The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Data Extraction
2.4. Assessment of Risk of Bias
2.5. Data Synthesis and Statistics
3. Results
3.1. Search Results
3.2. Characteristics of the Studies
3.3. Methodological Quality
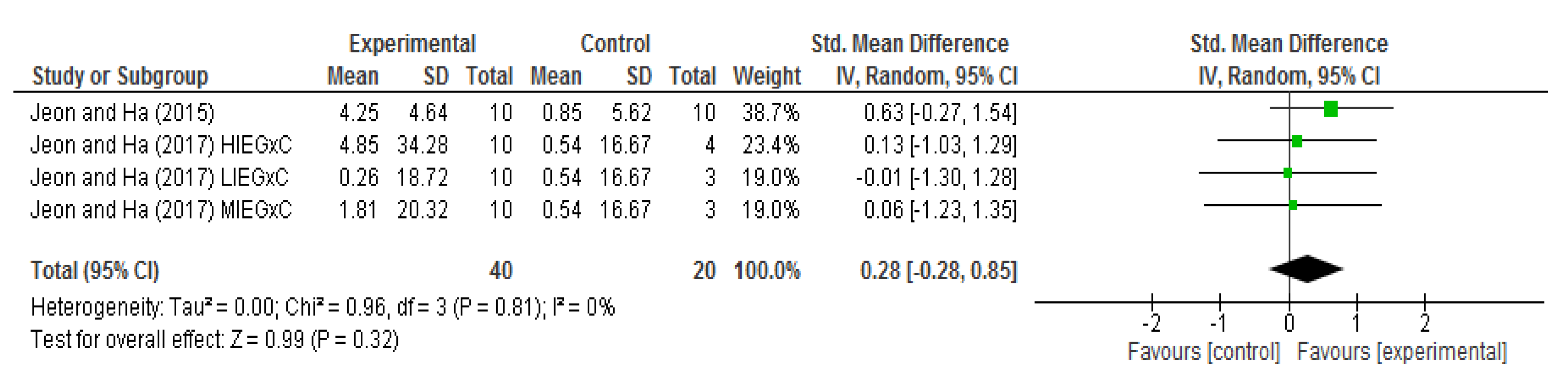
3.4. Meta-Analysis of Effects on BDNF Levels
4. Discussion
4.1. Practical Applications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSM | American College of Sports Medicine |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body mass index |
| HIEG | High-intensity aerobic exercise group |
| HR | Heart rate |
| HRmax | Maximum heart rate |
| LIEG | Low-intensity aerobic exercise group |
| MIEG | Moderate-intensity aerobic exercise group |
| Non-RCTs | Non-randomized controlled trials |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCTs | Randomized controlled trials |
| RM | Maximum resistance |
| RoB | Risk of bias |
| ROBINS-I | Risk of Bias in Non-Randomized Studies—of Interventions |
| SMD | Standardised mean differences |
| VO2max | Maximum oxygen consumption |
| VO2R | Reserve oxygen consumption |
References
- Blakemore, S.J.; Mills, K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Clin. Psychol. 2014, 65, 187–207. [Google Scholar] [CrossRef]
- Galván, A. Insights about adolescent behavior, plasticity, and policy from neuroscience research. Neuron 2014, 83, 262–265. [Google Scholar]
- Esteban-Cornejo, I.; Tejero-Gonzalez, C.M.; Sallis, J.F.; Veiga, O.L. Physical activity and cognition in adolescents: A systematic review. J. Sci. Med. Sport 2015, 18, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Goldfield, G.S.; Kenny, G.P.; Prud’homme, D.; Holcik, M.; Alberga, A.S.; Fahnestock, M.; Cameron, J.D.; Doucette, S.; Hadjiyannakis, S.; Tulloch, H.; et al. Effects of aerobic training, resistance training, or both on brain-derived neurotrophic factor in adolescents with obesity: The hearty randomized controlled trial. Physiol. Behav. 2018, 191, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Feter, N.; Alt, R.; Dias, M.G.; Rombaldi, A.J. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci. Sports 2019, 34, 293–304. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity—Exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Wilke, J.; Giesche, F.; Klier, K.; Vogt, L.; Herrmann, E.; Banzer, W. Acute Effects of Resistance Exercise on Cognitive Function in Healthy Adults: A Systematic Review with Multilevel Meta-Analysis. Sports Med. 2019, 49, 905–916. [Google Scholar] [CrossRef]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. Expression of brain-derived neurotrophic factor, IGF-1 and cortisol elicited by regular aerobic exercise in adolescents. J. Phys. Ther. Sci. 2015, 27, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [Green Version]
- Gunnell, K.E.; Poitras, V.J.; LeBlanc, A.; Schibli, K.; Barbeau, K.; Hedayati, N.; Ponitfex, M.B.; Goldfield, G.S.; Dunlap, C.; Lehan, E.; et al. Physical activity and brain structure, brain function, and cognition in children and youth: A systematic review of randomized controlled trials. Ment. Health Phys. Act. 2019, 16, 105–127. [Google Scholar] [CrossRef]
- Li, J.W.; O’Connor, H.; O’Dwyer, N.; Orr, R. The effect of acute and chronic exercise on cognitive function and academic performance in adolescents: A systematic review. J. Sci. Med. Sport 2017, 20, 841–848. [Google Scholar] [CrossRef]
- Moreau, D.; Chou, E. The Acute Effect of High-Intensity Exercise on Executive Function: A Meta-Analysis. Perspect. Psychol. Sci. 2019, 14, 734–764. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Yang, Y.; Huang, T. Effects of chronic exercise interventions on executive function among children and adolescents: A systematic review with meta-analysis. Br. J. Sports Med. 2019, 53, 1397. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Esteban-Cornejo, I.; Brown, B.; Bender, C.M.B.; Erickson, K.I. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020, 1–11. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, K.P.M.; Segundo, V.H.O.; Medeiros, G.C.B.S.; Mata, A.N.S.; García, D.A.; Leitão, J.C.G.C.; Knackfuss, M.I.; Piuvezam, G. Effects of exercise on the levels of BDNF and executive function in adolescents: A protocol for systematic review and meta-analysis. Medicine 2019, 98, e16445. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsley, T.; Dingwall, O.; Sampson, M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst. Rev. 2011, 8, MR000026. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Review Manager (RevMan) [Computer Program]; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014.
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; (updated July 2019); John Wiley & Sons: Cochrane, AB, Canada, 2019; Available online: www.training.cochrane.org/handbook (accessed on 31 May 2020).
- Kim, H.T.; Song, Y.E.; Kang, E.B.; Cho, J.Y.; Kim, B.W.; Kim, C.H. The effects of combined exercise on basic physical fitness, neurotrophic factors and working memory of elementary students. Exerc. Sci. 2015, 24, 243–251. [Google Scholar]
- Lee, S.S.; Yoo, J.H.; Kang, S.; Woo, J.H.; Shin, K.O.; Kim, K.B.; Cho, S.Y.; Roh, H.T.; Kim, Y.I.I. The Effects of 12 Weeks Regular Aerobic Exercise on Brain-derived Neurotrophic Factor and Inflammatory Factors in Juvenile Obesity and Type 2 Diabetes Mellitus. J. Phys. Ther. Sci. 2014, 26, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.M.; Kim, C.H. Effects of Combined Exercise on Body Composition, Blood Lipids, and BDNF in Obese Adolescents. J. Life Sci. 2012, 22, 1231–1236. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Maldonado, A.; Álvarez-Buylla, E.R.; Montero, S.; Melnikov, V.; Castro-Rodríguez, E.; Gamboa-Domínguez, A.; Rodríguez-Hernández, A.; Lemus, M.; Murguía, J.M. Chronic exercise increases plasma brain-derived neurotrophic factor levels, pancreatic islet size, and insulin tolerance in a TrkB-dependent manner. PLoS ONE 2014, 9, e115177. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Coelho, F.G.M.; Gobbi, S.; Andreatto, C.A.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduróz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Herold, F.; Müller, P.; Gronwald, T.; Müller, N.G. Dose–Response Matters!—A Perspective on the Exercise Prescription in Exercise–Cognition Research. Front. Psychol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Müllers, P.; Taubert, M.; Müller, N.G. Physical Exercise as Personalized Medicine for Dementia Prevention? Front. Physiol. 2019, 10, 2–5. [Google Scholar] [CrossRef]
- Rehfeld, K.; Lüders, A.; Hökelmann, A.; Lessmann, V.; Kaufmann, J.; Brigadski, T.; Müller, P.; Müller, N.G. Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS ONE 2018, 13, e0196636. [Google Scholar] [CrossRef] [Green Version]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höfler, M.; Hoyer, J. Population size matters: Bias in conventional meta-analysis. Int. J. Soc. Res. Methodol. 2014, 17, 585–597. [Google Scholar] [CrossRef]
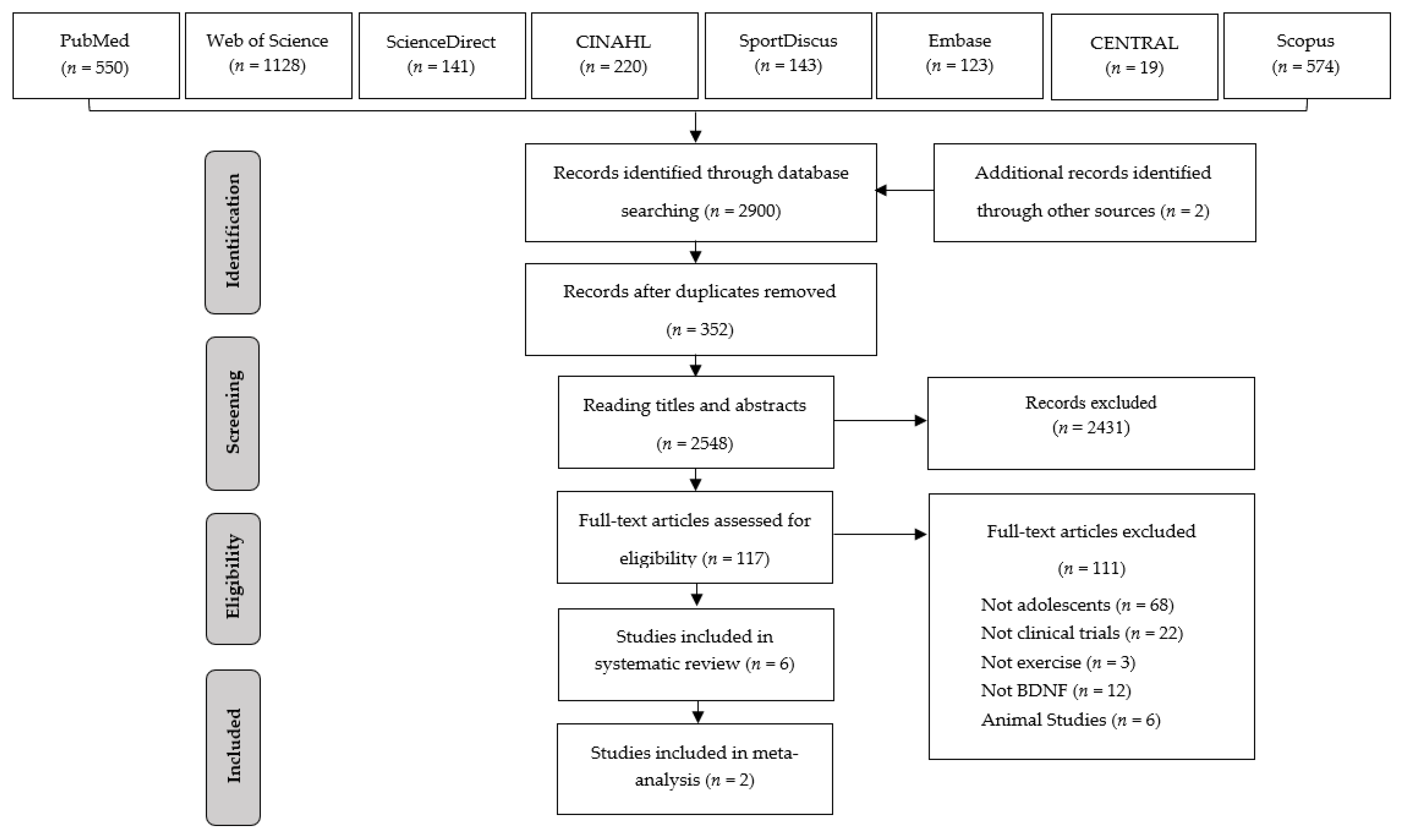
| Author (Year) | Country | Design | Participants | BMI | Training Status |
|---|---|---|---|---|---|
| Goldfield et al. (2018) [4] | Canada | RCT (Parallel-group) | 282 (84 boys, 198 girls) Aerobic: n = 69 (15.5 ± 1.3 y) Resistance: n = 70 (15.8 ± 1.5 y) Combined: n = 74 (15.5 ± 1.3 y) Control: n = 69 (15.6 ± 1.3 y) | Aerobic = 34.6 ± 4.2
Resistance = 35.3 ± 4.8 Combined = 34.5 ± 4.1 Control = 34.3 ± 5.0 | Irregularly active |
| Jeon and Ha (2015) [15] | South Korea | RCT (Parallel-group) | 20 (boys) Exercise: n = 10 (15 y) Control: n = 10 (15 y) | Exercise = 19.8 ± 4.6
Control = 18.9 ± 3.8 | Irregularly active |
| Jeon and Ha (2017) [16] | South Korea | RCT (Parallel-group) | 40 (boys) Low-intensity: n = 10 (15.06 ± 0.7 y) Moderate-intensity: n = 10 (15.47 ± 0.8 y) High-intensity: n = 10 (15.15 ± 0.3 y) SG: n = 10 (15.05 ± 0.4 y) | LIEG = 18.05 ± 1.88 MIEG = 17.15 ± 2.01 HIEG = 18.34 ± 2.54 SG = 19.35 ± 1.44 | Sedentary |
| Kim et al. (2015) [33] | South Korea | RCT (Parallel-group) | 30 boys Combined group: n = 15 (10.93 ± 0.3 y) Control group: n = 15 (11.00 ± 0.0 y) | Combined group = 17.90 ± 2.09 Control group = 18.15 ± 1.47 | ND |
| Lee et al. (2014) [34] | South Korea | Non-RCT (Parallel-group) | 19 (15 boys, 4 girls) Obesity group: n = 8 (16.3 ± 0.9 y) Control group: n = 11 (16.4 ± 1.4 y) | Obesity group = 27.47 ± 2.51 Control group = 22.35 ± 3.94 | ND |
| Shim and Kim (2012) [35] | South Korea | Non-RCT (Parallel-group) | 18 boys Exercise group: n= 9 (13.0 ± 0.71 y) Control group: n= 9 (12.78 ± 0.83 y) | Exercise group: 27.0 ± 2.39 Control group: 27.0 ± 2.88 | Sedentary |
| Author (Year) | Follow-Up | Intervention | Control | Outcomes | Main Results |
|---|---|---|---|---|---|
| Goldfield et al. (2018) RCT [4] | 6 months (4 days per week) | (1) Aerobic (20–45 min, 65–85% HRmax) (2) Resistance (20–45 min, exercises with 2 × 15 progressing to 3 × 8, 8RM) (3) Combined (Complete aerobic training program plus resistance training program during each session) | The control group received only dietary counselling with no exercise prescription | Serum BDNF (ng/mL) | No significant within- or between-group changes in BDNF Aerobic group (△ = +1.80) Resistance group (△ = −2.00) Combined group (△ = −1.70) |
| Jeon and Ha 2015) RCT [15] | 8 weeks (3 days per week) | Exercise was performed on treadmills and the exercise intensity was set between 40% and 60%VO2R | The control group were asked to continue their daily normal and sedentary activities | Serum BDNF (pg/mL) | The exercise group showed a significant increase in BDNF levels (p < 0.001) Exercise (△ = +4.25) Control (△ = +0.85) |
| Jeon and Ha (2017) RCT [16] | 12 weeks (4 days per week) | (1) Low-intensity aerobic exercise (43.34 ± 3.59 min, 40% VO2R) (2) Moderate-intensity aerobic exercise (33.33 ± 3.64 min, 55% VO2R) (3) High-intensity aerobic exercise (25.76 ± 2.10 min, 70% VO2R) | The SG performed whole-body stretching for 30 min at the same time, frequency and location as the aerobic exercise groups. | Serum BDNF (ng/mL) | The MIEG (p < 0.05) and HIEG (p < 0.01) groups showed a significant increase in BDNF levels LIEG (△ = +0.26) MIEG (△ = +1.81) HIEG (△ = +4.85) SG (△ = +0.54) |
| Kim et al. (2015) RCT [33] | 12 weeks (5 days per week) | Combined exercise group (CEG): taekwondo movement-based exercise program (60 min, 50–80% HR) | No exercise | Serum BDNF (ng/mL) | The CEG showed improvements in BDNF levels, but there was no significant difference between the groups Combined group (△ = +2.58) Control group (△ = +0.07) |
| Lee et al. (2014) Non-RCT [34] | 12 weeks (3 days per week) | (1) Obesity group: aerobic exercise (40–60 min, 40–60% VO2max) | No exercise | Serum BDNF (pg/mL) | Only GO showed a significant increase in BDNF levels (p < 0.05) Obesity group (△ = +11.62) Control group (△ = −0.51) |
| Shim and Kim (2012) Non-RCT [35] | 12 weeks (3 days per week) | (1) Exercise group (20–25 min, 55–75% HRmax) and resistance (20–45 min, exercises with 3 × 8 progressing to 3 × 15, 50–70% 1RM) | No exercise | Serum BDNF (ng/mL) | The EG showed improvements in BDNF levels, but there was no significant difference between the groups Exercise group (△ = +2.58) Control group (△ = +0.07) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, K.P.M.d.; de Oliveira, V.H.; Medeiros, G.C.B.S.d.; Mata, Á.N.d.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. https://doi.org/10.3390/ijerph17176056
Azevedo KPMd, de Oliveira VH, Medeiros GCBSd, Mata ÁNdS, García DÁ, Martínez DG, Leitão JC, Knackfuss MI, Piuvezam G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(17):6056. https://doi.org/10.3390/ijerph17176056
Chicago/Turabian StyleAzevedo, Kesley Pablo Morais de, Victor Hugo de Oliveira, Gidyenne Christine Bandeira Silva de Medeiros, Ádala Nayana de Sousa Mata, Daniel Ángel García, Daniel Guillén Martínez, José Carlos Leitão, Maria Irany Knackfuss, and Grasiela Piuvezam. 2020. "The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 17: 6056. https://doi.org/10.3390/ijerph17176056
APA StyleAzevedo, K. P. M. d., de Oliveira, V. H., Medeiros, G. C. B. S. d., Mata, Á. N. d. S., García, D. Á., Martínez, D. G., Leitão, J. C., Knackfuss, M. I., & Piuvezam, G. (2020). The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health, 17(17), 6056. https://doi.org/10.3390/ijerph17176056






