Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Setup
2.3. Experimental Procedure
3. Results and Discussion
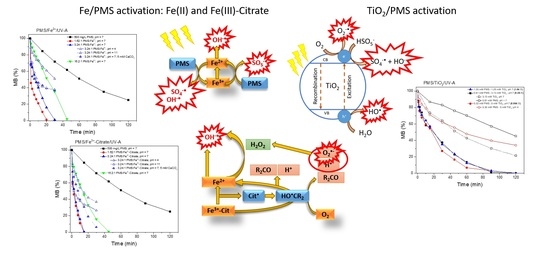
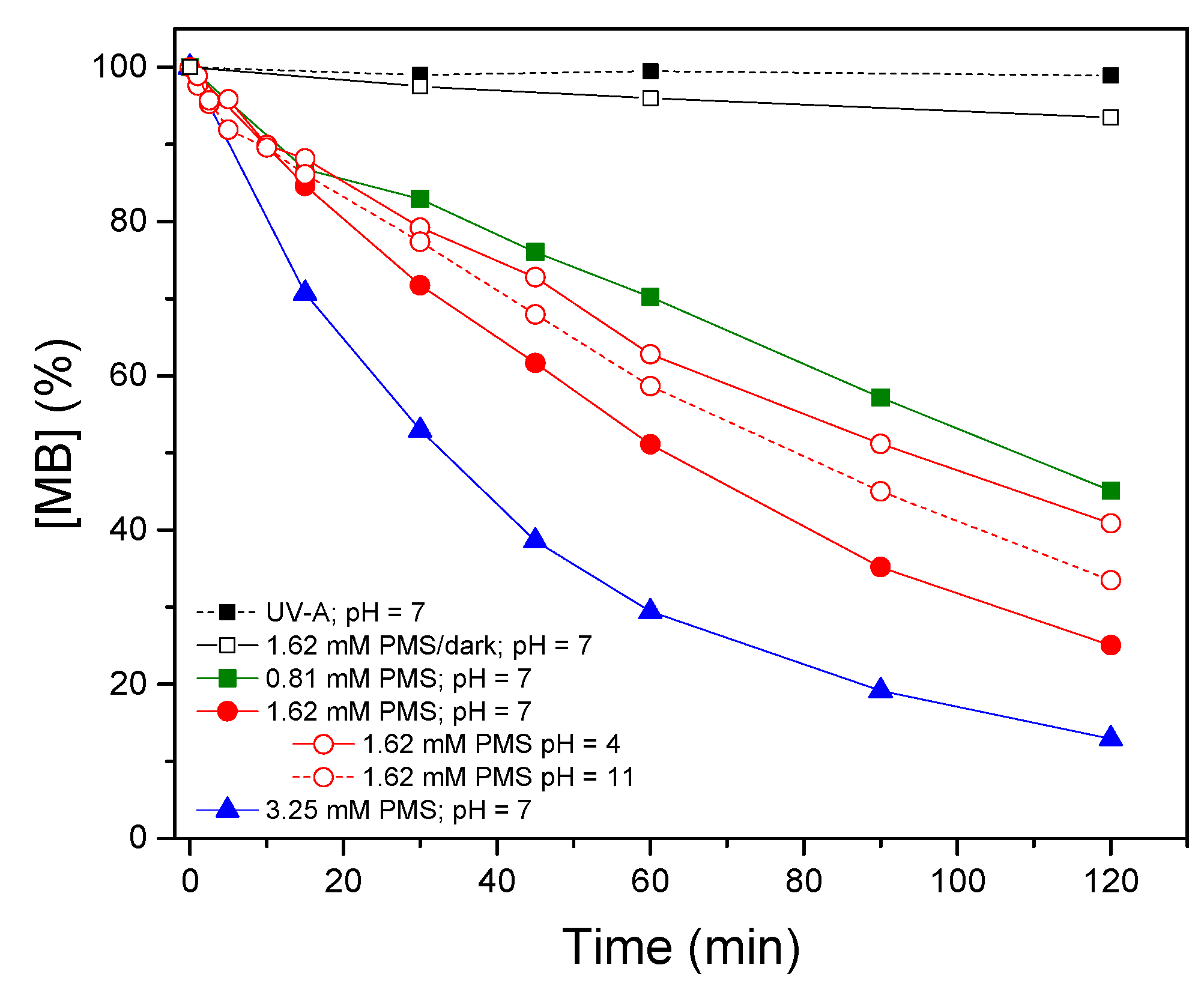
3.1. Photolytic Activation of Peroxymonosulfate and TiO2 as a Benchmark
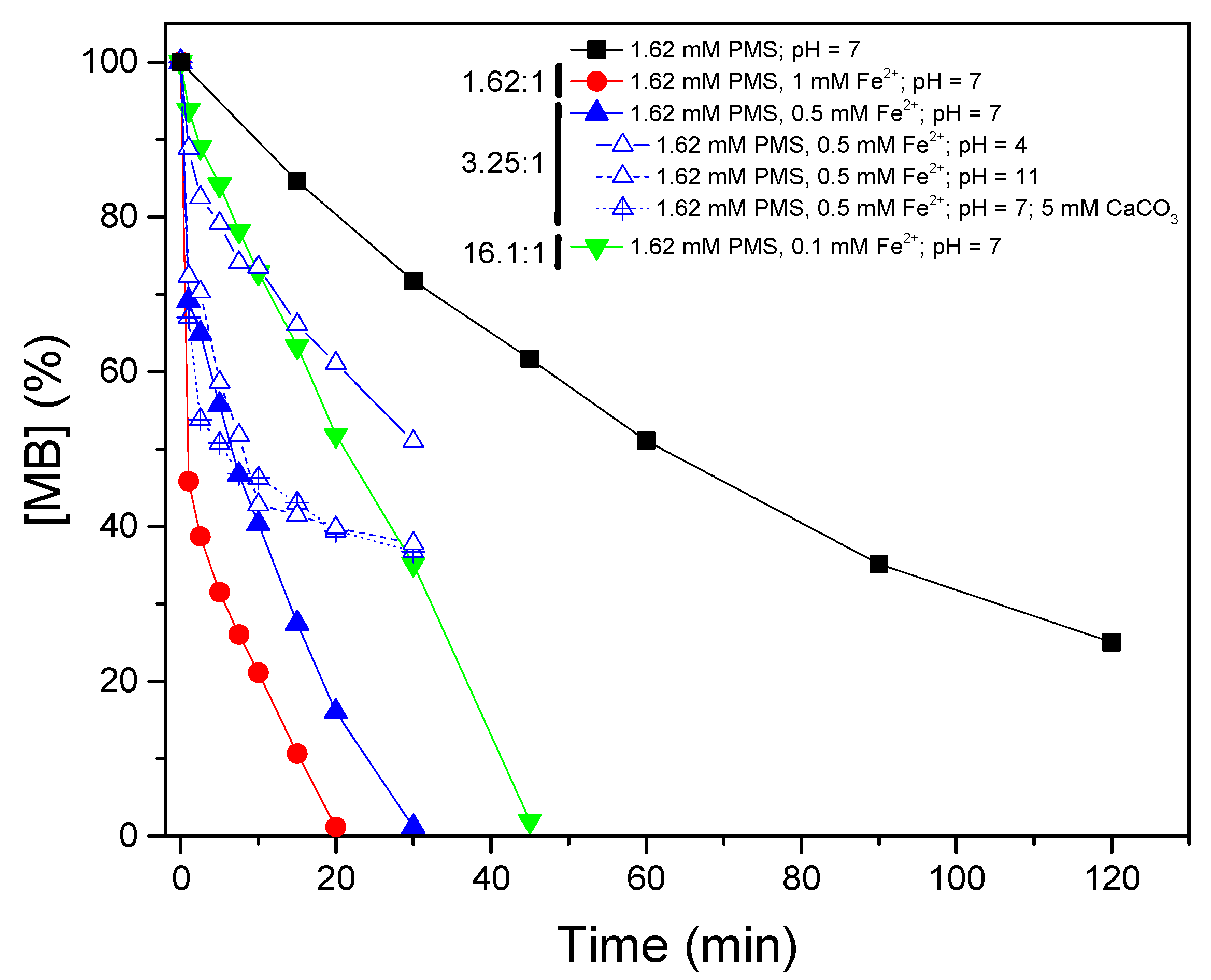
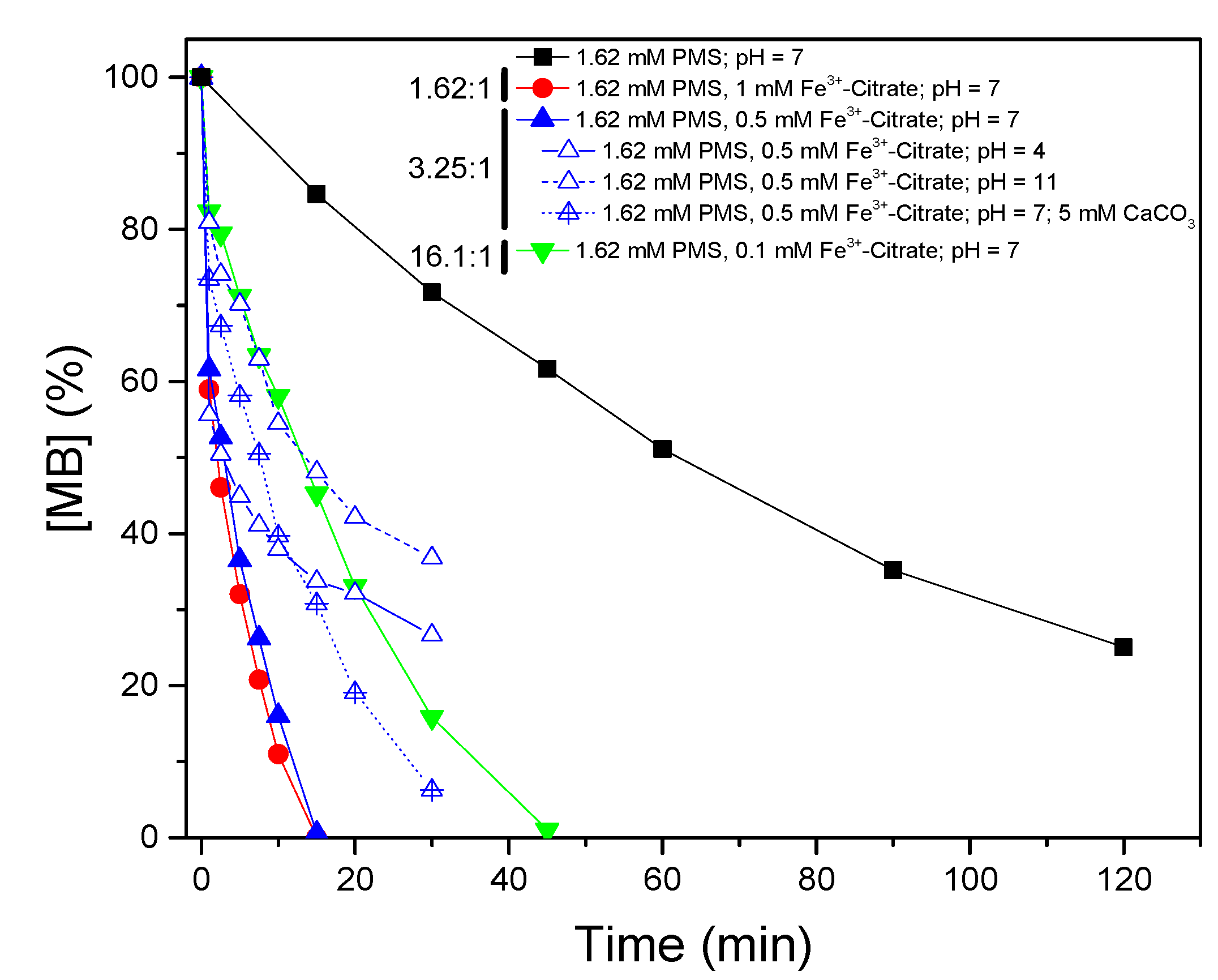
3.2. Photocatalytic Activation of Peroxymonosulfate by Iron Species
3.3. Peroxymonosulfate/TiO2/UV-A Synergistic Efficiency for Methylene Blue Removal
4. Conclusions
- Low dosages of PMS (0.81–3.25 mM) are enough to remove organic pollutants like MB. Activation of PMS by UV-A radiation increases the removal rate, reducing the contact time to reach complete removal of pollutants.
- The pH plays an important role. Neutral conditions reached the best efficiencies, while efficiency was reduced at acidic (pH 4) and basic (pH 11) conditions as a consequence of scavenger reactions.
- Photocatalytic activation of PMS with iron species accelerates the generation of sulfate radicals and therefore the MB removal. The molar ratio of PMS:Fe is crucial, because an excess of PMS scavenges the oxidant radicals.
- The use of Fe3+-citrate as a catalyst showed the best performance, reaching total removal of MB in 15 min of reaction. The reason is related to the generation of hydrogen peroxide (H2O2) and superoxide radical (O2−) by the photolysis of Fe3+-citrate. In addition, the variations in pH do not affect the reaction because of the complexation of Fe3+, which avoids the formation of oxyhydroxides of Fe3+.
- PMS is successfully activated by TiO2, reaching the total removal of MB in 90 min. The PMS activation occurs because of the reaction with the free electron on the surface of TiO2, where there is no influence of the molar ratio of PMS:TiO2 (1:04:1; 0.26:1; 0.064:1).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Fisher, S.; Suk, W.A.; Sly, P.; Chiles, T.C.; Bose-O’Reilly, S. Pollution and children’s health. Sci. Total Environ. 2019, 650, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment, and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef]
- Nzila, A.; Razzak, S.A.; Zhu, J. Bioaugmentation: An emerging stratey of industrial wastewater treatment for reuse and discharge. Int. J. Environ. Res. Public Health 2016, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jiang, C.; Lin, Z.; Zou, Z. Microwave-hydrothermal treated grape peel as an efficient biosorbent for methylene blue removal. Int. J. Environ. Res. Public Health 2018, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, L.; Meehan, B.J.; Jones, O.A.H. Regulated and emerging disinfection by-products in recycled waters. Sci. Total Environ. 2018, 637–638, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, L.; Wang, Z.; Lu, X.; Zhou, Q. Using Fe(III)-coagulant-modified colloidal gas aphrons to remove bio-recalcitrant dissolved organic matter and colorants from cassava distillery wastewater. Bioresour. Technol. 2018, 268, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; García-Cañibano, C.; Lepistö, R.-J.; Encinas, Á.; Pellinen, J.; Marugán, J. Intensification of UV-C tertiary treatment: Disinfection and removal of micropollutants by sulfate radical based Advanced Oxidation Processes. J. Hazard. Mater. 2018. [Google Scholar] [CrossRef]
- Shalla, A.H.; Bhat, M.A.; Yaseen, Z. Hydrogels for removal of recalcitrant organic dyes: A conceptual overview. J. Environ. Chem. Eng. 2018, 6, 5938–5949. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016, 320, 469–478. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Kinetics and mechanism for transparent polyethylene-TiO2 films mediated self-cleaning leading to MB dye discoloration under sunlight irradiation. Appl. Catal. B Environ. 2015, 162, 236–244. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Mediano, A.; Pueyo, N.; García-Suescun, I.; Mosteo, R.; Ormad, M.P. Degradation of chloroform by Fenton-like treatment induced by electromagnetic fields: A case of study. Chem. Eng. Sci. 2016, 156, 89–96. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Marjanovic, M.; Giannakis, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Effect of μM Fe addition, mild heat and solar UV on sulfate radical-mediated inactivation of bacteria, viruses, and micropollutant degradation in water. Water Res. 2018, 140, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Amor, C.; Silva, T.; Dionysiou, D.D.; Li Puma, G.; Lucas, M.S.; Peres, J.A. Treatment of winery wastewater by sulphate radicals: HSO5−/transition metal/UV-A LEDs. Chem. Eng. J. 2017, 310, 473–483. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, C.; Moon, G.; Lee, J.; An, T.; Choi, W. Activation of peroxymonosulfate on visible light irradiated TiO2 via a charge transfer complex path. Chem. Eng. J. 2018, 346, 249–257. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Xiao, H.; Hong, C.; Zhu, F.; Yao, Y.; Xue, Z. Accelerated TiO2 photocatalytic degradation of Acid Orange 7 under visible light mediated by peroxymonosulfate. Chem. Eng. J. 2012, 193–194, 290–295. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Ghanbari, F.; Yousefi, M.; Madihi-Bidgoli, S. Photocatalytic degradation of food dye by Fe3O4–TiO2 nanoparticles in presence of peroxymonosulfate: The effect of UV sources. J. Environ. Chem. Eng. 2017, 5, 2459–2468. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Xie, R.; Ji, J.; Guo, K.; Lei, D.; Fan, Q.; Leung, D.Y.C.; Huang, H. Wet scrubber coupled with UV/PMS process for efficient removal of gaseous VOCs: Roles of sulfate and hydroxyl radicals. Chem. Eng. J. 2019, 356, 632–640. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Moreira, S.I.; Lucas, M.S.; Fernandes, J.R.; Tavares, P.B.; Sampaio, A.; Peres, J.A. Disinfection of simulated and real winery wastewater using sulphate radicals: Peroxymonosulphate/transition metal/UV-A LED oxidation. J. Clean. Prod. 2017, 149, 805–817. [Google Scholar] [CrossRef]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Pi, Y.; Feng, J.; Sun, J.; Sun, J. Facile, effective, and environment-friendly degradation of sulfamonomethoxine in aqueous solution with the aid of a UV/Oxone oxidative process. Environ. Sci. Pollut. Res. 2013, 20, 8621–8628. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Amor, C.; Mota, J.; Lucas, M.S.; Peres, J.A. Oxidation of winery wastewater by sulphate radicals: Catalytic and solar photocatalytic activations. Environ. Sci. Pollut. Res. 2017, 24, 22414–22426. [Google Scholar] [CrossRef] [PubMed]
- Bandala, E.R.; Peláez, M.A.; Dionysiou, D.D.; Gelover, S.; Garcia, J.; Macías, D. Degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) using cobalt-peroxymonosulfate in Fenton-like process. J. Photochem. Photobiol. A Chem. 2007, 186, 357–363. [Google Scholar] [CrossRef]
- Fernandez, J.; Maruthamuthu, P.; Kiwi, J. Photobleaching and mineralization of Orange II by oxone and metal-ions involving Fenton-like chemistry under visible light. J. Photochem. Photobiol. A Chem. 2004, 161, 185–192. [Google Scholar] [CrossRef]
- Sun, Y.; Pignatello, J.J. Evidence for a surface dual hole-radical mechanism in the titanium dioxide photocatalytic oxidation of 2,4-D. Environ. Sci. Technol. 1995, 29, 2065–2072. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, J.; Qu, R.; Feng, M.; Wang, Z. Degradation of octafluorodibenzo-p-dioxin by UV/Fe(II)/potassium monopersulfate system: Kinetics, influence of coexisting chemicals, degradation products and pathways. Chem. Eng. J. 2017, 319, 98–107. [Google Scholar] [CrossRef]
- Sun, J.; Song, M.; Feng, J.; Pi, Y. Highly efficient degradation of ofloxacin by UV/Oxone/Co2+ oxidation process. Environ. Sci. Pollut. Res. 2011, 19, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Fang, C.; Liu, W.; Lou, X.; Liu, J.J. Importance of reagent addition order in contaminant degradation in an Fe(II)/PMS system. RSC Adv. 2016, 6, 70271–70276. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, D.; Dai, D.; Yang, Z.; Chen, L.; Liu, Q.; He, J.; Yao, Y. Synergistic effects of persistent free radicals and visible radiation on peroxymonosulfate activation by ferric citrate for the decomposition of organic contaminants. Appl. Catal. B. Environ. 2017, 205, 404–411. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, D.; Fan, C.; Shang, C. A Fe(II)/citrate/UV/PMS process for carbamazepine degradation at a very low Fe(II)/PMS ratio and neutral pH: The mechanisms. Water Res. 2017, 124, 446–453. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, D.; Fang, J.; Fan, C.; Shang, C. A novel Fe(II)/citrate/UV/peroxymonosulfate process for micropollutant degradation: Optimization by response surface methodology and effects of water matrices. Chemosphere 2017, 184, 417–428. [Google Scholar] [CrossRef]
- Spuhler, D.; Rengifo-Herrera, J.A.; Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl. Catal. B Environ. 2010, 96, 126–141. [Google Scholar] [CrossRef]

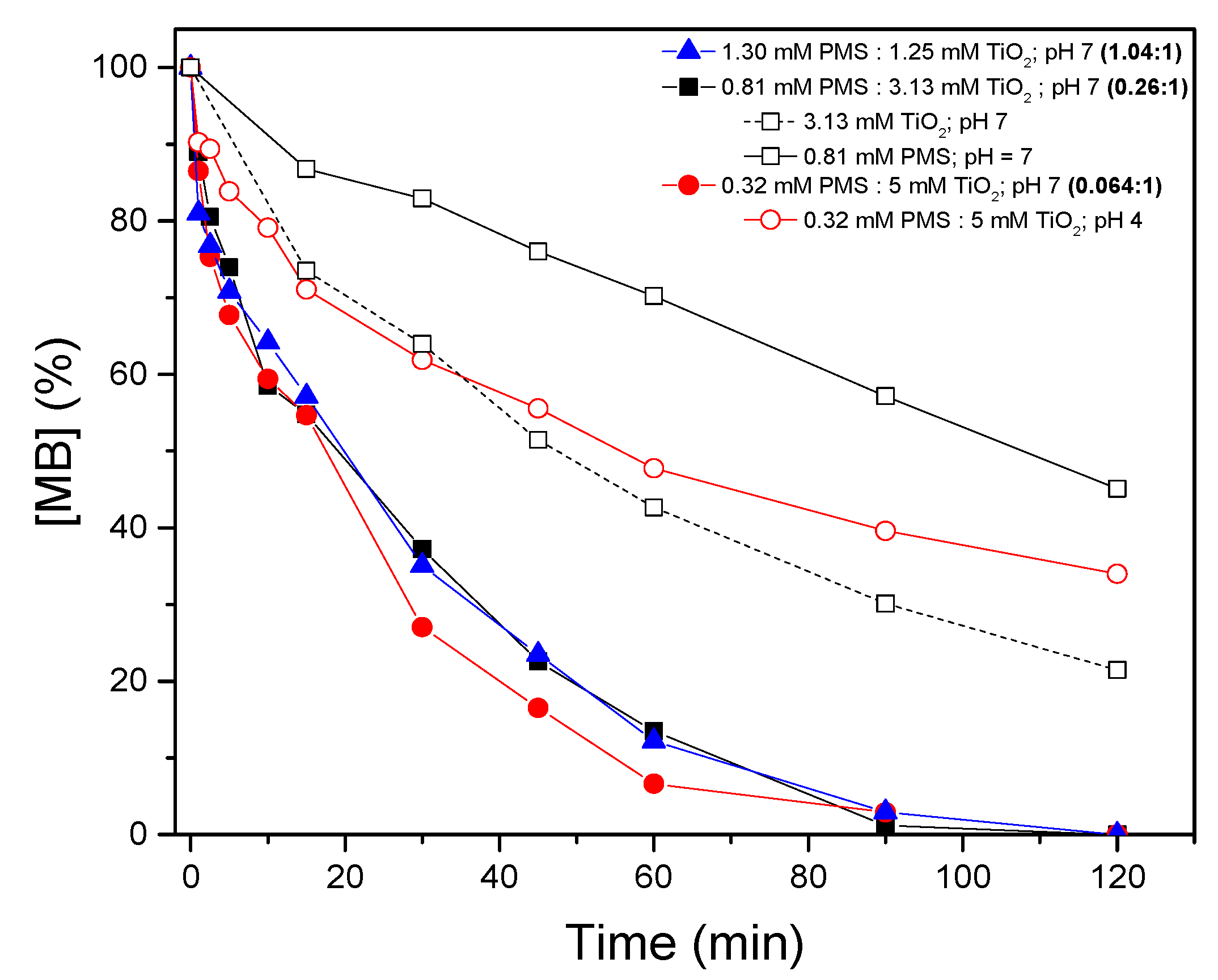
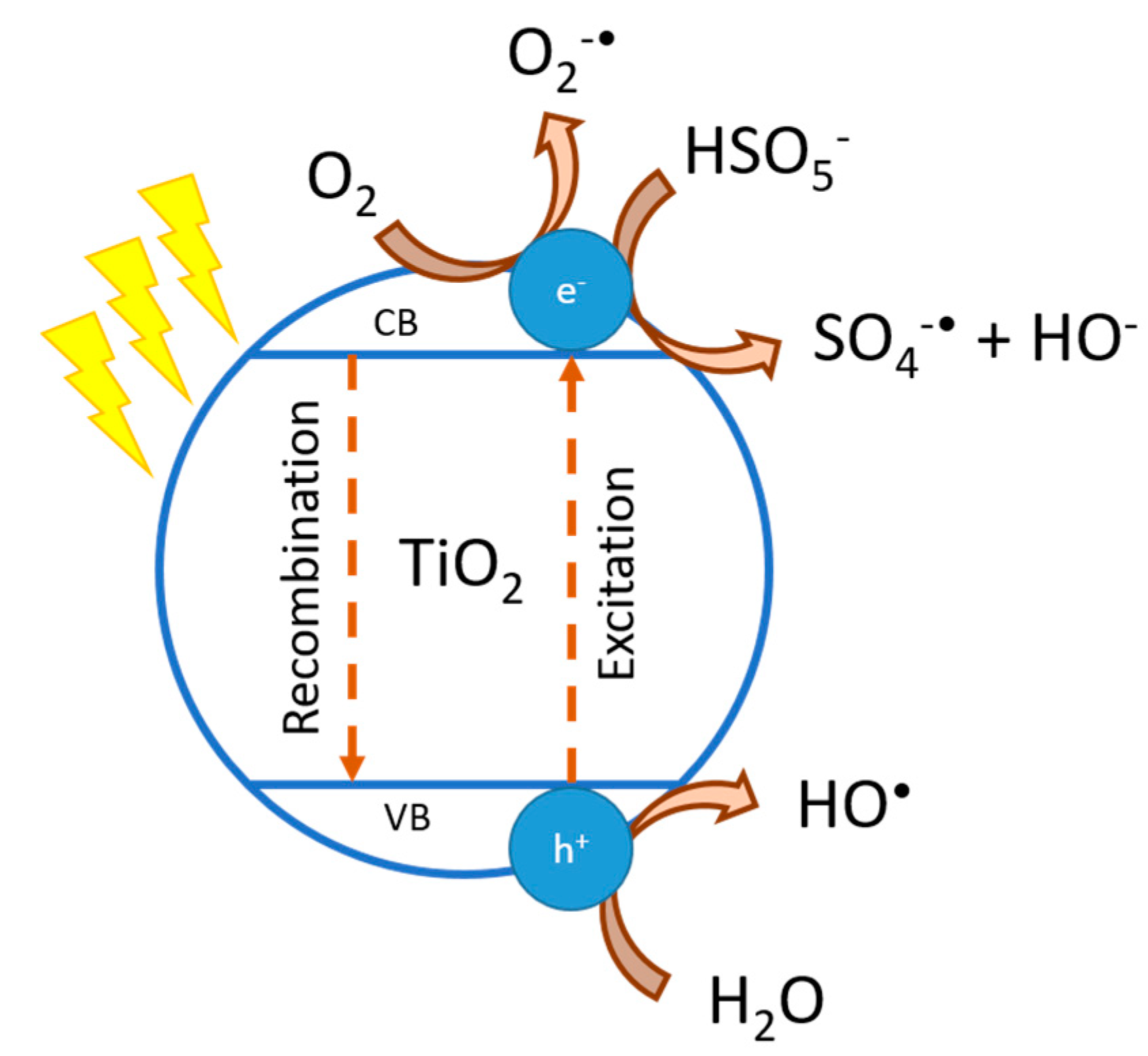
| Experimental Conditions | pH | Rate Constant (min−1) | R2 |
|---|---|---|---|
| 0.81 mM PMS/UV-A | 7 | 0.006 | 0.99 |
| 1.62 mM PMS/UV-A | 7 | 0.011 | 0.99 |
| 1.62 mM PMS/UV-A | 4 | 0.008 | 0.99 |
| 1.62 mM PMS/UV-A | 11 | 0.009 | 0.99 |
| 3.25 mM PMS/UV-A | 7 | 0.018 | 0.98 |
| 3.16 mM TiO2/UV-A | 7 | 0.013 | 0.98 |
| 3.16 mM TiO2/UV-A | 4 | 0.011 | 0.67 |
| 6.26 mM TiO2/UV-A | 7 | 0.020 | 0.98 |
| 12.52 mM TiO2/UV-A | 7 | 0.025 | 0.94 |
| Experimental Conditions | pH | Rate Constant (min−1) | R2 |
|---|---|---|---|
| 1.62 mM PMS/1 mM Fe2+/UV-A | 7 | 0.165 | 0.70 |
| 1.62 mM PMS/0.5 mM Fe2+/UV-A | 7 | 0.092 | 0.94 |
| 1.62 mM PMS/0.5 mM Fe2+/UV-A + 5 mM CaCO3 | 7 | 0.092 | 0.82 |
| 1.62 mM PMS/0.5 mM Fe2+/UV-A | 4 | N.F. | |
| 1.62 mM PMS/0.5 mM Fe2+/UV-A | 11 | 0.025 | 0.82 |
| 1.62 mM PMS/0.1 mM Fe2+/UV-A | 7 | 0.034 | 0.99 |
| 1.62 mM PMS/1 mM Fe3+-Citrate/UV-A | 7 | 0.223 | 0.95 |
| 1.62 mM PMS/0.5 mM Fe3+-Citrate /UV-A | 7 | 0.188 | 0.94 |
| 1.62 mM PMS/0.5 mM Fe3+-Citrate /UV-A + 5 mM CaCO3 | 7 | 0.065 | 0.80 |
| 1.62 mM PMS/0.5 mM Fe3+-Citrate /UV-A | 4 | 0.088 | 0.98 |
| 1.62 mM PMS/0.5 mM Fe3+-Citrate /UV-A | 11 | N.F. | |
| 1.62 mM PMS/0.1 mM Fe3+-Citrate /UV-A | 7 | 0.059 | 0.98 |
| Experimental Conditions | pH | Rate Constant (min−1) | R2 |
|---|---|---|---|
| 1.30 mM PMS/1.25 mM TiO2/UV-A | 7 | 0.035 | 0.97 |
| 0.81 mM PMS/3.16 mM TiO2/UV-A | 7 | 0.034 | 0.97 |
| 0.32 mM PMS/5 mM TiO2/UV-A | 7 | 0.044 | 0.98 |
| 0.32 mM PMS/5 mM TiO2/UV-A | 4 | 0.014 | 0.87 |
| Experiment | Experimental Conditions | First-Order Rate Constant (min−1) | Synergistic Factor |
|---|---|---|---|
| 1 | 0.81 mM PMS/UV-A | 0.006 | 1.79 |
| 2 | 3.16 mM TiO2/UV-A | 0.013 | |
| 3 | 0.81:3.16 mM PMS:TiO2/UV-A | 0.034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Chueca, J.; Alonso, E.; Singh, D.N. Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study. Int. J. Environ. Res. Public Health 2019, 16, 198. https://doi.org/10.3390/ijerph16020198
Rodríguez-Chueca J, Alonso E, Singh DN. Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study. International Journal of Environmental Research and Public Health. 2019; 16(2):198. https://doi.org/10.3390/ijerph16020198
Chicago/Turabian StyleRodríguez-Chueca, Jorge, Esther Alonso, and Devendra Narain Singh. 2019. "Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study" International Journal of Environmental Research and Public Health 16, no. 2: 198. https://doi.org/10.3390/ijerph16020198
APA StyleRodríguez-Chueca, J., Alonso, E., & Singh, D. N. (2019). Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study. International Journal of Environmental Research and Public Health, 16(2), 198. https://doi.org/10.3390/ijerph16020198