Minireview: The Epigenetic Modulation of KISS1 in Reproduction and Cancer
Abstract
1. Introduction
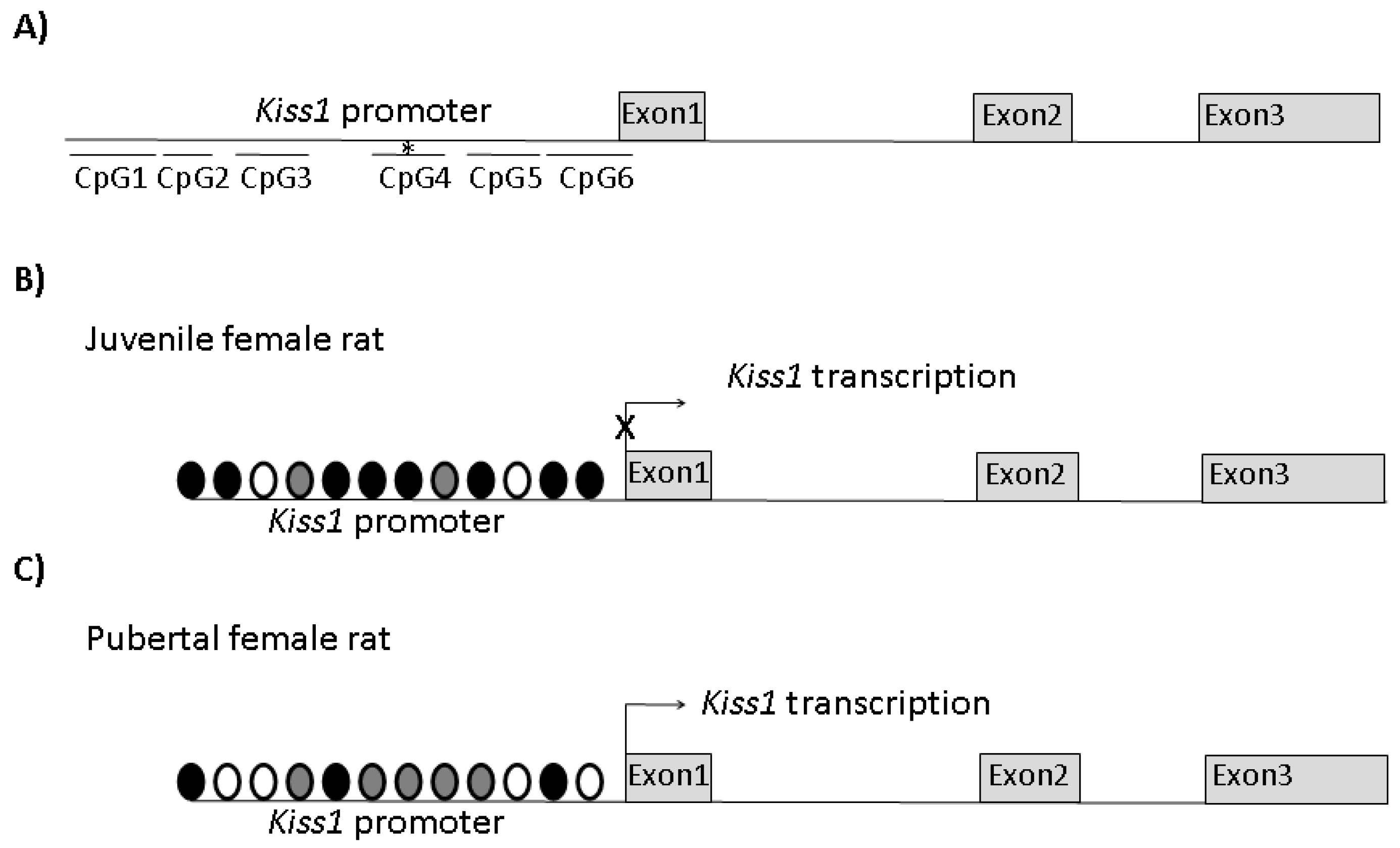
2. The Epigenetic Modulation of KISS1 in Reproduction
3. The Epigenetic Modulation of KISS1 in Cancer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waddington, C.H. Canalization of development and the inheritance of acquired characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- Kim, J.K.; Samaranayake, M.; Pradhan, S. Epigenetic mechanisms in mammals. Cell. Mol. Life Sci. 2009, 66, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Ferguson-Smith, A.C. Genomic imprinting: The emergence of an epigenetic paradigm. Nat. Rev. Genet. 2011, 12, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, A.F.; Eliza, S.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.J.; Pang, K.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-coding RNAs: Regulators of disease. J. Pathol. 2010, 220, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Brookes, E.; Shi, Y. Diverse Epigenetic Mechanisms of Human Disease. Annu. Rev. Genet. 2014, 48, 237–268. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Kamaleddin, M.A.; Aalishah, K.A. Comprehensive review on exosomes and microvesicles as epigenetic factors. Curr. Stem Cell Res. Ther. 2017, 12, 31–36. [Google Scholar] [CrossRef]
- Qian, Z.; Shen, Q.; Yang, X.; Qiu, Y.; Zhang, W. The role of extracellular vesicles: An epigenetic view of the cancer microenvironment. BioMed Res. Int. 2015, 2015, 649161. [Google Scholar] [CrossRef]
- Motti, M.L.; D’Angelo, S.; Meccariello, R. MicroRNAs, cancer and diet: Facts and new exciting perspectives. Curr. Mol. Pharmacol. 2018, 11, 90–96. [Google Scholar] [CrossRef]
- Gelato, K.A.; Shaikhibrahim, Z.; Ocker, M.; Haendler, B. Targeting epigenetic regulators for cancer therapy: Modulation of bromodomain proteins, methyltransferases, demethylases, and microRNAs. Expert Opin. Ther. Targets 2016, 20, 783–799. [Google Scholar] [CrossRef]
- Nervi, C.; De Marinis, E.; Codacci-Pisanelli, G. Epigenetic treatment of solid tumours: A review of clinical trials. Clin. Epigenetics 2015, 7, 127. [Google Scholar] [CrossRef]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 2018, 19, 774–790. [Google Scholar] [CrossRef]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef]
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, estrogens and male fertility. Curr. Med. Chem. 2016, 23, 4070–4091. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Chianese, R.; Chioccarelli, T.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Cobellis, G. Intratesticular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front. Endocrinol. 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Atika, B.; Shahab, M.; Behr, R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat. Rev. Urol. 2016, 13, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Bre’zillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.I.; Chamberlain, L.; Elshourbagy, N.A.; Michalovich, D.; Moore, D.J.; Calamari, A.; Szekeres, P.G.; Sarau, H.M.; Chambers, J.K.; Murdock, P.; et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 2001, 276, 28969–28975. [Google Scholar] [CrossRef]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.K.; McDonald, T.P.; Wang, R.; Xie, G.; O’Dowd, B.F.; George, S.R.; Austin, C.P.; Liu, Q. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem. Biophys. Res. Commun. 2001, 284, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin signaling in the brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Colledge, W.H.; Fasano, S.; Meccariello, R. (Eds.) The Multiple Facets of Kisspeptin Activity in Biological Systems; Frontiers in Endocrinology; Frontiers Media: Lausanne, Switzerland, 2019. [Google Scholar] [CrossRef]
- Chianese, R.; Colledge, W.H.; Fasano, S.; Meccariello, R. Editorial: The Multiple Facets of Kisspeptin Activity in Biological Systems. Front. Endocrinol. (Lausanne) 2018, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141. [Google Scholar] [PubMed]
- Handa, R.J.; Weiser, M.J. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014, 35, 197–220. [Google Scholar] [CrossRef]
- Arena, B.; Maffulli, N.; Maffulli, F.; Morleo, M.A. Reproductive hormones and menstrual changes with exercise in female athletes. Sports Med. 1995, 19, 278–287. [Google Scholar] [CrossRef]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunck, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019. accepted for publication on 19 July 2019. [Google Scholar]
- Kurian, J.R.; Keen, K.L.; Terasawa, E. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation. Endocrinology 2010, 151, 5359–5368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lehman, M.N.; Hileman, S.M.; Goodman, R.L. Neuroanatomy of the kisspeptin signaling system in mammals: Comparative and developmental aspects. Adv. Exp. Med. Biol. 2013, 784, 27–62. [Google Scholar] [PubMed]
- Semaan, S.J.; Kauffman, A.S. Emerging concepts on the epigenetic and transcriptional regulation of the Kiss1 gene. Int. J. Dev. Neurosci. 2013, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, T.; Otsuka, F.; Tsukamoto, N.; Nakamura, E.; Inagaki, K.; Toma, K.; Ogura-Ochi, K.; Glidewell-Kenney, C.; Lawson, M.A.; Makino, H. Mutual interaction of kisspeptin, estrogen and bone morphogenetic protein-4 activity in GnRH regulation by GT1-7 cells. Mol. Cell. Endocrinol. 2013, 381, 8–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uenoyama, Y.; Tomikawa, J.; Inoue, N.; Goto, T.; Minabe, S.; Ieda, N.; Nakamura, S.; Watanabe, Y.; Ikegami, K.; Matsuda, F.; et al. Molecular and Epigenetic Mechanism Regulating Hypothalamic Kiss1 Gene Expression in Mammals. Neuroendocrinology 2016, 103, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Tomikawa, J.; Uenoyama, Y.; Ozawa, M.; Fukanuma, T.; Takase, K.; Goto, T.; Abe, H.; Ieda, N.; Minabe, S.; Deura, C.; et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc. Natl. Acad. Sci. USA 2012, 109, E1294–E1301. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Dhillo, W.S.; Jayasena, C.N. Comprehensive Review on Kisspeptin and Its Role in Reproductive Disorders. Endocrinol. Metab. 2015, 30, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M. Deciphering puberty: Novel partners, novel mechanisms. Eur. J. Endocrinol. 2012, 167, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, M.S.; Vazquez, M.J.; Tena-Sempere, M. Disentangling puberty: Novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Update 2017, 23, 737–763. [Google Scholar] [CrossRef]
- Wyatt, A.K.; Zavodna, M.; Viljoen, J.L.; Stanton, J.A.; Gemmell, N.J.; Jasoni, C.L. Changes in methylation patterns of kiss1 and kiss1r gene promoters across puberty. Genet. Epigenetics 2013, 5, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lomniczi, A.; Loche, A.; Castellano, J.M.; Ronnekleiv, O.K.; Bosch, M.; Kaidar, G.; Knoll, J.G.; Wright, H.; Pfeifer, G.P.; Ojeda, S.R. Epigenetic control of female puberty. Nat. Neurosci. 2013, 16, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.A.; Wright, H.; Aylwin, C.F.; Ojeda, S.R.; Lomniczi, A. Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nat. Commun. 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.J.; Toro, C.A.; Castellano, J.M.; Ruiz-Pino, F.; Roa, J.; Beiroa, D.; Heras, V.; Velasco, I.; Dieguez, C.; Pinilla, L.; et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun. 2018, 9, 4194. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Issa, J.P.; Baylin, S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.C.; Witt, H.; Piro, R.M.; Gu, L.; Zuyderduyn, S.; Stütz, A.M.; Wang, X.; Gallo, M.; Garzia, L.; Zayne, K.; et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 2014, 506, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Zhang, Y.; Mina, A.; Altman, J.K.; Sukhanova, M.; Frankfurt, O.; Jennings, L.; Lu, X.; Behdad, A.; Chen, Q.; et al. An integrative approach reveals genetic complexity and epigenetic perturbation in acute Promyelocytic leukemia: A single institution experience. J. Hum. Pathol. 2019. [Google Scholar] [CrossRef]
- Vidoni, C.; Ferraresi, A.; Secomandi, E.; Vallino, L.; Dhanasekaran, D.N.; Isidoro, C. Epigenetic targeting of autophagy for cancer prevention and treatment by natural compounds. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, R.; Gupta, S. Epigenetic modifications in cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Villar-Garea, A.; Boix-Chornet, M.; Espada, J.; Schotta, G.; Bonaldi, T.; Haydon, C.; Ropero, S.; Petrie, K.; et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005, 37, 391–400. [Google Scholar] [CrossRef]
- Hosseini, A.; Minucci, S. Alterations of histone modifications in cancer. In Epigenetics in Human Disease, 2nd ed.; Tollefsbol, T.O., Ed.; Translational Epigenetics Series; Academic Press Elsevier: London, UK, 2018; Volume 6, pp. 141–217. [Google Scholar]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Liz, J.; Esteller, M. lncRNAs and microRNAs with a role in cancer development. Biochim. Biophys. Acta 2015, 1859, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.A.; Muller, C.P.; Turner, J.D. DNA methylation: Conducting the orchestra from exposure to phenotype? Clin. Epigenetics 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357, eaal2380. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.; Brackstone, M.; Radovick, S.; Babwah, A.V.; Bhattacharya, M.M. KISS1/KISS1R in Cancer: Friend or Foe? Front. Endocrinol. (Lausanne) 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Fratangelo, F.; Carriero, M.V.; Motti, M.L. Controversial Role of Kisspeptins/KiSS-1R Signaling System in Tumor Development. Front. Endocrinol. (Lausanne) 2018, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, V.; Della Corte, C.M.; Ciardiello, F.; Morgillo, F. Kisspeptin and Cancer: Molecular Interaction, Biological Functions, and Future Perspectives. Front. Endocrinol. (Lausanne) 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Corno, C.; Perego, P. KiSS1 in regulation of metastasis and response to antitumor drugs. Drug Resist. Updates 2019, 42, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ingangi, V.; Minopoli, M.; Ragone, C.; Motti, M.L.; Carriero, M.V. Role of Microenvironment on the Fate of Disseminating Cancer Stem Cells. Front. Oncol. 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.H.; Welch, D.R. The KiSS1 metastasis suppressor: A good night kiss for disseminated cancer cells. Eur. J. Cancer 2010, 46, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Chen, Z.H.; Lin, S.Y.; Dai, Q.B.; Fu, L.X.; Chen, R.Q. KISS1 methylation and expression as predictors of disease progression in colorectal cancer patients. World J. Gastroenterol. 2014, 20, 10071–10081. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Esteban, S.; Fernandez-Suarez, A.; Maestro, M.; Morente, M.; Sánchez-Carbayo, M. KiSS-1 methylation and protein expression patterns contribute to diagnostic and prognostic assessments in tissue specimens for colorectal cancer. Tumour Biol. 2013, 34, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, V.; Fierro, M.; Orenes-Piñero, E.; Grau, L.; Moya, P.; Ecke, T.; Alvarez, M.; Gil, M.; Algaba, F.; Bellmunt, J.; et al. KISS1 methylation and expression as tumor stratification biomarkers and clinical outcome prognosticators for bladder cancer patients. Am. J. Pathol. 2011, 179, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Z.; Zhu, Z.; Zheng, X.; Liu, J.; Han, Z.; Ma, X.; Zhang, Y. Upregulated UHRF1 Promotes Bladder Cancer Cell Invasion by Epigenetic Silencing of KiSS1. PLoS ONE 2014, 9, e104252. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Attar, R.; Bageshlooyafshar, B.; Sabitaliyevich, U.Y.; Nurmurzayevich, S.B.; Yelekenova, A.B.; Gormus, U. Regulation of Kisspeptin mediated signaling by non-coding RNAs in different cancers: The beginning of a new era. Cell. Mol. Biol. (Noisy-le-grand) 2019, 65, 72–75. [Google Scholar] [CrossRef]
- Shen, Z.L.; Wang, B.; Jiang, K.W.; Ye, C.X.; Cheng, C.; Yan, Y.C.; Zhang, J.Z.; Yang, Y.; Gao, Z.D.; Ye, Y.J.; et al. Downregulation of miR-199b is associated with distant metastasis in colorectal cancer via activation of SIRT1 and inhibition of CREB/KISS1 signaling. Oncotarget 2016, 7, 35092–35105. [Google Scholar] [CrossRef] [PubMed]
- Dotterweich, J.; Tower, R.J.; Brandl, A.; Müller, M.; Hofbauer, L.C.; Beilhack, A.; Ebert, R.; Glüer, C.C.; Tiwari, S.; Schütze, N.; et al. The KISS1 Receptor as an In Vivo Microenvironment Imaging Biomarker of Multiple Myeloma Bone Disease. PLoS ONE 2016, 11, e0155087. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, I.; Cocco, C.; Sorrentino, C.; Angelucci, D.; Di Meo, S.; Manzoli, L.; Esposito, S.; Ribatti, D.; Bertolotto, M.; Iezzi, L.; et al. Interleukin-30 Promotes Breast Cancer Growth and Progression. Cancer Res. 2016, 76, 6218–6229. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motti, M.L.; Meccariello, R. Minireview: The Epigenetic Modulation of KISS1 in Reproduction and Cancer. Int. J. Environ. Res. Public Health 2019, 16, 2607. https://doi.org/10.3390/ijerph16142607
Motti ML, Meccariello R. Minireview: The Epigenetic Modulation of KISS1 in Reproduction and Cancer. International Journal of Environmental Research and Public Health. 2019; 16(14):2607. https://doi.org/10.3390/ijerph16142607
Chicago/Turabian StyleMotti, Maria Letizia, and Rosaria Meccariello. 2019. "Minireview: The Epigenetic Modulation of KISS1 in Reproduction and Cancer" International Journal of Environmental Research and Public Health 16, no. 14: 2607. https://doi.org/10.3390/ijerph16142607
APA StyleMotti, M. L., & Meccariello, R. (2019). Minireview: The Epigenetic Modulation of KISS1 in Reproduction and Cancer. International Journal of Environmental Research and Public Health, 16(14), 2607. https://doi.org/10.3390/ijerph16142607







