.; Brooks, K.R.; Nebe, T.; Witten, J.; Obi-Osius, N.; Kruse, H. Immune function biomarkers in children exposed to lead and organochlorine compounds: A cross-sectional study. Environ. Health 2005, 4, 5. [Google Scholar] [CrossRef]
- Dietert, R.R. New Developments in the Assessment of Developmental Immunotoxicology. J. Immunotoxicol. 2005, 2, 185–189. [Google Scholar] [CrossRef]
- Rodosthenous, R.; Burris, H.H.; Svensson, K.; Amarasiriwardena, C.J.; Cantoral, A.; Schnaas, L.; García, A.M.; Coull, B.; Wright, R.O.; Rojo, M.M.T.; et al. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environ. Int. 2017, 99, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Skerfving, S.; Löfmark, L.; Lundh, T.; Mikoczy, Z.; Strömberg, U. Late effects of low blood lead concentrations in children on school performance and cognitive functions. NeuroToxicology 2015, 49, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Kordas, K.; Golding, J.; Emond, A.M. Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. NeuroToxicology 2017, 62, 162–169. [Google Scholar] [CrossRef]
- Kostial, K.; Maljković, T.; Jugo, S. Lead acetate toxicity in rats in relation to age and sex. Arch. Toxicol. 1974, 31, 265–269. [Google Scholar] [CrossRef]
- Faith, R.E.; Luster, M.I.; A Kimmel, C. Effect of chronic developmental lead exposure on cell-mediated immune functions. Clin. Exp. Immunol. 1979, 35, 413–420. [Google Scholar] [PubMed]
- Canadian Cancer Society. The Thymus. Thymus Cancer. 2019. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/thymus/thymus-cancer/the-thymus/?region=on (accessed on 2 March 2018).
- Hu, H.; Rabinowitz, M.; Smith, D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: Conceptual paradigms. Environ. Health Perspect. 1998, 106, 1–8. [Google Scholar] [CrossRef]
- Wittmers, L.E.; Wallgren, J.; Alich, A.; Aufderheide, A.C.; Rapp, G. Lead in Bone. IV. Distribution of Lead in the Human Skeleton. Arch. Environ. Health Int. J. 1988, 43, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Muenchhoff, M.; Goulder, P.J.R. Sex Differences in Pediatric Infectious Diseases. J. Infect. Dis. 2014, 209 (Suppl. S3), S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Boule, L.A.; Lawrence, B.P. Influence of Early-Life Environmental Exposures on Immune Function Across the Life Span. In Environmental Influences on the Immune System; Springer: Vienna, Austria, 2016; pp. 21–54. [Google Scholar]
- Shah-Kulkarni, S.; Ha, M.; Kim, B.-M.; Kim, E.; Hong, Y.-C.; Park, H.; Kim, Y.; Kim, B.-N.; Chang, N.; Oh, S.-Y.; et al. Neurodevelopment in Early Childhood Affected by Prenatal Lead Exposure and Iron Intake. Medicine 2016, 95, e2508. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, W.; Hirota, Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine 2017, 35, 4796–4800. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Peak Lead <3 µg/dL (N = 499) n (%) | Peak Lead ≥3 µg/dL (N = 118) n (%) | p-Value 2 |
|---|---|---|---|
| Child characteristics | |||
| Age (months) | 0.8368 | ||
| 0.0–5.9 | 191 (38) | 47 (40) | |
| 6.0–11.9 | 119 (24) | 27 (23) | |
| 12.0–23.9 | 137 (27) | 29 (25) | |
| ≥24.0 | 52 (10) | 15 (13) | |
| Median age (months) | 9.1 | 8.0 | |
| Race | <0.0001 | ||
| White | 210 (42) | 32 (27) | |
| Black | 204 (41) | 80 (69) | |
| Other | 85 (17) | 6 (5) | |
| Ethnicity | 0.0824 | ||
| Non-Hispanic | 440 (88) | 97 (82) | |
| Hispanic | 59 (12) | 21 (18) | |
| Insurance | 0.4282 | ||
| Private | 377 (76) | 85 (72) | |
| Public/Self-Pay | 122 (24) | 33 (28) | |
| Sex | 0.1424 | ||
| Male | 270 (54) | 55 (47) | |
| Female | 229 (46) | 63 (53) | |
| Maternal Characteristics | |||
| Maternal Age (years) | <0.0001 | ||
| <20 | 27 (5) | 17 (14) | |
| 20–24 | 133 (27) | 42 (36) | |
| 25–30 | 143 (29) | 36 (31) | |
| ≥35 | 196 (39) | 23 (19) | |
| Median age (years) | 27.00 | 24.50 | |
| Parity | 0.3057 | ||
| 0 | 165 (33) | 44 (37) | |
| 1 | 179 (36) | 32 (27) | |
| 2 | 92 (18) | 27 (23) | |
| ≥3 | 63 (13) | 15 (13) | |
| Feeding Type During Hospitalization | 0.2484 | ||
| Formula only | 114 (23) | 19 (16) | |
| BM and Formula | 275 (55) | 73 (62) | |
| Breastmilk only | 110 (22) | 26 (22) | |
| Pre/Perinatal Smoker | 0.0383 | ||
| No | 417 (84) | 89 (75) | |
| Yes | 82 (16) | 29 (25) | |
| Area-Level Characteristics 3 | |||
| High Proportion Unemployment | 0.1357 | ||
| No | 258 (52) | 52 (44) | |
| Yes | 241 (48) | 66 (56) | |
| High Proportion Less than College Education | 0.0376 | ||
| No | 226 (45) | 41 (35) | |
| Yes | 273 (55) | 77 (65) | |
| High Proportion Below Poverty Level | 0.8910 | ||
| No | 253 (51) | 59 (50) | |
| Yes | 246 (49) | 59 (50) | |
| High Proportion Houses Built Before 1980 | 0.3279 | ||
| No | 266 (53) | 57 (48) | |
| Yes | 233 (47) | 61 (52) |
| Characteristics | With Blood Lead Values | Without Blood Lead Values | p-Value 2 |
|---|---|---|---|
| (N = 617) | (N = 661) | ||
| n (%) | n (%) | ||
| Child characteristics | |||
| Age (months) | <0.0001 | ||
| 0.0–5.9 | 191 (38) | 333 (50) | |
| 6.0–11.9 | 119 (24) | 143 (22) | |
| 12.0–23.9 | 137 (27) | 114 (17) | |
| ≥24.0 | 52 (10) | 71 (11) | |
| Median age (months) | 9.1 | 6 | |
| Race | <0.0001 | ||
| White | 210 (42) | 490 (74) | |
| Black | 204 (41) | 124 (19) | |
| Other | 85 (17) | 47 (7) | |
| Ethnicity | 0.0112 | ||
| Non-Hispanic | 440 (88) | 604 (91) | |
| Hispanic | 59 (12) | 57 (9) | |
| Insurance | 0.0647 | ||
| Private | 377 (76) | 524 (79) | |
| Public/Self-Pay | 122 (24) | 137 (21) | |
| Sex | 0.4102 | ||
| Male | 270 (54) | 364 (55) | |
| Female | 229 (46) | 297 (45) | |
| Maternal Characteristics | |||
| Maternal Age (years) | <0.0001 | ||
| <20 | 27 (5) | 21 (3) | |
| 20–24 | 133 (27) | 106 (16) | |
| 25–30 | 143 (29) | 161 (24) | |
| ≥35 | 196 (39) | 373 (56) | |
| Median age (years) | 27 | 30 | |
| Parity | 0.6794 | ||
| 0 | 165 (33) | 231 (35) | |
| 1 | 179 (36) | 208 (31) | |
| 2 | 92 (18) | 140 (21) | |
| ≥3 | 63 (13) | 82 (12) | |
| Feeding Type During Hospitalization | <0.0001 | ||
| Formula only | |||
| BM and Formula | 114 (23) | 219 (33) | |
| Breastmilk only | 275 (55) | 319 (48) | |
| 110 (22) | 123 (19) | ||
| Pre/Perinatal Smoker | 0.7529 | ||
| No | 417 (84) | 547 (83) | |
| Yes | 82 (16) | 114 (17) | |
| Area-Level Characteristics3 | |||
| High Proportion Unemployment | 0.0483 | ||
| No | 258 (52) | 295 (45) | |
| Yes | 241 (48) | 366 (55) | |
| High Proportion Less than College Education | 0.0003 | ||
| No | 226 (45) | 354 (54) | |
| Yes | 273 (55) | 307 (46) | |
| High Proportion Below Poverty Level | 0.1492 | ||
| No | 253 (51) | 306 (46) | |
| Yes | 246 (49) | 355 (54) | |
| High Proportion Houses Built Before 1980 | 0.94 | ||
| No | 266 (53) | 347 (53) | |
| Yes | 233 (47) | 314 (47) |
| Peak Lead | Larger Sample | Reduced Sample | ||
|---|---|---|---|---|
| 1 n = 558/2105 | 2 n = 123/494 | |||
| Crude OR | Adjusted OR 3 | Crude OR | Adjusted OR 4 | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| LOQ Split (1 µg/dL) | ||||
| <LOQ | 1 | 1 | 1 | 1 |
| ≥LOQ | 1.17 (0.97, 1.42) | 1.19 (0.98, 1.46) | 0.97 (0.65, 1.45) | 0.95 (0.60, 1.49) |
| 3 µg/dL cut-point | ||||
| <3 µg/dL | 1 | 1 | 1 | 1 |
| ≥3 µg/dL | 1.16 (0.92, 1.46) | 1.15 (0.90, 1.46) | 0.97 (0.58, 1.60) | 0.93 (0.53, 1.63) |
| Categories | ||||
| <1 µg/dL | 1 | 1 | 1 | 1 |
| ≥1 to <3 µg/dL | 1.15 (0.92, 1.44) | 1.18 (0.93, 1.49) | 0.97 (0.59, 1.60) | 0.97 (0.56, 1.66) |
| ≥3 µg/dL | 1.21 (0.95, 1.53) | 1.22 (0.94, 1.57) | 0.96 (0.57, 1.62) | 0.90 (0.50, 1.62) |
| Peak Lead | Larger Sample | Reduced Sample | ||
|---|---|---|---|---|
| 1 n = 209/2454 | 2 n = 49/568 | |||
| Crude OR | Adjusted OR 3 | Crude OR | Adjusted OR 4 | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| LOQ Split (1 µg/dL) | ||||
| <LOQ | 1 | 1 | 1 | 1 |
| ≥LOQ | 1.54 (1.16, 2.05) | 1.53 (1.13, 2.08) | 1.59 (0.89, 2.85) | 1.34 (0.65, 2.75) |
| 3 µg/dL cut-point | ||||
| <3.00 µg/dL | 1 | 1 | 1 | 1 |
| ≥3.00 µg/dL | 1.36 (0.97, 1.89) | 1.33 (0.93, 1.89) | 1.25 (0.62, 2.52) | 0.92 (0.40, 2.14) |
| Categories | ||||
| <1 µg/dL | 1 | 1 | 1 | 1 |
| ≥1 to <3 µg/dL | 1.53 (1.10, 2.15) | 1.51 (1.06, 2.16) | 1.70 (0.85, 3.39) | 1.52 (0.69, 3.37) |
| ≥3 µg/dL | 1.57 (1.09, 2.22) | 1.56 (1.07, 2.29) | 1.47 (0.70, 3.10) | 1.12 (0.45, 2.82) |
| RSV Adjusted OR1 (95% CI) | Influenza Adjusted OR 1 (95% CI) | |||
|---|---|---|---|---|
| Peak Lead | Males | Females | Males | Females |
| 2 n = 303/1141 | 2 n = 255/964 | 2 n = 116/1328 | 2 n = 93/1126 | |
| LOQ Split (1 µg/dL) | ||||
| <LOQ | 1 | 1 | 1 | 1 |
| ≥LOQ | 1.09 (0.82, 1.43) | 1.34 (0.99, 1.81) | 1.89 (1.25, 2.86) | 1.17 (0.74, 1.86) |
| 3 µg/dL cut-point | ||||
| <3.00 µg/dL | 1 | 1 | 1 | 1 |
| ≥3.00 µg/dL | 1.31 (0.95, 1.81) | 0.99 (0.68, 1.44) | 1.43 (0.90, 2.28) | 1.13 (0.65, 1.97) |
| Categories | ||||
| <1 µg/dL | 1 | 1 | 1 | 1 |
| ≥1 to <3 µg/dL | 0.93 (0.66, 1.31) | 1.48 (1.05, 2.07) | 1.93 (1.19, 3.12) | 1.15 (0.68, 1.96) |
| ≥3 µg/dL | 1.28 (0.91, 1.80) | 1.15 (0.77, 1.72) | 1.84 (1.11, 3.06) | 1.19 (0.66, 2.17) |
| Peak Lead | Influenza | No Influenza | Crude RR | Adjusted RR 2 (95% CI) | Adjusted RR 3 (95% CI) |
|---|---|---|---|---|---|
| N = 49 | N = 568 | (95% CI) | N = 49/568 | N = 49/568 | |
| n (%) | n (%) | 1 N = 49/568 | |||
| LOQ Split (1 µg/dL) | |||||
| <LOQ | 24 (49) | 343 (60) | 1 | 1 | 1 |
| ≥LOQ | 25 (51) | 225 (40) | 1.59 (0.89, 2.85) | 1.93 (1.02, 3.67) | 1.34 (0.65, 2.75) |
| 3 µg/dL cut-point | |||||
| <3.00 µg/dL | 38 (78) | 461 (81) | 1 | 1 | 1 |
| ≥3.00 µg/dL | 11(22) | 107 (19) | 1.25 (0.62, 2.52) | 1.42 (0.66, 2.04) | 0.92 (0.40, 2.14) |
| Categories | |||||
| <1 µg/dL | 24 (49) | 343 (60) | 1 | 1 | 1 |
| ≥1 to <3 µg/dL | 14 (29) | 118 (21) | 1.70 (0.85, 3.39) | 2.00 (0.96, 4.16) | 1.52 (0.69, 3.37) |
| ≥3 µg/dL | 11 (22) | 107 (19) | 1.47 (0.70, 3.10) | 1.84 (0.81, 1.11) | 1.12 (0.45, 2.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oktapodas Feiler, M.; Caserta, M.T.; van Wijngaarden, E.; Thevenet-Morrison, K.; Hardy, D.J.; Zhang, Y.V.; Dozier, A.M.; Lawrence, B.P.; Jusko, T.A. Environmental Lead Exposure and Influenza and Respiratory Syncytial Virus Diagnoses in Young Children: A Test-Negative Case-Control Study. Int. J. Environ. Res. Public Health 2020, 17, 7625. https://doi.org/10.3390/ijerph17207625
Oktapodas Feiler M, Caserta MT, van Wijngaarden E, Thevenet-Morrison K, Hardy DJ, Zhang YV, Dozier AM, Lawrence BP, Jusko TA. Environmental Lead Exposure and Influenza and Respiratory Syncytial Virus Diagnoses in Young Children: A Test-Negative Case-Control Study. International Journal of Environmental Research and Public Health. 2020; 17(20):7625. https://doi.org/10.3390/ijerph17207625
Chicago/Turabian StyleOktapodas Feiler, Marina, Mary T. Caserta, Edwin van Wijngaarden, Kelly Thevenet-Morrison, Dwight J. Hardy, Yan Victoria Zhang, Ann M. Dozier, B. Paige Lawrence, and Todd A. Jusko. 2020. "Environmental Lead Exposure and Influenza and Respiratory Syncytial Virus Diagnoses in Young Children: A Test-Negative Case-Control Study" International Journal of Environmental Research and Public Health 17, no. 20: 7625. https://doi.org/10.3390/ijerph17207625
APA StyleOktapodas Feiler, M., Caserta, M. T., van Wijngaarden, E., Thevenet-Morrison, K., Hardy, D. J., Zhang, Y. V., Dozier, A. M., Lawrence, B. P., & Jusko, T. A. (2020). Environmental Lead Exposure and Influenza and Respiratory Syncytial Virus Diagnoses in Young Children: A Test-Negative Case-Control Study. International Journal of Environmental Research and Public Health, 17(20), 7625. https://doi.org/10.3390/ijerph17207625






