Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Personal Data and Biological Samples
2.3. DNA Extraction and Methylation Analysis
2.4. Telomere Length Analysis
2.5. Statistical Analysis
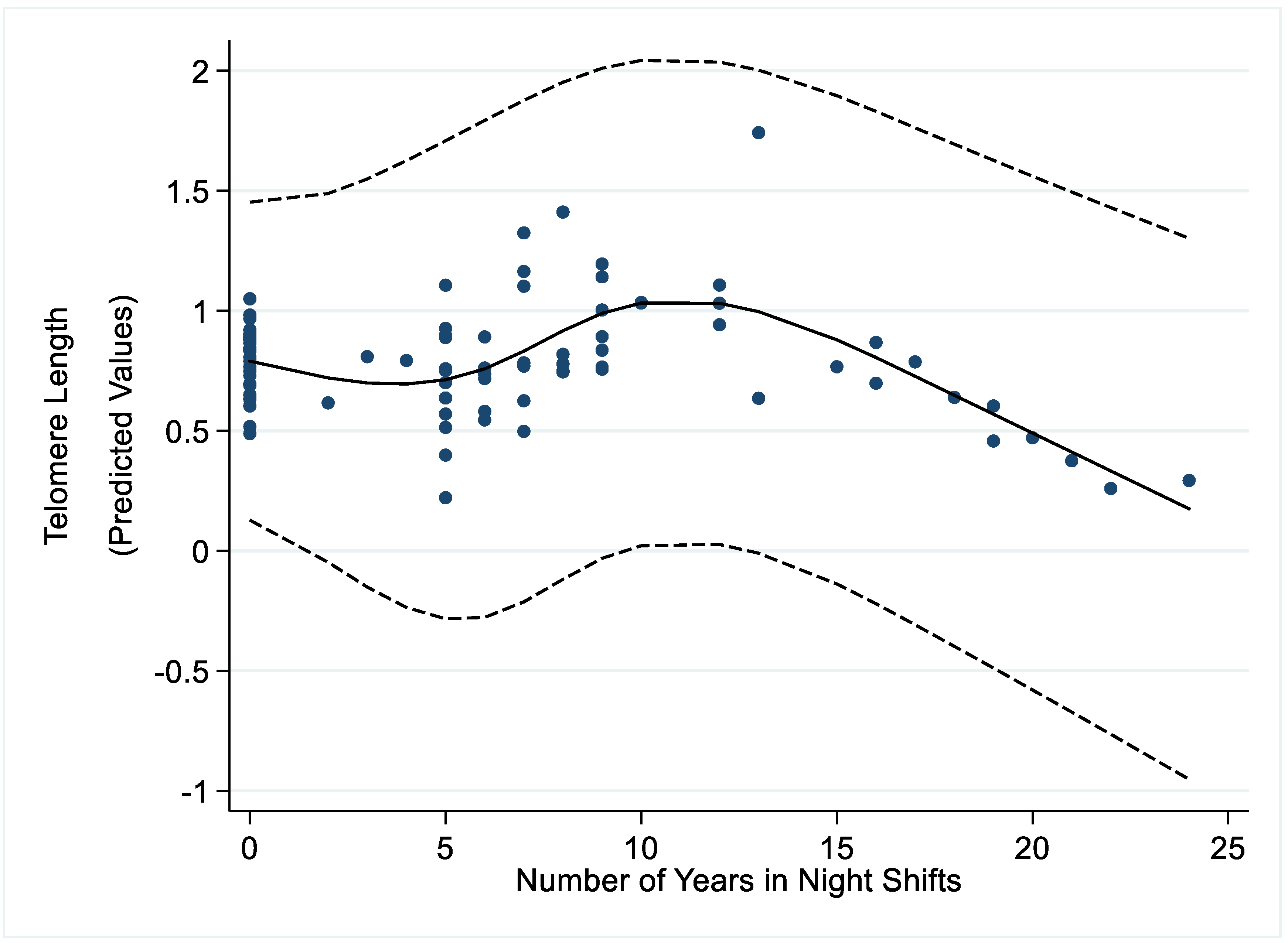
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V.; et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Kroenke, C.H.; Laden, F.; Hankinson, S.E. Night work and risk of breast cancer. Epidemiology 2006, 17, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S.; et al. Rotating night-shift work and the risk of breast cancer in the nurses' health studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Lie, J.A.; Kjuus, H.; Zienolddiny, S.; Haugen, A.; Stevens, R.G.; Kjaerheim, K. Night work and breast cancer risk among norwegian nurses: Assessment by different exposure metrics. Am. J. Epidemiol. 2011, 173, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J. Night shift work and risk of breast cancer. Curr. Environ. Health Rep. 2017, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Haus, E.; Stevens, R. Shift work and cancer—Considerations on rationale, mechanisms, and epidemiology. Scand. J. Work Environ. Health 2010, 36, 163–179. [Google Scholar] [CrossRef] [PubMed]
- How Kit, A.; Nielsen, H.M.; Tost, J. DNA methylation based biomarkers: Practical considerations and applications. Biochimie 2012, 94, 2314–2337. [Google Scholar] [CrossRef]
- Santos-Reboucas, C.B.; Pimentel, M.M. Implication of abnormal epigenetic patterns for human diseases. Eur. J. Hum. Genet. EJHG 2007, 15, 10–17. [Google Scholar] [CrossRef]
- Baccarelli, A.; Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009, 21, 243–251. [Google Scholar] [CrossRef]
- Auclair, G.; Weber, M. Mechanisms of DNA methylation and demethylation in mammals. Biochimie 2012, 94, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [PubMed]
- Dean, W.; Lucifero, D.; Santos, F. DNA methylation in mammalian development and disease. Birth Defects Res. Part C Embryo Today Rev. 2005, 75, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Gravina, S.; Vijg, J. Epigenetic factors in aging and longevity. Pflug. Arch. Eur. J. Physiol. 2010, 459, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Starkweather, A.R.; Alhaeeri, A.A.; Montpetit, A.; Brumelle, J.; Filler, K.; Montpetit, M.; Mohanraj, L.; Lyon, D.E.; Jackson-Cook, C.K. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs. Res. 2014, 63, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Schernhammer, E.; Qi, L.; Gao, X.; De Vivo, I.; Han, J. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS ONE 2011, 6, e23462. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.G.; DeRoo, L.A.; Miller, D.B.; McCanlies, E.C.; Cawthon, R.M.; Sandler, D.P. Employment and work schedule are related to telomere length in women. Occup. Environ. Med. 2011, 68, 582–589. [Google Scholar] [CrossRef]
- Chen, W.D.; Wen, M.S.; Shie, S.S.; Lo, Y.L.; Wo, H.T.; Wang, C.C.; Hsieh, I.C.; Lee, T.H.; Wang, C.Y. The circadian rhythm controls telomeres and telomerase activity. Biochem. Biophys. Res. Commun. 2014, 451, 408–414. [Google Scholar] [CrossRef]
- De Lange, T. Telomere-related genome instability in cancer. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 197–204. [Google Scholar] [CrossRef]
- Fritschi, L.; Glass, D.C.; Heyworth, J.S.; Aronson, K.; Girschik, J.; Boyle, T.; Grundy, A.; Erren, T.C. Hypotheses for mechanisms linking shiftwork and cancer. Med. Hypotheses 2011, 77, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Paul, S. The breast cancer susceptibility genes (brca) in breast and ovarian cancers. Front. Biosci. 2014, 19, 605–618. [Google Scholar] [CrossRef]
- Shin, E.; Jung, W.H.; Koo, J.S. Expression of p16 and prb in invasive breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8209–8217. [Google Scholar] [PubMed]
- Duffy, M.J. Estrogen receptors: Role in breast cancer. Crit. Rev. Clin. Lab. Sci. 2006, 43, 325–347. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World medical association declaration of helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373–374. [Google Scholar]
- Barton, J.; Spelten, E.; Totterdell, P.; Smith, L.; Folkard, S.; Costa, G. The standard shiftwork index—A battery of questionnaires for assessing shiftwork-related problems. Work Stress 1995, 9, 4–30. [Google Scholar] [CrossRef]
- Bollati, V.; Iodice, S.; Favero, C.; Angelici, L.; Albetti, B.; Cacace, R.; Cantone, L.; Carugno, M.; Cavalleri, T.; De Giorgio, B.; et al. Susceptibility to particle health effects, mirna and exosomes: Rationale and study protocol of the sphere study. BMC Public Health 2014, 14, 1137. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative pcr method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Iodice, S.; Hoxha, M.; Ferrari, L.; Carbone, I.F.; Anceschi, C.; Miragoli, M.; Pesatori, A.C.; Persico, N.; Bollati, V. Particulate air pollution, blood mitochondrial DNA copy number, and telomere length in mothers in the first trimester of pregnancy: Effects on fetal growth. Oxidative Med. Cell. Longev. 2018, 2018, 5162905. [Google Scholar] [CrossRef]
- Ridout, K.K.; Levandowski, M.; Ridout, S.J.; Gantz, L.; Goonan, K.; Palermo, D.; Price, L.H.; Tyrka, A.R. Early life adversity and telomere length: A meta-analysis. Mol. Psychiatry 2018, 23, 858–871. [Google Scholar] [CrossRef]
- Verde, G.; De Llobet, L.I.; Wright, R.H.G.; Quilez, J.; Peiro, S.; Le Dily, F.; Beato, M. Unliganded progesterone receptor governs estrogen receptor gene expression by regulating DNA methylation in breast cancer cells. Cancers 2018, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Lin, C.Y. Oestrogen receptors in breast cancer: Basic mechanisms and clinical implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar] [PubMed]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Yaghjyan, L.; Colditz, G.A.; Collins, L.C.; Schnitt, S.J.; Rosner, B.; Vachon, C.; Tamimi, R.M. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J. Natl. Cancer Inst. 2011, 103, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.M.; Stiemsma, L.T.; Keller, K.; van Otterdijk, S.D.; Mericq, V.; Pereira, A.; Santos, J.L.; Shepherd, J.; Michels, K.B. Inverse association between estrogen receptor-alpha DNA methylation and breast composition in adolescent chilean girls. Clin. Epigenet. 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift work: Disrupted circadian rhythms and sleep-implications for health and well-being. Curr. Sleep Med. Rep. 2017, 3, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, M.D.; Sanchez-Barcelo, E.J.; Tan, D.X.; Manchester, L.; Reiter, R.J. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr. Med. Chem. 2010, 17, 4462–4481. [Google Scholar] [CrossRef] [PubMed]
- Koifman, G.; Aloni-Grinstein, R.; Rotter, V. P53 balances between tissue hierarchy and anarchy. J. Mol. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Friedenson, B. The brca1/2 pathway prevents hematologic cancers in addition to breast and ovarian cancers. BMC Cancer 2007, 7, 152. [Google Scholar] [CrossRef]
- Bosviel, R.; Michard, E.; Lavediaux, G.; Kwiatkowski, F.; Bignon, Y.J.; Bernard-Gallon, D.J. Peripheral blood DNA methylation detected in the brca1 or brca2 promoter for sporadic ovarian cancer patients and controls. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 1472–1475. [Google Scholar] [CrossRef]
- Zhu, Y.; Stevens, R.G.; Hoffman, A.E.; Tjonneland, A.; Vogel, U.B.; Zheng, T.; Hansen, J. Epigenetic impact of long-term shiftwork: Pilot evidence from circadian genes and whole-genome methylation analysis. Chronobiol. Int. 2011, 28, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Amos, C.I.; Zhu, Y.; Zhao, H.; Grossman, B.H.; Shay, J.W.; Luo, S.; Hong, W.K.; Spitz, M.R. Telomere dysfunction: A potential cancer predisposition factor. J. Natl. Cancer Inst. 2003, 95, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, I.; Prescott, J.; Wong, J.Y.Y.; Kraft, P.; Hankinson, S.E.; Hunter, D.J. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sandler, D.P.; Carswell, G.; De Roo, L.A.; Parks, C.G.; Cawthon, R.; Weinberg, C.R.; Taylor, J.A. Telomere length in peripheral blood and breast cancer risk in a prospective case-cohort analysis: Results from the sister study. Cancer Cause Control 2011, 22, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, A.J.; Wolff, R.K.; Torres-Mejia, G.; John, E.M.; Herrick, J.S.; Lundgreen, A.; Baumgartner, K.B.; Giuliano, A.R.; Hines, L.M.; Fejerman, L.; et al. Telomere length, telomere-related genes, and breast cancer risk: The breast cancer health disparities study. Gene Chromosome Cancer 2013, 52, 595–609. [Google Scholar] [CrossRef]
- Martinez-Delgado, B.; Gallardo, M.; Tanic, M.; Yanowsky, K.; Inglada-Perez, L.; Barroso, A.; Rodriguez-Pinilla, M.; Canamero, M.; Blasco, M.A.; Benitez, J.; et al. Short telomeres are frequent in hereditary breast tumors and are associated with high tumor grade. Breast Cancer Res. Treat. 2013, 141, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kammori, M.; Sugishita, Y.; Okamoto, T.; Kobayashi, M.; Yamazaki, K.; Yamada, E.; Yamada, T. Telomere shortening in breast cancer correlates with the pathological features of tumor progression. Oncol. Rep. 2015, 34, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Rangel, H.A.; Sanchez-Suarez, P.; Castellanos-Juarez, E.; Penaroja-Flores, R.; Arenas-Aranda, D.J.; Gariglio, P.; Benitez-Bribiesca, L. Shorter telomeres and high telomerase activity correlate with a highly aggressive phenotype in breast cancer cell lines. Tumour Biol. 2016, 37, 11917–11926. [Google Scholar] [CrossRef]
- Erdem, J.S.; Noto, H.O.; Skare, O.; Lie, J.A.S.; Petersen-Overleir, M.; Reszka, E.; Peplonska, B.; Zienolddiny, S. Mechanisms of breast cancer risk in shift workers: Association of telomere shortening with the duration and intensity of night work. Cancer Med. 2017, 6, 1988–1997. [Google Scholar] [CrossRef]
- Bollati, V.; Baccarelli, A.; Sartori, S.; Tarantini, L.; Motta, V.; Rota, F.; Costa, G. Epigenetic effects of shiftwork on blood DNA methylation. Chronobiol. Int. 2010, 27, 1093–1104. [Google Scholar] [CrossRef]
- deHaro, D.; Kines, K.J.; Sokolowski, M.; Dauchy, R.T.; Streva, V.A.; Hill, S.M.; Hanifin, J.P.; Brainard, G.C.; Blask, D.E.; Belancio, V.P.; et al. Regulation of l1 expression and retrotransposition by melatonin and its receptor: Implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 2014, 42, 7694–7707. [Google Scholar] [CrossRef] [PubMed]
- Belancio, V.P. Line-1 activity as molecular basis for genomic instability associated with light exposure at night. Mob. Genet. Elem. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Abrahamowicz, M.; duBerger, R.; Grover, S.A. Flexible modeling of the effects of serum cholesterol on coronary heart disease mortality. Am. J. Epidemiol. 1997, 145, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Ewertz, M.; Duffy, S.W.; Adami, H.O.; Kvale, G.; Lund, E.; Meirik, O.; Mellemgaard, A.; Soini, I.; Tulinius, H. Age at 1st birth, parity and risk of breast-cancer—A metaanalysis of 8 studies from the nordic countries. Int. J. Cancer 1990, 46, 597–603. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Current night shift | |||
|---|---|---|---|---|
| Mean ± SD, N (%) | Total | No (51) | Yes (46) | p * |
| Age | 35.9 ± 5.4 | 36.5 ± 5.3 | 35.3 ± 5.6 | 0.31 |
| Length of service | 11.8 ± 6.9 | 12.7 ± 7.3 | 10.7 ± 6.3 | 0.17 |
| BMI | 22.7 ± 3.2 | 22.2 ± 3.3 | 23.2 ± 3.0 | 0.14 |
| Smoking habit | ||||
| Former/Never | 67 (71.3) | 38 (76.0) | 29 (65.9) | |
| Current | 27 (28.7) | 12 (24.0) | 15 (34.1) | 0.36 |
| Oral contraceptive use | ||||
| No | 58 (63.0) | 33 (67.4) | 25 (58.1) | |
| Yes | 34 (37.0) | 16 (32.6) | 18 (41.9) | 0.36 |
| Number of children | ||||
| 0 | 70 (72.1) | 29 (56.9) | 41 (89.1) | |
| 1 | 12 (12.4) | 10 (19.6) | 2 (4.4) | |
| 2+ | 15 (15.5) | 12 (23.5) | 3 (6.5) | 0.002 |
| Marital status/Age at marriage | ||||
| Not married | 55 (56.7) | 24 (47.1) | 31 (67.4) | |
| Married at 30+ years | 16 (16.5) | 11 (21.6) | 5 (10.9) | |
| Married at 25–29 years | 20 (20.6) | 12 (23.5) | 8 (17.4) | |
| Married at <25 years | 6 (6.2) | 4 (7.8) | 2 (4.3) | 0.23 |
| Biological Markers | Unadjusted | Adjusted * | ||||
|---|---|---|---|---|---|---|
| β | (95%CI) | p | β | (95%CI) | p | |
| TP53 | −0.32 | (−0.93 ; 0.30) | 0.31 | −0.19 | (−0.97 ; 0.59) | 0.63 |
| CDKN2A | 0.25 | (−0.12 ; 0.62) | 0.19 | 0.16 | (−0.26 ; 0.58) | 0.46 |
| BRCA1 | −0.68 | (−1.13 ; −0.23) | 0.004 | −0.42 | (−1.00 ; 0.15) | 0.15 |
| BRCA2 | −0.07 | (−0.92 ; 0.78) | 0.87 | −0.15 | (−1.26 ; 0.95) | 0.78 |
| ESR1 | −1.67 | (−2.58 ; −0.76) | <0.001 | −1.85 | (−3.03 ; −0.67) | 0.003 |
| ESR2 | −0.12 | (−3.13 ; 2.88) | 0.94 | 0.47 | (−3.24 ; 4.18) | 0.80 |
| LINE-1 | −0.17 | (−0.58 ; 0.24) | 0.40 | −0.16 | (−0.66 ; 0.34) | 0.52 |
| Alu | 0.30 | (−0.25 ; 0.85) | 0.29 | −0.12 | (−0.73 ; 0.50) | 0.71 |
| TL | 0.03 | (−0.07 ; 0.14) | 0.53 | 0.05 | (−0.07 ; 0.18) | 0.39 |
| Biological Markers | Unadjusted | Adjusted * | ||||
|---|---|---|---|---|---|---|
| β | (95%CI) | p | β | (95%CI) | p | |
| TP53 | −0.85 | (−1.50 ; −0.20) | 0.01 | −0.93 | (−1.73 ; −0.12) | 0.03 |
| CDKN2A | 0.22 | (−0.18 ; 0.63) | 0.27 | 0.09 | (−0.37 ; 0.54) | 0.71 |
| BRCA1 | −1.25 | (−1.70 ; −0.80) | <0.001 | −1.14 | (−1.71 ; −0.58) | <0.001 |
| BRCA2 | −0.09 | (−1.01 ; 0.83) | 0.85 | −0.31 | (−1.47 ; 0.85) | 0.60 |
| ESR1 | −1.53 | (−2.55 ; −0.51) | 0.004 | −1.52 | (−2.81 ; −0.22) | 0.02 |
| ESR2 | 1.63 | (−1.59 ; 4.85) | 0.32 | 2.52 | (−1.38 ; 6.43) | 0.20 |
| LINE-1 | −0.58 | (−1.02 ; −0.15) | 0.009 | −0.42 | (−0.96 ; 0.11) | 0.12 |
| Alu | −0.001 | (−0.61 ; 0.61) | 0.99 | −0.13 | (−0.79 ; 0.53) | 0.70 |
| TL | 0.02 | (−0.10 ; 0.14) | 0.70 | 0.03 | (−0.10 ; 0.17) | 0.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carugno, M.; Maggioni, C.; Crespi, E.; Bonzini, M.; Cuocina, S.; Dioni, L.; Tarantini, L.; Consonni, D.; Ferrari, L.; Pesatori, A.C. Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses. Int. J. Environ. Res. Public Health 2019, 16, 2292. https://doi.org/10.3390/ijerph16132292
Carugno M, Maggioni C, Crespi E, Bonzini M, Cuocina S, Dioni L, Tarantini L, Consonni D, Ferrari L, Pesatori AC. Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses. International Journal of Environmental Research and Public Health. 2019; 16(13):2292. https://doi.org/10.3390/ijerph16132292
Chicago/Turabian StyleCarugno, Michele, Cristina Maggioni, Eleonora Crespi, Matteo Bonzini, Simone Cuocina, Laura Dioni, Letizia Tarantini, Dario Consonni, Luca Ferrari, and Angela Cecilia Pesatori. 2019. "Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses" International Journal of Environmental Research and Public Health 16, no. 13: 2292. https://doi.org/10.3390/ijerph16132292
APA StyleCarugno, M., Maggioni, C., Crespi, E., Bonzini, M., Cuocina, S., Dioni, L., Tarantini, L., Consonni, D., Ferrari, L., & Pesatori, A. C. (2019). Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses. International Journal of Environmental Research and Public Health, 16(13), 2292. https://doi.org/10.3390/ijerph16132292






