Evaluation of Wastewater Treatment by Microcosms of Vertical Subsurface Wetlands in Partially Saturated Conditions Planted with Ornamental Plants and Filled with Mineral and Plastic Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Field of Study
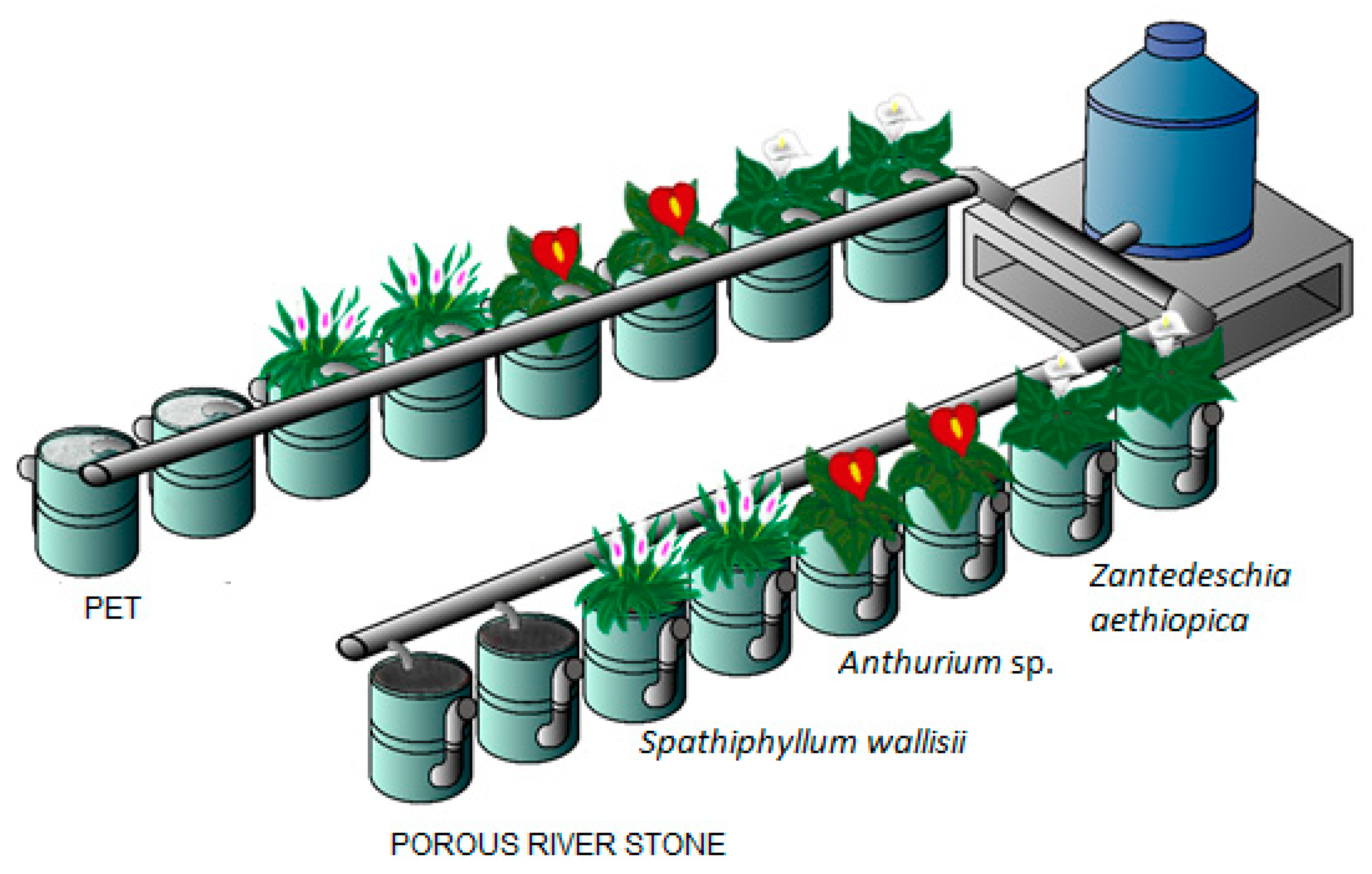
2.2. Characteristics of the FD-CWs
2.3. Experimental Design
2.4. Sampling and Analysis
2.5. Statistical Analysis
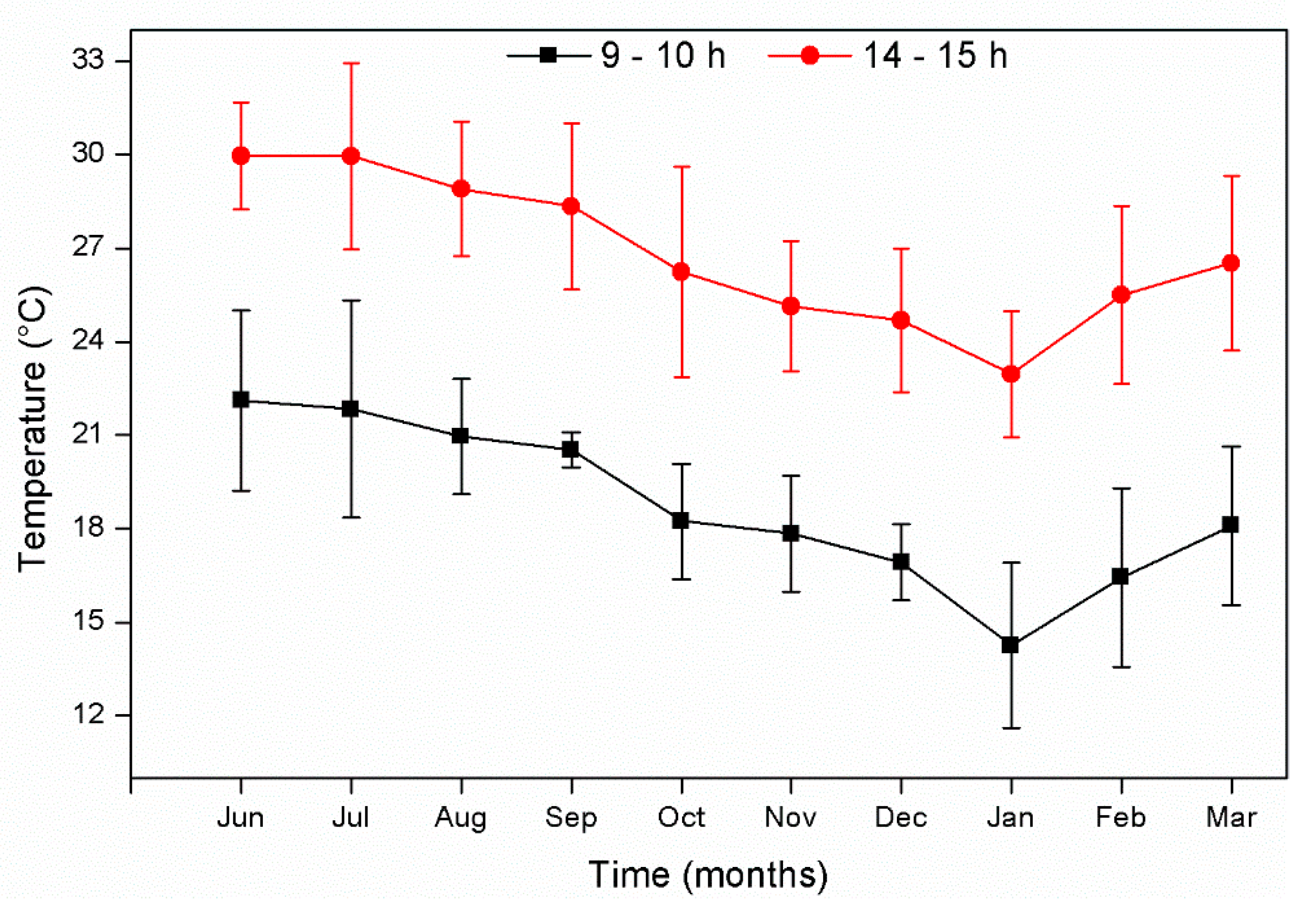
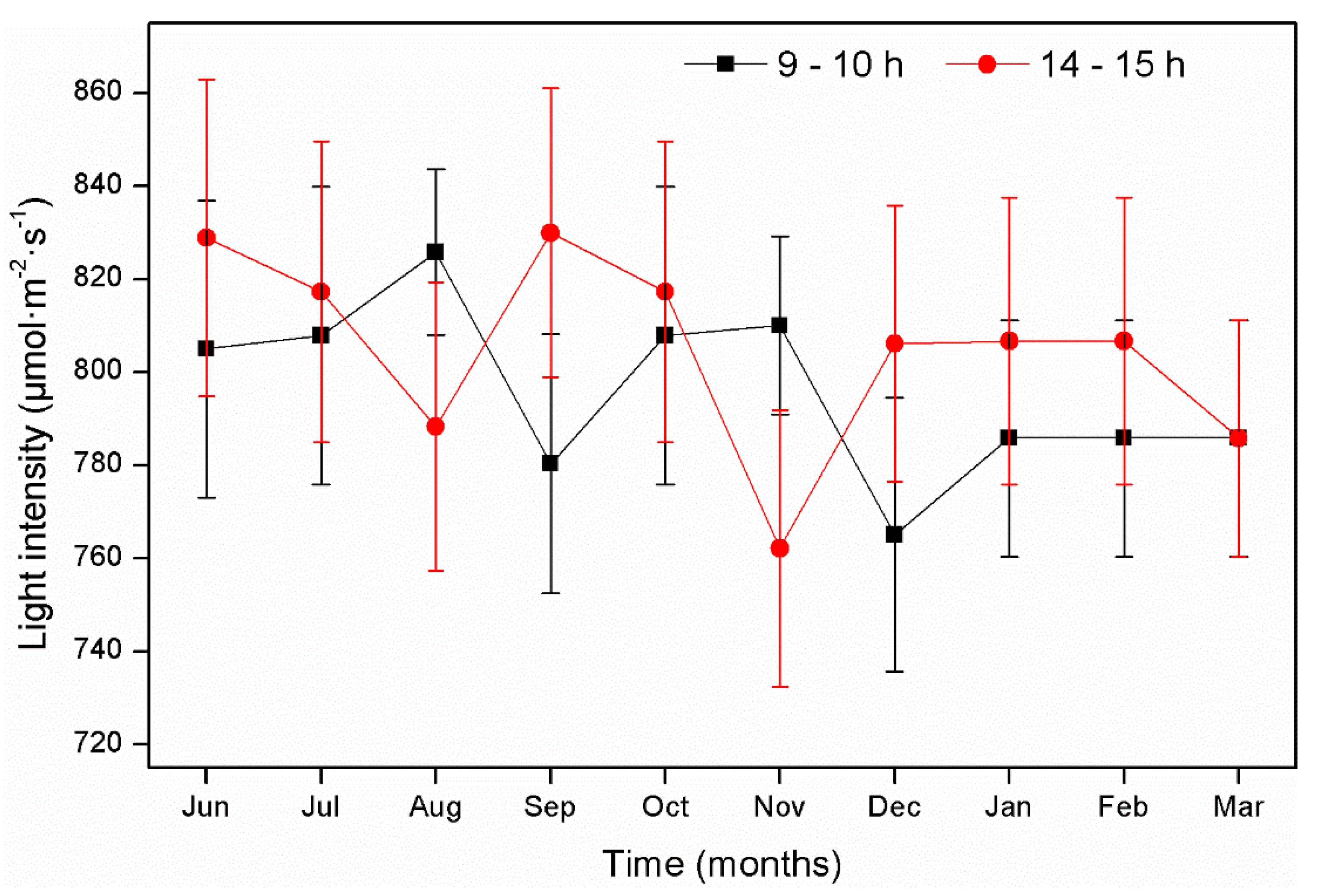
3. Results and Discussion
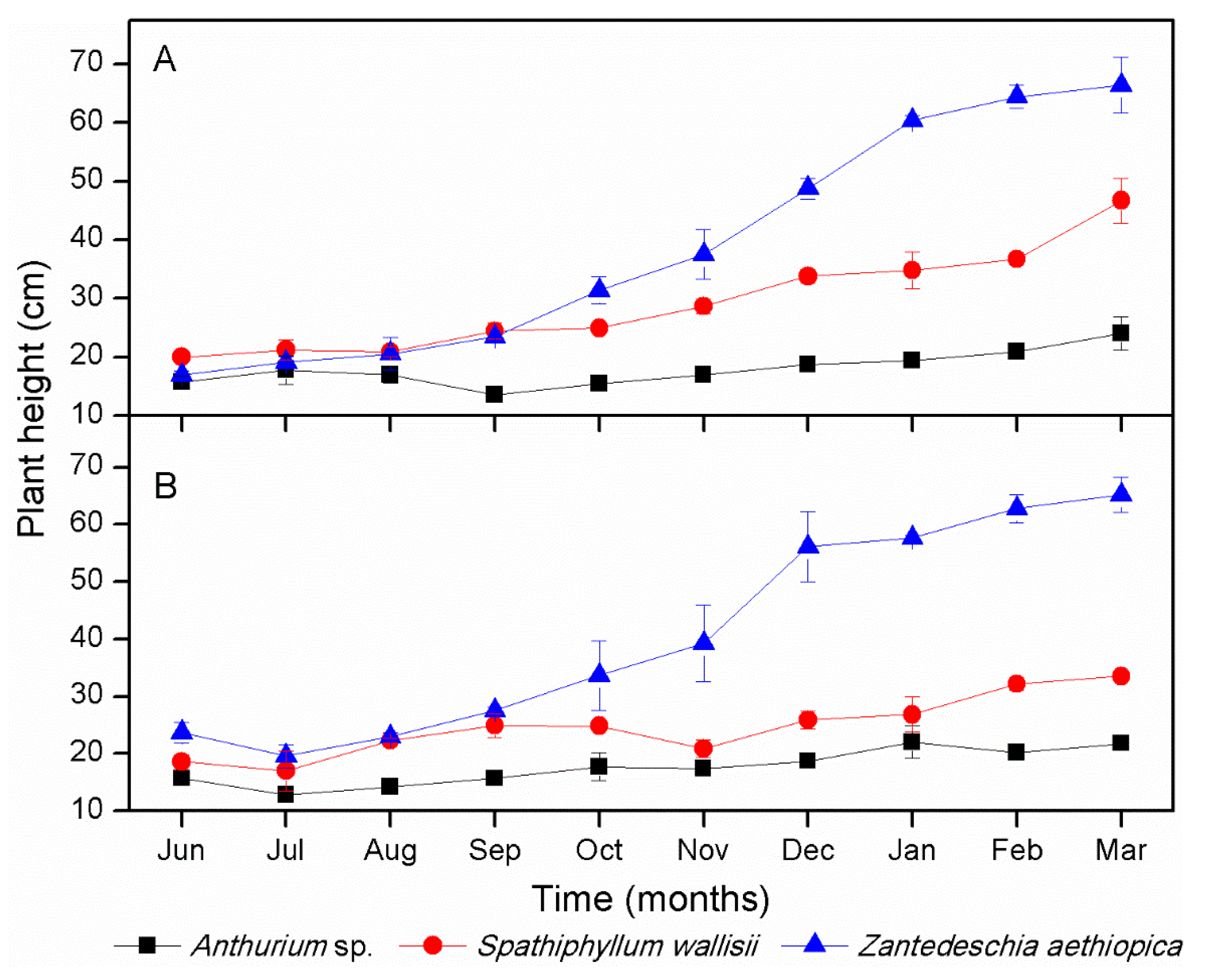
3.1. Plant Growth and Flower Production in Microcosms
3.2. Pollutant Concentration and Removal in Microcosms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Upadhyay, A.K.; Bankoti, N.S.; Rai, U.N. Studies on sustainability of simulated constructed wetland system for treatment of urban waste: Design and operation. J. Environ. Manag. 2016, 169, 285–292. [Google Scholar] [CrossRef]
- Oteng-Peprah, M.; Acheampong, M.A. Greywater Characteristics, Treatment Systems, Reuse Strategies and User Perception—A Review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Salazar, A.B.; Moreno-Seceña, J.C.; Sandoval-Herazo, L.C. Tratamiento de aguas residuales industriales en México: Una aproximación a su situación actual y retos por atender. RINDERESU 2018, 2, 75–87. [Google Scholar]
- WWAP (Programa Mundial de Evaluación de los Recursos Hídricos de las Naciones Unidas). Informe Mundial de las Naciones Unidas sobre el Desarrollo de los Recursos Hídricos 2017. Aguas residuales: El recurso desaprovechado; UNESCO: París, France, 2017. [Google Scholar]
- Long, S.; Zhao, L.; Shi, T.; Li, J.; Yang, J.; Liu, H.; Yang, Y. Pollution control and cost analysis of wastewater treatment at industrial parks in Taihu and Haihe water basins, China. J. Clean. Prod. 2018, 172, 2435. [Google Scholar] [CrossRef]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Liu, H. A review on the sustainability of constructed wetlands for waste water treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Sandoval-Herazo, L.C.; Alvarado-Lassman, A.; Marín-Muñiz, J.L.; Méndez-Contreras, J.M.; Zamora-Castro, S.A. Effects of the Use of Ornamental Plants and Different Substrates in the Removal of Wastewater Pollutants through Microcosms of Constructed Wetlands. Sustainability 2018, 10, 1594. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Golinielli, G.; Abou-Taleb, E.M.; Hellal, M.S. Municipal wastewater treatment in horizontal and vertical flows constructed wetlands. Ecol. Eng. 2013, 61, 460–468. [Google Scholar] [CrossRef]
- Martínez, N.B.; Tejeda, A.; Del Toro, A.; Sánchez, M.P.; Zurita, F. Nitrogen removal in pilot-scale partially saturated vertical wetlands with and without an internal source of carbon. Sci. Total Environ. 2018, 645, 524–532. [Google Scholar] [CrossRef]
- Yalcuk, A.; Ugurlu, A. Comparison of horizontal and vertical constructed wetland systems for landfill leachate treatment. Bioresour. Technol. 2009, 100, 2521–2526. [Google Scholar] [CrossRef]
- Kantawanichkul, S.; Wannasri, S. Wastewater treatment performances of horizontal and vertical subsurface flow constructed wetland systems in tropical climate. Songklanakarin J. Sci. Technol. 2013, 35, 599–603. [Google Scholar]
- Sochacki, A.; Surmacz-Gorska, J.; Faure, O.; Guy, B. Polishing of synthetic electroplating wastewater in microcosm upflow constructed wetlands: Effect of operating conditions. Chem. Eng. J. 2014, 237, 250–258. [Google Scholar] [CrossRef]
- García, J.A.; Paredes, D.; Cubillos, J.A. Effect of plants and the combination of wetland treatment type systems on pathogen removal in tropical climate conditions. Ecol. Eng. 2013, 58, 57. [Google Scholar] [CrossRef]
- Yin, H.; Yan, X.; Gu, X. Evaluation of thermally-modified calcium-rich attapulgite as a low-cost substrate for rapid phosphorus removal in constructed wetlands. Water Res. 2017, 115, 329. [Google Scholar] [CrossRef] [PubMed]
- Morvannou, A.; Forquet, N.; Vanclooster, M.; Molle, P. Characterizing hydraulic properties of filter material of a vertical flow constructed wetland. Ecol. Eng. 2013, 60, 325. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, H.; Chen, X.; Wang, R.; Liu, J. Composition and distribution of microbial communities in natural river wetlands and corresponding constructed wetlands. Ecol. Eng. 2017, 98, 40–48. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Austin, D.; Liu, L.; Dong, R. Performance of integrated household constructed wetland for domestic wastewater treatment in rural areas. Ecol. Eng. 2011, 37, 948–954. [Google Scholar] [CrossRef]
- Yam, R.S.W.; Fan, Y.-T.; Wang, T.-T. Importance of Macrophyte Quality in Determining Life-History Traits of the Apple Snails Pomacea canaliculata: Implications for Bottom-Up Management of an Invasive Herbivorous Pest in Constructed Wetlands. Int. J. Environ. Res. Public Health 2016, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.E. Ornamental wetlands with community participation for the improvement of municipal water in Mexico. RINDERESU 2016, 1, 1–12. (In Spainish) [Google Scholar]
- Latune, R.L.; Laporte-Daube, O.; Fina, N.; Peyrat, S.; Pelus, L.; Molle, P. Which plants are needed for a French vertical-flow constructed wetland under a tropical climate? Water. Sci. Technol. 2017, 75, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Caselles-Osorio, A.; Vega, H.; Lancheros, J.C.; Casierra-Martínez, H.A.; Mosquera, J.E. Horizontal subsurface-flow constructed wetland removal efficiency using Cyperus articulatus L. Ecol. Eng. 2017, 99, 479–485. [Google Scholar] [CrossRef]
- Casierra-Martínez, H.A.; Charris-Olmos, J.C.; Caselles-Osorio, A.; Parody-Muñoz, A.E. Organic matter and nutrients removal in tropical constructed wetlands using cyperusligularis (cyperaceae) and echinocloacolona (poaceae). Water Air Soil Pollut. 2017, 9, 228–338. [Google Scholar] [CrossRef]
- Zhang, X.B.; Peng, L.I.U.; Yang, Y.S.; Chen, W.R. Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J. Environ. Sci. 2007, 19, 902–909. [Google Scholar] [CrossRef]
- Konnerup, D.; Koottatep, T.; Brix, H. Treatment of domestic wastewater in tropical, subsurface flow constructed wetlands planted with Canna and Heliconia. Ecol. Eng. 2009, 35, 248–257. [Google Scholar] [CrossRef]
- Zurita, F.; De Anda, J.; Belmont, M.A. Treatment of domestic wastewater and production of commercial flowers in vertical and horizontal subsurface-flow constructed wetlands. Ecol. Eng. 2009, 35, 861–869. [Google Scholar] [CrossRef]
- Calheiros, C.S.; Bessa, V.S.; Mesquita, R.B.; Brix, H.; Rangel, A.O.; Castro, P.M. Constructed wetland with a polyculture of ornamental plants for wastewater treatment at a rural tourism facility. Ecol. Eng. 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Türker, O.C.; Türe, C.; Böcük, H.; Cicek, A.; Yakar, A. Role of plants and vegetation structure on boron (B) removal process in constructed wetlands. Ecol. Eng. 2016, 88, 143–152. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; García-González, M.C.; Ruelas-Monjardín, L.C.; Moreno-Casasola, P. Influence of different porous media and ornamental vegetation on wastewater pollutant removal in vertical subsurface flow wetland microcosms. Environ. Eng. Sci. 2018, 35, 88–94. [Google Scholar] [CrossRef]
- Bedell, M.; Brown, M.; Kiziltas, A.; Mielewski, D.; Mukerjee, S.; Tabor, R. A case for closed-loop recycling of post-consumer PET for automotive foams. Waste Manag. 2018, 71, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.R. The context and evolution of the integrated solid waste management program at Universidad Iberoamericana Mexico city. Revista Internacional de Contaminación Ambiental 2017, 33, 337–346. [Google Scholar] [CrossRef]
- Lou, C.W.; Lee, M.C.; Guo, Y.L.; Chen, C.K.; Lin, Z.I.; Lin, J.H. Effects of Triclosan Contents on 432 Antimicrobial Efficacy of Environmentally-friendly Biodegradable Polylactic Acid Composite Films. DEStech Trans. Eng. Technol. Res. 2017, 1, 2159–2164. [Google Scholar] [CrossRef]
- Inegi. Instituto Nacional de Geografía Estadística y Procesamiento de Datos. Manual de información geográfica municipal de los Estados Unidos Mexicanos. 2009. Available online: http://www.inegi.gob.mx (accessed on 20 April 2018).
- Inegi. Instituto Nacional de Geografía Estadística y Procesamiento de Datos. Anuario estadístico y geográfico de Veracruz de Ignacio de la Llave. 2014. Available online: http://www.inegi.gob.mx (accessed on 20 April 2018).
- Zurita, F.; Belmont, M.A.; De Anda, J.; Cervantes-Martinez, J. Stress detection by laser-induced fluorescence in Zantedeschi aaethiopica planted in subsurface-flow treatment wetlands. Ecol. Eng. 2008, 33, 110–118. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Jin, M. Using Free Water Surface Constructed Wetland for the Mitigation of High pH Secondary Effluent from Municipal Wastewater Treatment Plant. Available online: https://search.proquest.com/openview/a8d82faf37a669ce3338c7cee0d726c1/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 21 April 2018).
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Kyambadde, J.; Kansiime, F.; Dalhammar, G. Nitrogen and phosphorus removal in substrate-free pilot constructed wetlands with horizontal surface flow in Uganda. Water Air Soil Pollut. 2005, 165, 37–59. [Google Scholar] [CrossRef]
- Zurita, F.; Carreón-Álvarez, A. Performance of three pilot-scale hybrid constructed wetlands for total coliforms and Escherichia coli removal from primary effluent–a 2-year study in a subtropical climate. J. Water Health 2015, 13, 446–458. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; González-Portela, R.E.; López-Vela, M. Constructed wetland mesocosms for the treatment of diluted sugarcane molasses stillage from ethanol production using Pontederia sagittata. Water Res. 2008, 42, 3659–3666. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, F.; Liu, L.; Meng, F. Effects of COD/N ratio and DO concentration on simultaneous nitrification and denitrification in an airlift internal circulation membrane bioreactor. J. Environ. Sci. 2008, 20, 933–939. [Google Scholar] [CrossRef]
- Morgan, J.A.; Martin, J.F.; Bouchard, V. Identifying plant species with root associated bacteria that promote nitrification and denitrification in ecological treatment systems. Wetlands 2008, 28, 220–231. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J. Wetlands; John Wiley and Sons Inc.: New York, NY, USA, 2015; Volume 5, p. 456. [Google Scholar]
- Prochaska, C.A.; Zouboulis, A.I. Removal of phosphates by pilot vertical-flow constructed wetlands using a mixture of sand and dolomite as substrate. Ecol. Eng. 2006, 26, 293–303. [Google Scholar] [CrossRef]
- Melián, J.H.; Rodríguez, A.M.; Araña, J.; Díaz, O.G.; Henríquez, J.G. Hybrid constructed wetlands for wastewater treatment and reuse in the Canary Islands. Ecol. Eng. 2010, 36, 891–899. [Google Scholar] [CrossRef]
- Comino, E.; Riggio, V.; Rosso, M. Mountain cheese factory wastewater treatment with the use of a hybrid constructed wetland. Ecol. Eng. 2011, 37, 1673–1680. [Google Scholar] [CrossRef]
- Toscano, A.; Marzo, A.; Milani, M.; Cirelli, G.L.; Barbagallo, S. Comparison of removal efficiencies in Mediterranean pilot constructed wetlands vegetated with different plant species. Ecol. Eng. 2015, 75, 155–160. [Google Scholar] [CrossRef]
- Öövel, M.; Tooming, A.; Mauring, T.; Mander, Ü. Schoolhouse wastewater purification in a LWA-filled hybrid constructed wetland in Estonia. Ecol. Eng. 2007, 29, 17–26. [Google Scholar] [CrossRef]
- Haghshenas-Adarmanabadi, A.; Heidarpour, M.; Tarkesh-Esfahani, S. Evaluation of horizontal–vertical subsurface hybrid constructed wetlands for tertiary treatment of conventional treatment facilities effluents in developing countries. Water Air Soil Pollut. 2016, 1, 28. [Google Scholar] [CrossRef]
- Elfanssi, S.; Ouazzani, N.; Latrach, L.; Hejjaj, A.; Mandi, L. Phytoremediation of domestic wastewater using a hybrid constructed wetland in mountainous rural area. Int. J. Phytoremed. 2018, 1, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Qiu, J.; Liang, Y. Environmental factors influencing the distribution of total and fecal coliform bacteria in six water storage reservoirs in the Pearl River Delta Region, China. J. Environ. Sci. 2017, 22, 663–668. [Google Scholar] [CrossRef]
- Jesus, J.M.; Cassoni, A.C.; Danko, A.S.; Fiúza, A.; Borges, M.T. Role of three different plants on simultaneous salt and nutrient reduction from saline synthetic wastewater in lab-scale constructed wetlands. Sci. Total Environ. 2017, 579, 447–455. [Google Scholar] [CrossRef]

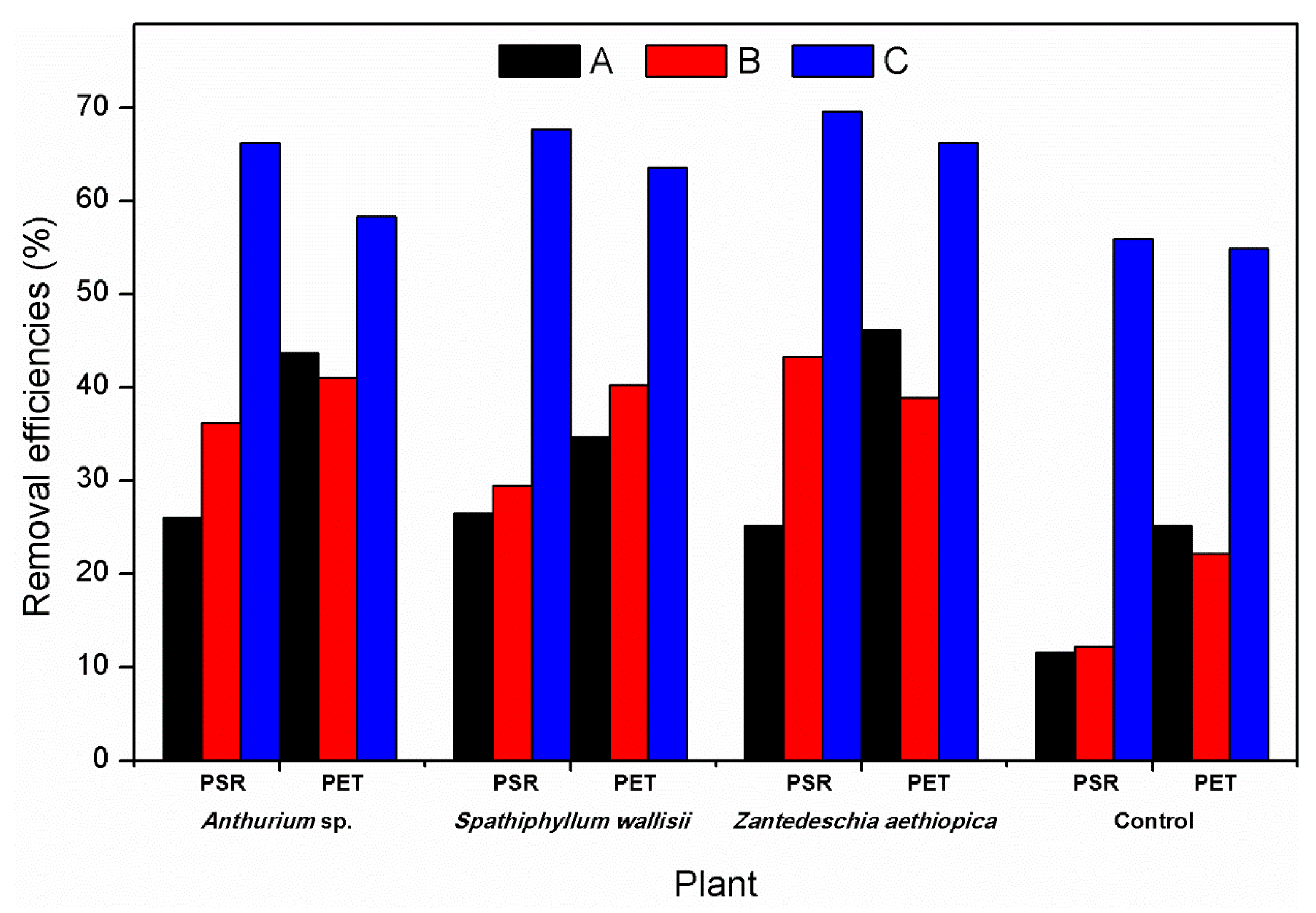
| Parameters | Influent | Anthurium sp. PET | Spathiphyllum wallisii PET | Zantedeschia aethiopica PET | Anthurium sp. PRS | Spathiphyllum wallisii PRS | Zantedeschia aethiopica PRS | Control PET | Control PRS |
|---|---|---|---|---|---|---|---|---|---|
| Water temperature (°C) | 23.34 ± 0.70 | 17.14 ± 0.17 | 16.99 ± 0.21 | 17.44 ± 0.19 | 17.18 ± 0.20 | 16.99 ± 0.16 | 14.44 ± 0.32 | 17.61 ± 0.16 | 17.48 ± 0.08 |
| pH | 7.9 ± 0.12 | 7.86 ± 0.06 | 7.70 ± 0.06 | 7.09 ± 0.05 | 7.40 ± 0.06 | 7.12 ± 0.06 | 7.17 ± 0.06 | 7.19 ± 0.06 | 7.04 ± 0.05 |
| EC (µS·cm−1) | 1306.45 ± 52.07 | 1116.98 ± 36.01 | 1167.94 ± 28.75 | 1118.48 ± 29.22 | 1019.78 ± 26.88 | 1080.12 ± 26.08 | 1032.80 ± 27.60 | 1050.2 ± 43.51 | 1042.26 ± 31.18 |
| TDS (mg·L−1) | 267.59 ± 5.94 | 169.55 ± 2.00 | 183.71 ± 1.67 | 168.50 ± 2.18 | 178.82 ± 1.28 | 183.82 ± 1.54 | 168.509 ± 2.35 | 190.43 ± 3.46 | 212.99 ± 2.92 |
| Total Flowers | |||
|---|---|---|---|
| Anthurium sp. | Spathiphyllum wallisii | Zantedeschia aethiopica | |
| PET | 2 | 12 | 10 |
| PRS | 2 | 9 | 7 |
| Vegetation Used in Microcosms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Anthurium sp. PET | Spathiphyllum wallisii PET | Zantedeschia aethiopica PET | Anthurium sp. PRS | Spathiphyllum wallisii PRS | Zantedeschia aethiopica PRS | Control PET | Control PRS |
| BOD5 | ||||||||
| Influent concentration (mg·L−1) | 115.96 ± 1.85 | 115.96 ± 1.85 | 115.96 ± 1.85 | 115.96 ± 1.85 | 115.96 ± 1.85 | 115.96 ± 1.85 | 115.96 ± 1.85 | 115.96 ± 1.85 |
| Effluent concentration (mgL−1) | 48.31 ± 2.19 | 42.20 ± 2.26 | 36.41 ± 2.00 | 39.15 ± 1.81 | 37.40 ± 2.03 | 35.21 ± 1.56 | 52.30 ± 2.88 | 51.12 ± 2.20 |
| N-NO3 | ||||||||
| Influent concentration (mg·L−1) | 12.08 ± 0.36 | 12.08 ± 0.36 | 12.08 ± 0.36 | 12.08 ± 0.36 | 12.08 ± 0.36 | 12.08 ± 0.36 | 12.08 ± 0.36 | 12.08 ± 0.36 |
| Effluent concentration (mg·L−1) | 7.12 ± 0.19 | 7.22 ± 0.18 | 7.38 ± 0.21 | 7.71 ± 0.14 | 8.52 ± 0.11 | 6.85 ± 0.18 | 9.40 ± 0.35 | 10.59 ± 0.12 |
| P-PO4 | ||||||||
| Influent concentration (mg·L−1) | 11.89 ± 0.38 | 11.89 ± 0.38 | 11.89 ± 0.38 | 11.89 ± 0.38 | 11.89 ± 0.38 | 11.89 ± 0.38 | 11.89 ± 0.38 | 11.89 ± 0.38 |
| Effluent concentration (mg·L−1) | 6.69 ± 0.44 | 7.77 ± 0.31 | 6.40 ± 0.34 | 8.80 ± 0.26 | 8.74 ± 0.27 | 8.66 ± 0.27 | 8.89 ± 0.27 | 10.51 ± 0.26 |
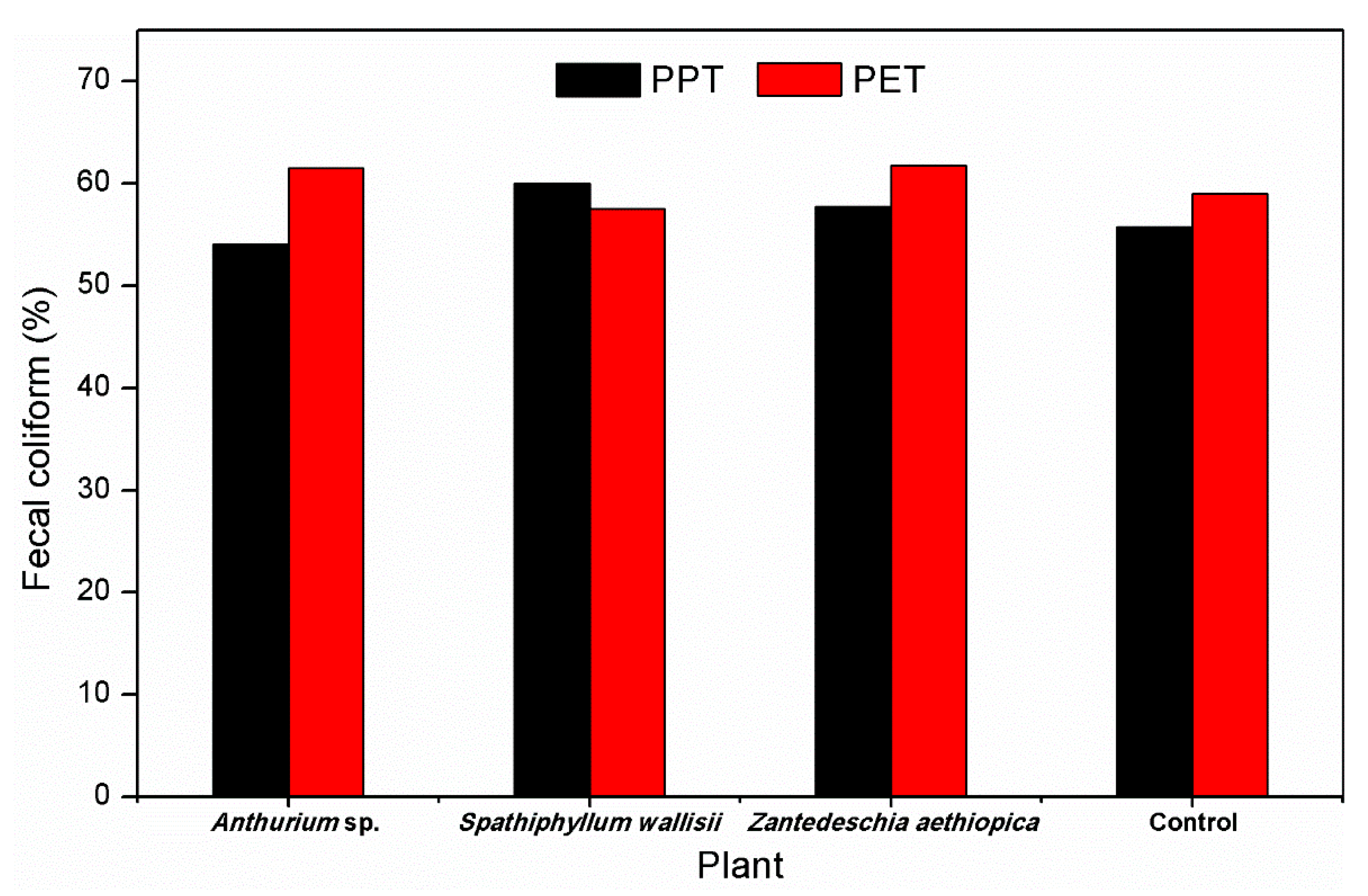
| FC | ||||||||
| Influent concentration (MPN·100 mL−1) | 3319.31 ± 64.41 | 3319.31 ± 64.41 | 3319.31 ± 64.41 | 3319.31 ± 64.41 | 3319.31 ± 64.41 | 3319.31 ± 64.41 | 3319.31 ± 64.41 | 3319.31 ± 64.41 |
| Effluent concentration (MPN·100 mL−1) | 1277.23 ± 94.71 | 1409.95 ± 84.72 | 1267.07 ± 95.54 | 1523.81 ± 90.05 | 1326.72 ± 102.79 | 1403.13 ± 93.22 | 1360.43 ± 89.01 | 1469.30 ± 90.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, L.; Marín-Muñiz, J.L.; Zamora-Castro, S.A.; Sandoval-Salas, F.; Alvarado-Lassman, A. Evaluation of Wastewater Treatment by Microcosms of Vertical Subsurface Wetlands in Partially Saturated Conditions Planted with Ornamental Plants and Filled with Mineral and Plastic Substrates. Int. J. Environ. Res. Public Health 2019, 16, 167. https://doi.org/10.3390/ijerph16020167
Sandoval L, Marín-Muñiz JL, Zamora-Castro SA, Sandoval-Salas F, Alvarado-Lassman A. Evaluation of Wastewater Treatment by Microcosms of Vertical Subsurface Wetlands in Partially Saturated Conditions Planted with Ornamental Plants and Filled with Mineral and Plastic Substrates. International Journal of Environmental Research and Public Health. 2019; 16(2):167. https://doi.org/10.3390/ijerph16020167
Chicago/Turabian StyleSandoval, Luis, José Luis Marín-Muñiz, Sergio Aurelio Zamora-Castro, Fabiola Sandoval-Salas, and Alejandro Alvarado-Lassman. 2019. "Evaluation of Wastewater Treatment by Microcosms of Vertical Subsurface Wetlands in Partially Saturated Conditions Planted with Ornamental Plants and Filled with Mineral and Plastic Substrates" International Journal of Environmental Research and Public Health 16, no. 2: 167. https://doi.org/10.3390/ijerph16020167
APA StyleSandoval, L., Marín-Muñiz, J. L., Zamora-Castro, S. A., Sandoval-Salas, F., & Alvarado-Lassman, A. (2019). Evaluation of Wastewater Treatment by Microcosms of Vertical Subsurface Wetlands in Partially Saturated Conditions Planted with Ornamental Plants and Filled with Mineral and Plastic Substrates. International Journal of Environmental Research and Public Health, 16(2), 167. https://doi.org/10.3390/ijerph16020167









