Prenatal Exposure to Cigarette Smoke and Anogenital Distance at 4 Years in the INMA-Asturias Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Smoking Status
2.3. AGD
2.4. Urinary Cotinine Levels
2.5. Potential Confounders
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, J.M. Tobacco and pregnancy: Overview of exposures and effects. Birth Defects Res. Part C Embryo Today Rev. 2008, 84, 1–15. [Google Scholar] [CrossRef]
- Sunyer, J.; Garcia-Esteban, R.; Castilla, A.M.; Aurrekoetxea, J.J.; Iñiguez, C.; Tardón, A.; Espada, M.; Lertxundi, A.; Chatzi, L.; Rebagliato, M.; et al. Exposure to second-hand smoke and reproductive outcomes depending on maternal asthma. Eur. Respir. J. 2012, 40, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.L.; Britton, J.; Leonardi-Bee, J. Second hand smoke exposure and the risk of invasive meningococcal disease in children: Systematic review and meta-analysis. BMC Public Health 2012, 12, 1062. [Google Scholar] [CrossRef]
- Simonetti, G.D.; Schwertz, R.; Klett, M.; Hoffmann, G.F.; Schaefer, F.; Wühl, E. Determinants of Blood Pressure in Preschool Children. Circulation 2011, 123, 292–298. [Google Scholar] [CrossRef]
- Hur, K.; Liang, J.; Lin, S.Y. The role of secondhand smoke in allergic rhinitis: A systematic review. Int. Forum Allergy Rhinol. 2014, 4, 110–116. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Portal Estadístico del SNS. Encuesta Nacional de Salud de España Determinantes de Salud (Valores Porcentuales y Medias). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE17_MOD3_REL.pdf (accessed on 16 July 2020).
- Gould, G.S.; Havard, A.; Lim, L.L.; Kumar, R. The PSANZ Smoking in Pregnancy Expert Group the PSANZ Exposure to Tobacco, Environmental Tobacco Smoke and Nicotine in Pregnancy: A Pragmatic Overview of Reviews of Maternal and Child Outcomes, Effectiveness of Interventions and Barriers and Facilitators to Quitting. Int. J. Environ. Res. Public Health 2020, 17, 2034. [Google Scholar] [CrossRef]
- Jauniaux, E. Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet. Gynecol. 1999, 93, 25–29. [Google Scholar] [CrossRef]
- Ness, R.B.; Grisso, J.A.; Hirschinger, N.; Markovic, N.; Shaw, L.M.; Day, N.L.; Kline, J. Cocaine and Tobacco Use and the Risk of Spontaneous Abortion. N. Engl. J. Med. 1999, 340, 333–339. [Google Scholar] [CrossRef]
- Bruin, J.E.; Gerstein, H.C.; Holloway, A.C. Long-Term Consequences of Fetal and Neonatal Nicotine Exposure: A Critical Review. Toxicol. Sci. 2010, 116, 364–374. [Google Scholar] [CrossRef]
- England, L.J.; Kendrick, J.S.; Gargiullo, P.M.; Zahniser, S.C.; Hannon, W.H. Measures of Maternal Tobacco Exposure and Infant Birth Weight at Term. Am. J. Epidemiol. 2001, 153, 954–960. [Google Scholar] [CrossRef]
- Baird, D.D.; Wilcox, A.J. Future fertility after prenatal exposure to cigarette smoke. Fertil. Steril. 1986, 46, 368–372. [Google Scholar] [CrossRef]
- Augood, C.; Duckitt, K.; Templeton, A.A. Smoking and female infertility: A systematic review and meta-analysis. Hum. Reprod. 1998, 13, 1532–1539. [Google Scholar] [CrossRef]
- Ernst, A.; Kristensen, S.; Toft, G.; Thulstrup, A.; Håkonsen, L.; Olsen, S.; Ramlau-Hansen, C. Maternal smoking during pregnancy and reproductive health of daughters: A follow-up study spanning two decades. Hum. Reprod. 2012, 27, 3593–3600. [Google Scholar] [CrossRef]
- Burton, A. Does the Smoke Ever Really Clear? Thirdhand Smoke Exposure Raises New Concerns. Environ. Health Perspect. 2011, 119, A70–A74. [Google Scholar] [CrossRef] [PubMed]
- Matt, G.E.; Quintana, P.J.E.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand Tobacco Smoke: Emerging Evidence and Arguments for a Multidisciplinary Research Agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Vitali, M. The New Danger of Thirdhand Smoke: Why Passive Smoking Does Not Stop at Secondhand Smoke. Environ. Health Perspect. 2011, 119, A422. [Google Scholar] [CrossRef] [PubMed]
- Winickoff, J.P.; Friebely, J.; Tanski, S.E.; Sherrod, C.; Matt, G.E.; Hovell, M.F.; McMillen, R.C. Beliefs About the Health Effects of "Thirdhand" Smoke and Home Smoking Bans. Pediatrics 2009, 123, e74–e79. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Cotinine as a Biomarker of Environmental Tobacco Smoke Exposure. Epidemiol. Rev. 1996, 18, 188–204. [Google Scholar] [CrossRef]
- Brouwers, M.M.; Feitz, W.F.J.; Roelofs, L.A.J.; Kiemeney, L.A.L.M.; De Gier, R.P.E.; Roeleveld, N. Risk factors for hypospadias. Eur. J. Nucl. Med. Mol. Imaging 2006, 166, 671–678. [Google Scholar] [CrossRef]
- Jensen, T.K.; Jørgensen, N.; Punab, M.; Haugen, T.B.; Suominen, J.; Zilaitiene, B.; Horte, A.; Andersen, A.-G.; Carlsen, E.; Magnus, Ø.; et al. Association of In Utero Exposure to Maternal Smoking with Reduced Semen Quality and Testis Size in Adulthood: A Cross-Sectional Study of 1770 Young Men from the General Population in Five European Countries. Am. J. Epidemiol. 2004, 159, 49–58. [Google Scholar] [CrossRef][Green Version]
- Storgaard, L.; Bonde, J.P.; Ernst, E.; Spanô, M.; Andersen, C.Y.; Frydenberg, M.; Olsen, J. Does Smoking During Pregnancy Affect Sons’ Sperm Counts? Epidemiology 2003, 14, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ravnborg, T.L.; Jensen, T.K.; Andersson, A.-M.; Toppari, J.; Skakkebaek, N.E.; Jørgensen, N. Prenatal and adult exposures to smoking are associated with adverse effects on reproductive hormones, semen quality, final height and body mass index. Hum. Reprod. 2011, 26, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Kunstmann, J.M.; Czyglik, F.; Jouannet, P. Decline in Semen Quality among Fertile Men in Paris during the Past 20 Years. N. Engl. J. Med. 1995, 332, 281–285. [Google Scholar] [CrossRef]
- Swan, S.H.; Elkin, E.P.; Fenster, L. Have sperm densities declined? A reanalysis of global trend data. Environ. Health Perspect. 1997, 105, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Strohsnitter, W.C.; Hatch, E.E.; Hyer, M.; Troisi, R.; Kaufman, R.H.; Robboy, S.J.; Palmer, J.R.; Titus-Ernstoff, L.; Anderson, D.; Hoover, R.N.; et al. The Association between In Utero Cigarette Smoke Exposure and Age at Menopause. Am. J. Epidemiol. 2007, 167, 727–733. [Google Scholar] [CrossRef]
- Ye, X.; Skjaerven, R.; Basso, O.; Baird, D.D.; Eggesbo, M.; Uicab, L.A.C.; Haug, K.; Longnecker, M.P. In utero exposure to tobacco smoke and subsequent reduced fertility in females. Hum. Reprod. 2010, 25, 2901–2906. [Google Scholar] [CrossRef]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.B.; Ternand, C.L.; Sullivan, S.; et al. Decrease in Anogenital Distance among Male Infants with Prenatal Phthalate Exposure. Environ. Health Perspect. 2005, 113, 1056–1061. [Google Scholar] [CrossRef]
- Thankamony, A.; Ong, K.K.; Dunger, D.B.; Acerini, C.L.; Hughes, I.A. Anogenital Distance from Birth to 2 Years: A Population Study. Environ. Health Perspect. 2009, 117, 1786–1790. [Google Scholar] [CrossRef]
- Welsh, M.; Saunders, P.T.K.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Investig. 2008, 118, 1479–1490. [Google Scholar] [CrossRef]
- Dean, A.; Sharpe, R.M. Anogenital Distance or Digit Length Ratio as Measures of Fetal Androgen Exposure: Relationship to Male Reproductive Development and Its Disorders. J. Clin. Endocrinol. Metab. 2013, 98, 2230–2238. [Google Scholar] [CrossRef]
- Swan, S.H.; Sathyanarayana, S.; Barrett, E.S.; Janssen, S.; Liu, F.; Nguyen, R.H.N.; Redmon, J.B.; The TIDES Study Team; Scher, E.; Stasenko, M.; et al. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 2015, 30, 963–972. [Google Scholar] [CrossRef]
- Loreto-Gómez, C.; Farías, P.; Moreno-Macías, H.; Guzmán, C.; Riojas-Rodriguez, H. Prenatal exposure to persistent organic compounds and their association with anogenital distance in infants. Reprod. Biomed. Online 2018, 37, 732–740. [Google Scholar] [CrossRef] [PubMed]
- García-Villarino, M.; Riaño-Galán, I.; Rodriguez-Dehli, A.C.; Vizcaíno, E.; Grimalt, J.O.; Tardón, A.; Fernández-Somoano, A. Prenatal Exposure to Persistent Organic Pollutants and Anogenital Distance in Children at 18 Months. Horm. Res. Paediatr. 2018, 90, 116–122. [Google Scholar] [CrossRef]
- García-Villarino, M.; Riaño-Galán, I.; Rodríguez-Dehli, A.C.; Freire, C.; Vizcaíno, E.; Grimalt, J.O.; Tardón, A.; Fernández-Somoano, A. Association between pre/perinatal exposure to POPs and children’s anogenital distance at age 4 years: A study from the INMA-Asturias cohort. Int. J. Hyg. Environ. Health 2020, 229, 113563. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Liang, H.; Yang, F.; Yuan, W.; Chen, A.; Liu, X.; Ji, H.; Wen, S.; Miao, M. Prenatal polybrominated diphenyl ethers exposure and anogenital distance in boys from a Shanghai birth cohort. Int. J. Hyg. Environ. Health 2019, 222, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.S.; Sathyanarayana, S.; Mbowe, O.; Thurston, S.W.; Redmon, J.B.; Nguyen, R.H.; Swan, S.H. First-Trimester Urinary Bisphenol A Concentration in Relation to Anogenital Distance, an Androgen-Sensitive Measure of Reproductive Development, in Infant Girls. Environ. Health Perspect. 2017, 125, 077008. [Google Scholar] [CrossRef]
- Barrett, E.S.; Hoeger, K.M.; Sathyanarayana, S.; Abbott, D.H.; Redmon, J.B.; Nguyen, R.H.N.; Swan, S.H. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J. Dev. Orig. Health Dis. 2018, 9, 307–314. [Google Scholar] [CrossRef]
- Bornehag, C.-G.; Carlstedt, F.; Jönsson, B.A.; Lindh, C.H.; Jensen, T.K.; Bodin, A.; Jonsson, C.; Janson, S.; Swan, S.H. Prenatal Phthalate Exposures and Anogenital Distance in Swedish Boys. Environ. Health Perspect. 2015, 123, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, A.K.; Lambright, C.S.; Ostby, J.S.; Parks-Saldutti, L.; Vandenbergh, J.G.; Gray, L.E. Prenatal Testosterone Exposure Permanently Masculinizes Anogenital Distance, Nipple Development, and Reproductive Tract Morphology in Female Sprague-Dawley Rats. Toxicol. Sci. 2006, 96, 335–345. [Google Scholar] [CrossRef]
- Callegari, C.; Everett, S.; Ross, M.; Brasel, J.A. Anogenital ratio: Measure of fetal virilization in premature and full-term newborn infants. J. Pediatr. 1987, 111, 240–243. [Google Scholar] [CrossRef]
- Mira-Escolano, M.P.; Mendiola, J.; Mínguez-Alarcón, L.; Melgarejo, M.; Cutillas-Tolín, A.; Roca, M.; López-Espín, J.J.; Noguera-Velasco, J.A.; Torres-Cantero, A.M. Longer anogenital distance is associated with higher testosterone levels in women: A cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Roca, M.; Mínguez-Alarcón, L.; Mira-Escolano, M.-P.; López-Espín, J.J.; Barrett, E.S.; Swan, S.H.; Torres-Cantero, A.M. Anogenital distance is related to ovarian follicular number in young Spanish women: A cross-sectional study. Environ. Health 2012, 11, 90. [Google Scholar] [CrossRef]
- Mendiola, J.; Stahlhut, R.W.; Jørgensen, N.; Liu, F.; Swan, S.H. Shorter Anogenital Distance Predicts Poorer Semen Quality in Young Men in Rochester, New York. Environ. Health Perspect. 2011, 119, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Breyer, B.N.; Eisenberg, M.L.; Baskin, L.S. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr. Urol. Rep. 2008, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, A.; Lek, N.; Carroll, D.; Williams, M.; Dunger, D.B.; Acerini, C.L.; Ong, K.K.; Hughes, I.A. Anogenital Distance and Penile Length in Infants with Hypospadias or Cryptorchidism:Comparison with Normative Data. Environ. Health Perspect. 2014, 122, 207–211. [Google Scholar] [CrossRef]
- Gallavan, R.H.; Holson, J.F.; Stump, D.G.; Knapp, J.F.; Reynolds, V.L. Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reprod. Toxicol. 1999, 13, 383–390. [Google Scholar] [CrossRef]
- Sultan, C.; Balaguer, P.; Terouanne, B.; Georget, V.; Paris, F.; Jeandel, C.; Lumbroso, S.; Nicolas, J.C. Environmental xen-oestrogens, antiandrogens and disorders of male sexual differentiation. Mol. Cell. Endocrinol. 2001, 178, 99–105. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Hsieh, M.H.; Walters, R.C.; Krasnow, R.; Lipshultz, L.I. The Relationship between Anogenital Distance, Fatherhood, and Fertility in Adult Men. PLoS ONE 2011, 6, e18973. [Google Scholar] [CrossRef]
- Fowler, P.A.; Bhattacharya, S.; Flannigan, S.; Drake, A.J.; O’Shaughnessy, P.J. Maternal Cigarette Smoking and Effects on Androgen Action in Male Offspring: Unexpected Effects on Second-Trimester Anogenital Distance. J. Clin. Endocrinol. Metab. 2011, 96, E1502–E1506. [Google Scholar] [CrossRef]
- Fowler, P.A.; Filis, P.; Bhattacharya, S.; Le Bizec, B.; Antignac, J.-P.; Morvan, M.-L.; Drake, A.J.; Soffientini, U.; O’Shaughnessy, P.J. Human anogenital distance: An update on fetal smoke-exposure and integration of the perinatal literature on sex differences. Hum. Reprod. 2016, 31, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kızılay, D.Ö.; Aydın, C.; Aygün, A.P.; Tuhan, H.Ü.; Olukman, Ö. Prenatal smoke exposure is associated with increased anogenital distance in female infants: A prospective case-control study. J. Pediatr. Endocrinol. Metab. 2021, 34, 79–88. [Google Scholar] [CrossRef]
- Riaño-Galán, I.; Fernández-Somoano, A.; Rodríguez-Dehli, C.; Valvi, D.; Vrijheid, M.; Tardón, A. Proatherogenic Lipid Profile in Early Childhood: Association with Weight Status at 4 Years and Parental Obesity. J. Pediatr. 2017, 187, 153–157.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Somoano, A.; Tardon, A. Socioeconomic status and exposure to outdoor NO2and benzene in the Asturias INMA birth cohort, Spain. J. Epidemiol. Community Health 2013, 68, 29–36. [Google Scholar] [CrossRef]
- Fernández-Somoano, A.; Estarlich, M.; Ballester, F.; Fernández-Patier, R.; Aguirre-Alfaro, A.; Herce-Garraleta, M.D.; Tardón, A. Outdoor NO2 and benzene exposure in the INMA (Environment and Childhood) Asturias cohort (Spain). Atmos. Environ. 2011, 45, 5240–5246. [Google Scholar] [CrossRef]
- Aurrekoetxea, J.J.; Murcia, M.; Rebagliato, M.; López, M.J.; Castilla, A.M.; Santa-Marina, L.; Guxens, M.; Fernández-Somoano, A.; Espada, M.; Lertxundi, A.; et al. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: A cross-sectional study. BMJ Open 2013, 3, e002034. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, J.J.; Murcia, M.; Rebagliato, M.; Fernández-Somoano, A.; Castilla, A.M.; Guxens, M.; López, M.J.; Lertxundi, A.; Espada, M.; Tardón, A.; et al. Factors associated with second-hand smoke exposure in non-smoking pregnant women in Spain: Self-reported exposure and urinary cotinine levels. Sci. Total Environ. 2014, 470–471, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, J.J.; Murcia, M.; Rebagliato, M.; Guxens, M.; Fernández-Somoano, A.; López, M.J.; Lertxundi, A.; Castilla, A.M.; Espada, M.; Tardón, A.; et al. Second-hand smoke exposure in 4-year-old children in Spain: Sources, associated factors and urinary cotinine. Environ. Res. 2016, 145, 116–125. [Google Scholar] [CrossRef]
- Salazar-Martinez, E.; Romano-Riquer, P.; Yanez-Marquez, E.; Longnecker, M.P.; Hernandez-Avila, M. Anogenital distance in human male and female newborns: A descriptive, cross-sectional study. Environ. Health 2004, 3, 8. [Google Scholar] [CrossRef]
- Textor, J.; Van Der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2017, 45, 1887–1894. [Google Scholar] [CrossRef]
- Zielińska-Danch, W.; Wardas, W.; Sobczak, A.; Szołtysek-Bołdys, I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers 2007, 12, 484–496. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-01. [Google Scholar]
- Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004, 6, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.; Martínez, D.; Aurrekoetxea, J.J.; Estarlich, M.L.E.; Somoano, A.F.; Íñiguez, C.; Santa-Marina, L.; Tardón, A.; Torrent, M.; Sunyer, J.; et al. The association between passive and active tobacco smoke exposure and child weight status among Spanish children. Obesity 2016, 24, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, C.; Ballester, F.; Costa, O.; Murcia, M.; Souto, A.; Santa-Marina, L.; Aurrekoetxea, J.J.; Espada, M.; Vrijheid, M.; Alvarez-Avellón, S.M.; et al. Maternal Smoking During Pregnancy and Fetal Biometry. Am. J. Epidemiol. 2013, 178, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.; Toft, G.; Thulstrup, A.M.; Bonde, J.P.; Olsen, J. Cryptorchidism According to Maternal Gestational Smoking. Epidemiology 2007, 18, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.A.; Cassie, S.; Rhind, S.M.; Brewer, M.J.; Collinson, J.M.; Lea, R.G.; Baker, P.J.; Bhattacharya, S.; O’Shaughnessy, P.J. Maternal Smoking during Pregnancy Specifically Reduces Human Fetal Desert Hedgehog Gene Expression during Testis Development. J. Clin. Endocrinol. Metab. 2008, 93, 619–626. [Google Scholar] [CrossRef][Green Version]
- O’Shaughnessy, P.J.; Baker, P.J.; Monteiro, A.; Cassie, S.; Bhattacharya, S.; Fowler, P.A. Developmental Changes in Human Fetal Testicular Cell Numbers and Messenger Ribonucleic Acid Levels during the Second Trimester. J. Clin. Endocrinol. Metab. 2007, 92, 4792–4801. [Google Scholar] [CrossRef]
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of Gonadotropins and Gonadotropin Receptors: Elucidating the Physiology and Pathophysiology of Pituitary-Gonadal Function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef]
- Flück, C.E.; Meyer-Böni, M.; Pandey, A.V.; Kempná, P.; Miller, W.L.; Schoenle, E.J.; Biason-Lauber, A. Why Boys Will Be Boys: Two Pathways of Fetal Testicular Androgen Biosynthesis Are Needed for Male Sexual Differentiation. Am. J. Hum. Genet. 2011, 89, 201–218. [Google Scholar] [CrossRef]
- Fowler, P.A.; Flannigan, S.; Mathers, A.; Gillanders, K.; Lea, R.G.; Wood, M.J.; Maheshwari, A.; Bhattacharya, S.; Collie-Duguid, E.S.R.; Baker, P.J.; et al. Gene Expression Analysis of Human Fetal Ovarian Primordial Follicle Formation. J. Clin. Endocrinol. Metab. 2009, 94, 1427–1435. [Google Scholar] [CrossRef]
- Adibi, J.J.; Lee, M.K.; Naimi, A.I.; Barrett, E.; Nguyen, R.H.; Sathyanarayana, S.; Zhao, Y.; Thiet, M.-P.; Redmon, J.B.; Swan, S.H. Human Chorionic Gonadotropin Partially Mediates Phthalate Association with Male and Female Anogenital Distance. J. Clin. Endocrinol. Metab. 2015, 100, E1216–E1224. [Google Scholar] [CrossRef]
- Matikainen, T.; Perez, G.I.; Jurisicova, A.; Pru, J.K.; Schlezinger, J.J.; Ryu, H.-Y.; Laine, J.; Sakai, T.; Korsmeyer, S.J.; Casper, R.F.; et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 2001, 28, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; McIlwain, L.; Coutts, S.; Kinnell, H.L.; Fowler, P.A.; Childs, A.J. Activation of the aryl hydrocarbon receptor by a component of cigarette smoke reduces germ cell proliferation in the human fetal ovary. Mol. Hum. Reprod. 2014, 20, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Vääräsmäki, M.; Lehtinen, M.; Zeleniuch-Jacquotte, A.; Lundin, E.; Rodgers, K.-G.; Lakso, H.-A.; Chen, T.; Schock, H.; Hallmans, G.; et al. Determinants of Maternal Sex Steroids During the First Half of Pregnancy. Obstet. Gynecol. 2011, 118, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J.; Inoue, S.; Tamura, T.; Yamamoto, T.; Honjo, H.; Higashiyama, T.; Osawa, Y.; Okada, H. Cigarette smoking during pregnancy lowers aromatase cytochrome P-450 in the human placenta. J. Steroid Biochem. Mol. Biol. 1993, 45, 485–491. [Google Scholar] [CrossRef]
- Sarasin, A.; Schlumpf, M.; Müller, M.; Fleischmann, I.; Lauber, M.E.; Lichtensteiger, W. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reprod. Toxicol. 2003, 17, 153–162. [Google Scholar] [CrossRef]
- Jain, V.G.; Goyal, V.; Chowdhary, V.; Swarup, N.; Singh, R.J.; Singal, A.; Shekhawat, P. Anogenital distance is determined during early gestation in humans. Hum. Reprod. 2018, 33, 1619–1627. [Google Scholar] [CrossRef]
- Andres, R.L.; Day, M.-C. Perinatal complications associated with maternal tobacco use. Semin. Neonatol. 2000, 5, 231–241. [Google Scholar] [CrossRef]
- Freire, C.; Ocón-Hernández, O.; Dávila-Arias, C.; Pérez-Lobato, R.; Calvente, I.; Ramos, R.; Olea, N.; Fernández, M.F. Anogenital distance and reproductive outcomes in 9- to 11-year-old boys: The INMA-Granada cohort study. Andrology 2018, 6, 874–881. [Google Scholar] [CrossRef]
- González, P.F.; Pérez, A.P.; Guillén, J.P.; Calvete, J.F. Neumonectomía en fibrosis quística. An. Pediatría 2003, 58, 55–58. [Google Scholar] [CrossRef]

| Variables | Female Children (n = 180) | Male Children (n = 201) | ||||
|---|---|---|---|---|---|---|
| Child characteristics | n | % | Mean (SD) | n | % | Mean (SD) |
| AGD at 4 years (mm) | 180 | 17.00 (4.89) | 201 | 33.92 (11.37) | ||
| AGI at 4 years (mm/kg) | 180 | 0.96 (0.27) | 201 | 1.83 (0.63) | ||
| Birth weight (kg) | 180 | 3.17 (0.48) | 201 | 3.34 (0.45) | ||
| Birth length (cm) | 180 | 49.23 (2.18) | 201 | 49.99 (2.1) | ||
| Weight at 4 years (kg) | 180 | 17.91 (2.78) | 201 | 18.86 (2.98) | ||
| Height at 4 years (cm) | 180 | 104.67 (4.53) | 201 | 107.05 (4.45) | ||
| BMI at 4 years (kg/m2) | 180 | 16.29 (1.79) | 201 | 16.38 (1.79) | ||
| Maternal characteristics | ||||||
| Age (years) | 180 | 31.73 (4.19) | 201 | 31.96 (4.40) | ||
| Gestational age (week) | 180 | 39.57 (1.68) | 201 | 39.35 (1.52) | ||
| Pre-pregnancy BMI | 180 | 201 | ||||
| Underweight (<18.5 kg/m2) | 3 | 1.67 | 11 | 5.47 | ||
| Normal (18.5–24.9 kg/m2) | 122 | 67.78 | 129 | 64.18 | ||
| Overweight (25.0–29.9 kg/m2) | 43 | 23.89 | 42 | 20.90 | ||
| Obese (≥30 kg/m2) | 12 | 6.67 | 19 | 9.45 | ||
| Weight (kg) | 180 | 62.74 (11.11) | 201 | 62.33 (11.31) | ||
| Height (cm) | 180 | 162.62 (5.60) | 201 | 162.48 (5.61) | ||
| Weight gain (kg) | 176 | 13.36 (5.55) | 195 | 13.99 (4.8) | ||
| Education | 180 | 201 | ||||
| Primary | 32 | 17.78 | 32 | 15.92 | ||
| Secondary | 83 | 46.11 | 84 | 41.79 | ||
| University | 65 | 36.11 | 85 | 42.29 | ||
| Social class | 180 | 200 | ||||
| I–II (highest) | 35 | 19.44 | 54 | 27.00 | ||
| III | 37 | 20.56 | 44 | 22.00 | ||
| IV–V (lowest) | 108 | 60.00 | 102 | 51.00 | ||
| Parity | 180 | 201 | ||||
| One | 106 | 58.89 | 126 | 62.69 | ||
| Two | 65 | 36.11 | 68 | 33.83 | ||
| Three or more | 9 | 5.00 | 7 | 3.48 | ||
| Cotinine (ng/mL) | 167 | 301.16 (806.92) | 178 | 351.1 (838.64) | ||
| Cotinine | 167 | 178 | ||||
| <27 ng/ml | 129 | 77.25 | 132 | 74.16 | ||
| ≥27 ng/ml | 38 | 22.75 | 46 | 25.84 | ||
| Cigarettes/day at the beginning of pregnancy a | 172 | 11.92 (9.01) | 192 | 13.19 (10.04) | ||
| Smoking at the beginning of pregnancy | 172 | 192 | ||||
| No | 126 | 73.26 | 138 | 71.88 | ||
| Yes | 46 | 26.74 | 54 | 28.12 | ||
| Cigarettes/day at week 12 of pregnancy a | 169 | 6.74 (4.93) | 192 | 7.74 (6.62) | ||
| Smoking at week 12 of pregnancy | 171 | 192 | ||||
| No | 145 | 84.80 | 158 | 82.29 | ||
| Yes | 26 | 15.20 | 34 | 17.71 | ||
| Cigarettes/day at week 32 of pregnancy a | 172 | 6.83 (5.12) | 192 | 5.77 (3.61) | ||
| Smoking at week 32 of pregnancy | 172 | 192 | ||||
| No | 147 | 85.47 | 159 | 82.81 | ||
| Yes | 25 | 14.53 | 33 | 17.19 | ||
| Passive smoke exposure during pregnancy | 172 | 192 | ||||
| No exposure | 96 | 55.81 | 99 | 51.56 | ||
| One between home/work/rest/leisure | 53 | 30.81 | 69 | 35.94 | ||
| More than one between home/work/rest/leisure | 23 | 13.37 | 24 | 12.50 | ||
| Female Children (n = 180) | Male Children (n = 201) | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Active smoke exposure | ||||||
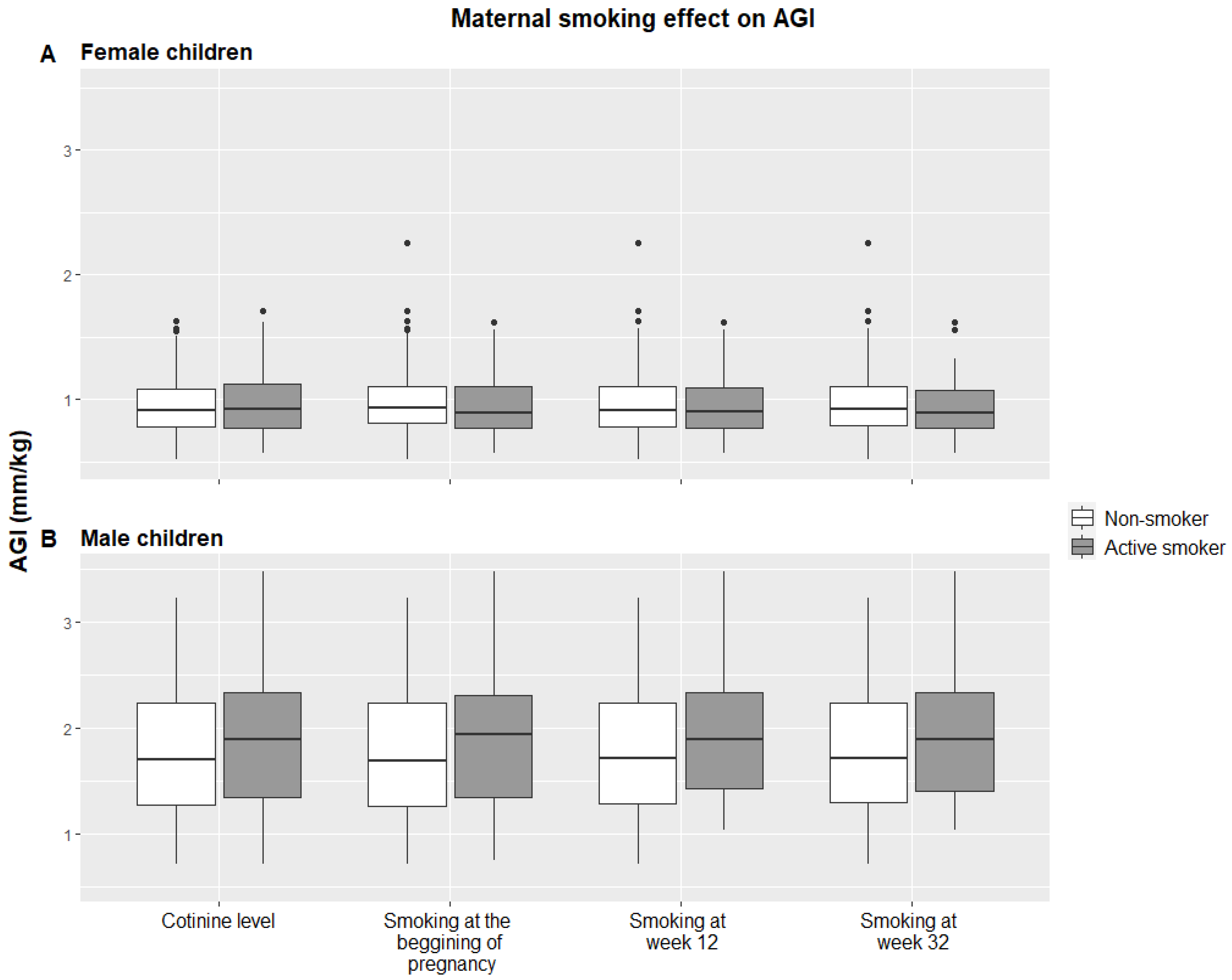
| Cotinine (continuous) 1 | 0.01 | (0.00, 0.03) | 0.07 | −0.01 | (−0.05, 0.03) | 0.66 |
| Cotinine (categorical) 2 | 0.06 | (−0.05, 0.17) | 0.30 | 0.11 | (−0.18, 0.36) | 0.45 |
| Cigarettes/day at the beginning of pregnancy 2 | 0.00 | (0.00, 0.01) | 0.54 | 0.01 | (−0.00, 0.03) | 0.17 |
| Smoking at the beginning of pregnancy 3 | 0.00 | (−0.10, 0.10) | 1.00 | 0.20 | (−0.05, 0.46) | 0.12 |
| Cigarettes/day at week 12 of pregnancy 2 | 0.00 | (−0.02, 0.02) | 0.90 | 0.02 | (−0.01, 0.04) | 0.27 |
| Smoking at week 12 of pregnancy 3 | 0.01 | (−0.12, 0.13) | 0.87 | 0.31 | (0.00, 0.63) | 0.05 |
| Cigarettes/day at week 32 of pregnancy 2 | 0.00 | (−0.02, 0.01) | 0.85 | 0.02 | (−0.03,0.06) | 0.46 |
| Smoking at week 32 of pregnancy 3 | 0.00 | (−0.13, 0.13) | 0.99 | 0.31 | (0.00, 0.63) | 0.05 |
| Passive smoke exposure 4 | ||||||
| One between home/work/rest/leisure | 0.04 | (−0.06, 0.14) | 0.45 | −0.13 | (−0.33, 0.08) | 0.23 |
| More than one between home/work/rest/leisure | 0.02 | (−0.11,0.15) | 0.81 | −0.02 | (−0.35, 0.32) | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Villarino, M.; Fernández-Iglesias, R.; Riaño-Galán, I.; Rodríguez-Dehli, C.; Babarro, I.; Fernández-Somoano, A.; Tardón, A. Prenatal Exposure to Cigarette Smoke and Anogenital Distance at 4 Years in the INMA-Asturias Cohort. Int. J. Environ. Res. Public Health 2021, 18, 4774. https://doi.org/10.3390/ijerph18094774
García-Villarino M, Fernández-Iglesias R, Riaño-Galán I, Rodríguez-Dehli C, Babarro I, Fernández-Somoano A, Tardón A. Prenatal Exposure to Cigarette Smoke and Anogenital Distance at 4 Years in the INMA-Asturias Cohort. International Journal of Environmental Research and Public Health. 2021; 18(9):4774. https://doi.org/10.3390/ijerph18094774
Chicago/Turabian StyleGarcía-Villarino, Miguel, Rocío Fernández-Iglesias, Isolina Riaño-Galán, Cristina Rodríguez-Dehli, Izaro Babarro, Ana Fernández-Somoano, and Adonina Tardón. 2021. "Prenatal Exposure to Cigarette Smoke and Anogenital Distance at 4 Years in the INMA-Asturias Cohort" International Journal of Environmental Research and Public Health 18, no. 9: 4774. https://doi.org/10.3390/ijerph18094774
APA StyleGarcía-Villarino, M., Fernández-Iglesias, R., Riaño-Galán, I., Rodríguez-Dehli, C., Babarro, I., Fernández-Somoano, A., & Tardón, A. (2021). Prenatal Exposure to Cigarette Smoke and Anogenital Distance at 4 Years in the INMA-Asturias Cohort. International Journal of Environmental Research and Public Health, 18(9), 4774. https://doi.org/10.3390/ijerph18094774








