Antioxidant and Antiproliferative Activities of Heated Sterilized Pepsin Hydrolysate Derived from Half-Fin Anchovy (Setipinna taty)
Abstract
:1. Introduction
2. Results and Discussion
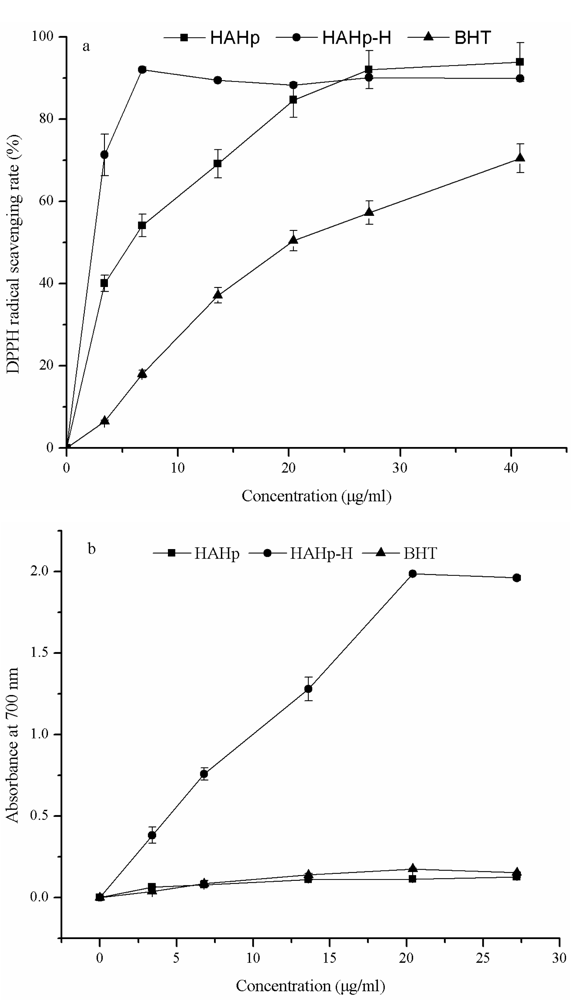
2.1. In Vitro Antioxidant Activity
2.2. Antiproliferative Activity
2.3. Chemical Profiles
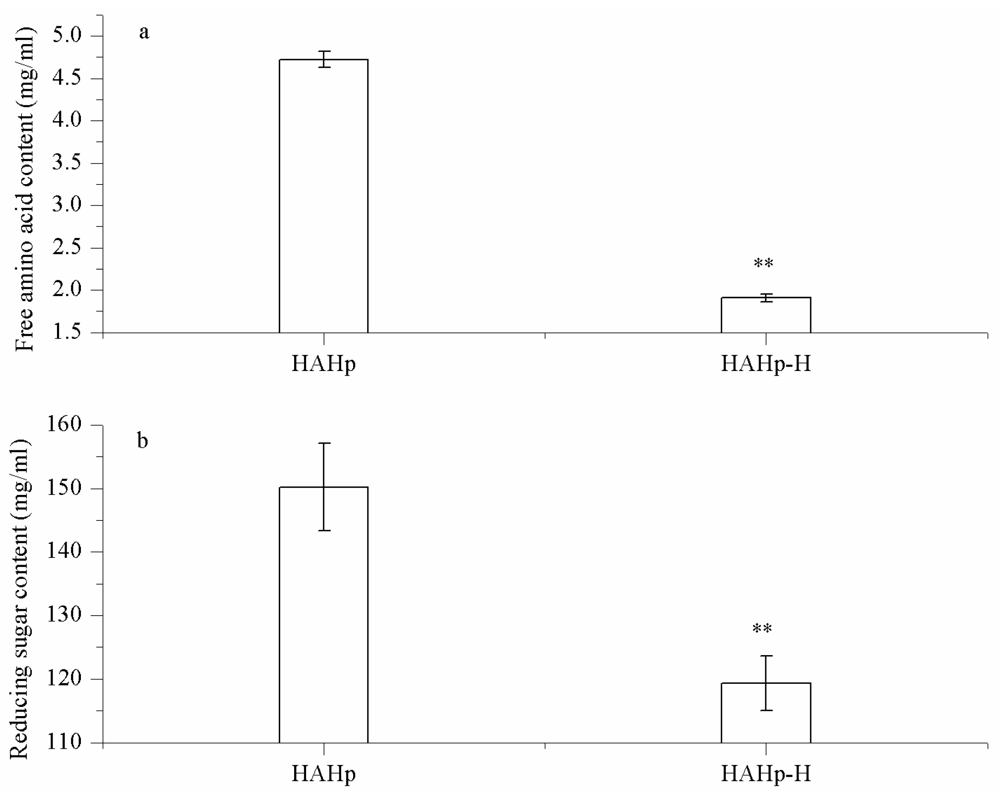
2.3.1. Changes in Free Amino Acid and Reducing Sugar Levels
2.3.2. Amino Acid Analysis
2.3.3. UV-Visible Spectra and Browning Intensity
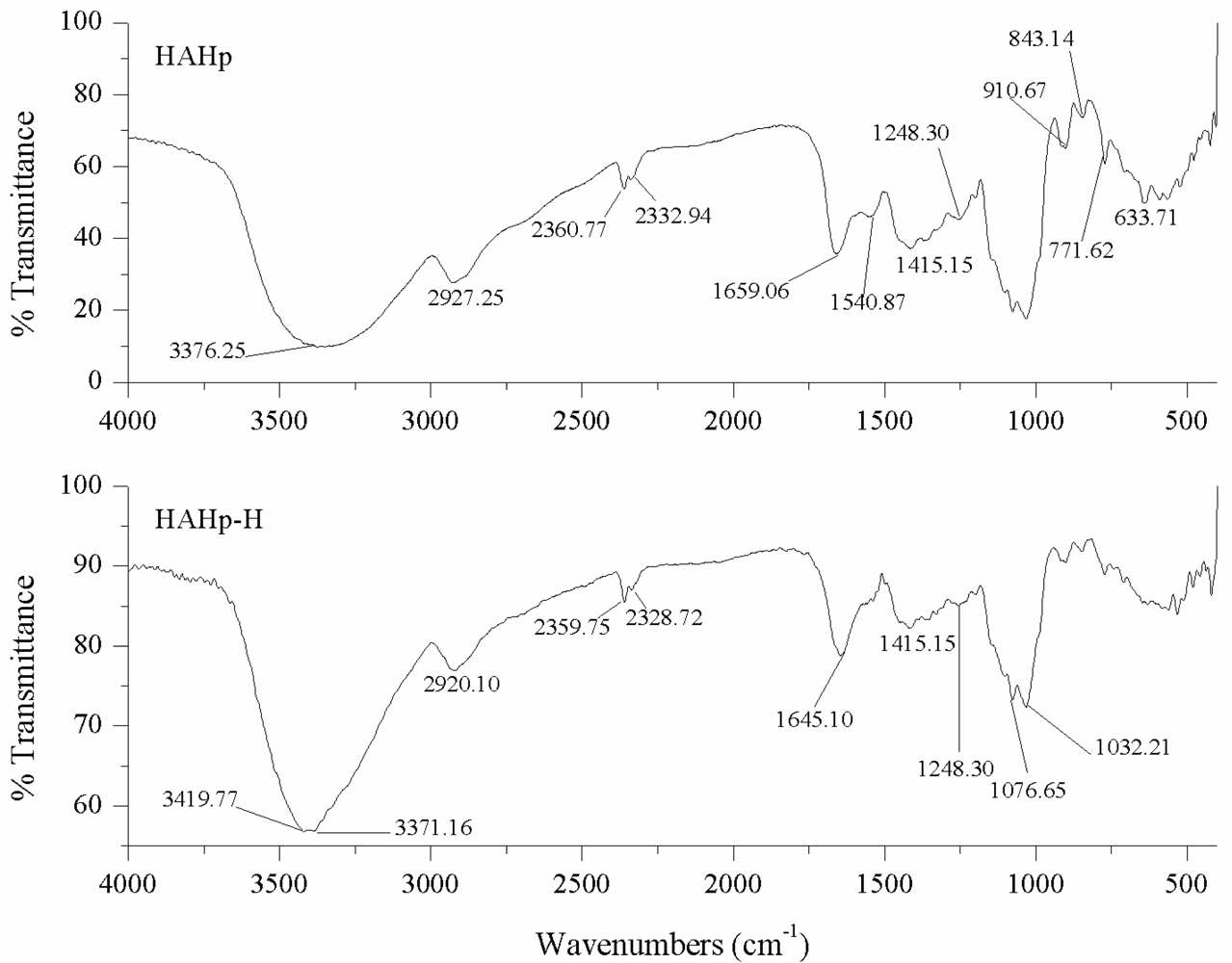
2.3.4. FT-IR Measurement
2.3.5. Molecular Weight (MW) Distribution
3. Experimental Section
3.1. Preparation of Heated Products from Half-Fin Anchovy Pepsin Hydrolysate
3.2. In Vitro Antioxidant Activity
3.2.1. DPPH Radical-Scavenging Assay
3.2.2. Reducing Power Assay
3.3. Antiproliferative Activity
3.3.1. Cell Lines and Cell Culture
3.3.2. MTT Assay
3.4. Measurement of Chemical Profiles
3.4.1. Free Amino Acid Content
3.4.2. Reducing Sugar Level
3.4.3. Amino Acid Analysis
3.4.4. UV-Visible Spectra and Browning Intensity
3.4.5. Fourier Transform Infrared Spectroscopy (FT-IR) Measurement
3.4.6. Molecular Weight (MW) Distribution
3.4.7. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Benjakul, S; Lertittikul, W; Bauer, F. Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system. Food Chem 2005, 93, 189–196. [Google Scholar]
- Lan, XH; Liu, P; Xia, SQ; Jia, CS; Mukunzi, D; Zhang, XM; Xia, WS; Tian, HX; Xiao, ZB. Temperature effect on the nonvolatile compounds of Maillard reaction products derived from xylose–soybean peptide system: further insights into thermal degradation and crosslinking. Food Chem 2010, 120, 967–972. [Google Scholar]
- Liu, P; Huang, MG; Song, SQ; Khizar, H; Zhang, XM; Xia, SQ; Jia, CS. Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Technol 2010. [Google Scholar] [CrossRef]
- Gu, FL; Kim, JM; Abbas, S; Zhang, XM; Xia, SQ; Chen, ZX. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem 2010, 120, 505–511. [Google Scholar]
- Rufián-Henares, JA; Morales, FJ. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res Int 2007, 40, 995–1002. [Google Scholar]
- Yen, GC; Tsai, LC. Antimutagenecity of partially fractionated Maillard reaction product. Food Chem 1993, 47, 11–15. [Google Scholar]
- Morales, FJ; Babbel, MB. Antiradical efficiency of Maillard reaction mixtures in a hydrophilic media. J Agric Food Chem 2002, 50, 2788–2792. [Google Scholar]
- Ogasawara, M; Katsumata, T; Egi, M. Taste properties of Maillard-reaction products preparedfrom 1000 to 5000 Da peptide. Food Chem 2006, 99, 600–604. [Google Scholar]
- Chawla, SP; Chander, R; Sharma, A. Antioxidant formation by γ-irradiation of glucose–amino acid model systems. Food Chem 2007, 103, 1297–1304. [Google Scholar]
- Martins, SIFS; Van Boekel, MAJS. A kinetic model for the glucose/glycine Maillard reaction pathway. Food Chem 2005, 90, 257–269. [Google Scholar]
- Song, R. Zhejiang Ocean University, Zhoushan, China. Unpublished work, 2011.
- Leuschner, C; Hansel, W. Membrane disrupting lytic peptides for cancer treatments. Curr Pharm Des 2004, 10, 2299–2310. [Google Scholar]
- Picot, L; Bordenave, S; Didelot, S; Fruitier-Arnaudin, I; Sannier, F; Thorkelsson, G; Bergé, JP; Guérard, F; Chabeaud, A; Piot, JM. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem 2006, 41, 1217–1222. [Google Scholar]
- Lee, YG; Lee, KW; Kim, JY; Kim, KH; Lee, HJ. Induction of apoptosis in a human lymphoma cell line by hydrophobic peptide fraction separated from anchovy sauce. Biofactors 2004, 21, 63–67. [Google Scholar]
- Hsu, KC; Li-Chan, ECY; Jao, CL. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem 2011, 126, 617–622. [Google Scholar]
- Lu, CY; Hao, ZG; Payne, R; Ho, CT. Effects of water content on volatile generation and peptide degradation in the Maillard reaction of glycine, diglycine, and triglycine. J Agric Food Chem 2005, 53, 6443–6447. [Google Scholar]
- Oluwaniyi, OO; Dosumu, OO; Awolola, GV. Effect of local processing methods (boiling, frying and roasting) on the amino acid composition of four marine fishes commonly consumed in Nigeria. Food Chem 2010, 123, 1000–1006. [Google Scholar]
- Ajandouz, EH; Desseaux, V; Tazi, S; Puigserver, A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem 2008, 107, 1244–1252. [Google Scholar]
- Chen, HM; Muramoto, K; Yamauchi, F; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agric Food Chem 1996, 44, 2619–2623. [Google Scholar]
- Rajapakse, N; Mendis, E; Byun, HG; Kim, SK. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem 2005, 16, 562–569. [Google Scholar]
- Hoskin, DW; Ramamoorthy, A. Studies on anticancer activites of antimicrobial peptides. Biochim Biophys Acta 2008, 1778, 357–375. [Google Scholar]
- Kroh, LW. Caramelisation in food and beverages. Food Chem 1994, 51, 373–379. [Google Scholar]
- Rafik, M; Mas, A; Elharfi, A; Schue, F. Decoloration de solutions sucrees par ultrafiltration sur une membrane a base de poly (organocyclophosphazene). Eur Polym J 1997, 33, 679–690. [Google Scholar]
- Kim, JS; Lee, YS. Study of Maillard reaction products derived from aqueous model systems with different peptide chain lengths. Food Chem 2009, 116, 846–853. [Google Scholar]
- Ajandouz, EH; Tchiakpe, LS; DalleOre, F; Benajiba, A; Puigserver, A. Effect of pH on caramelization and Maillard reaction kinetics in fructose–lysine model systems. J Food Sci 2001, 66, 926–931. [Google Scholar]
- Chang, MC; Tanaka, JZ. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar]
- Turner, JA; Sivasundaram, LR; Ottenhof, MA; Farhat, IA; Linforth, RST; Taylor, AJ. Monitoring chemical and physical changes during thermal flavor generation. J Agric Food Chem 2002, 50, 5406–5411. [Google Scholar]
- Vonhoff, S; Condliffe, J; Schiffter, H. Implementation of an FTIR calibration curve for fast and objective determination of changes in protein secondary structure during formulation development. J Pharm Biomed Anal 2010, 51, 39–45. [Google Scholar]
- Jing, H; Yap, M; Wong, PYY; Kitts, DD. Comparison of physicochemical and antioxidant properties of egg-white proteins and fructose and inulin Maillard reaction products. Food Bioprocess Technol, 2010. [Google Scholar] [CrossRef]
- Yoshimura, Y; Iijima, T; Watanabe, T; Nakazawa, H. Antioxidative effect of Maillard reaction products using glucose–glycine model system. J Agric Food Chem 1997, 45, 4106–4109. [Google Scholar]
- Song, R; Wei, RB; Zhang, B; Wang, DF. Optimization of the antibacterial activity of half-fin anchovy (Setipinna taty) hydrolysates. Food Bioprocess Technol, 2011. [Google Scholar] [CrossRef]
- Song, R; Wang, DF; Xie, C; Wang, X. In vitro antioxidant and antibacterial activities of pepsin hydrolysate of half-fin anchovy (Setipinna taty). Food Sci 2010, 31, 127–131. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 1976, 72, 248–254. [Google Scholar]
- Bersuder, P; Hole, M; Smith, G. Antioxidants from a heated histidine-glucose model system. I. Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc 1998, 75, 181–187. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 1986, 44, 307–315. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983, 65, 55–63. [Google Scholar]
- Doi, E; Shibata, D; Matoba, T. Modified colorimetric ninhydrin methods for peptidase assay. Anal Biochem 1981, 118, 173–184. [Google Scholar]
- Wu, MC. Food Analysis and Organoleptic Investigaation; China Agriculture Press: Beijing, China, 2002; pp. 85–87. [Google Scholar]
- Samaras, TS; Camburn, PA; Chandra, SX; Gordon, MH. Antioxidant properties of kilned and roasted malts. J Agric Food Chem 2005, 53, 8068–8074. [Google Scholar]

| Cell lines | Samples | Concentration (mg/mL) | |||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | ||
| DU-145 human prostate cancer cell | HAHp | 0.21 ± 0.11 aA | 8.39 ± 0.11 bA | 23.36 ± 0.69 cA | 44.40 ± 0.74 dB |
| HAHp-H | 17.31 ± 0.28 aC | 50.07 ± 0.76 bB | 71.28 ± 6.21 cB | 98.81 ± 0.34 dC | |
| 1299 human lung cancer cell | HAHp | – | 4.47 ± 2.05 aA | 20.62 ± 5.70 bA | 46.06 ± 0.95 cB |
| HAHp-H | 4.92 ± 1.10 aB | 9.27 ± 2.21 aA | 21.31 ± 7.93 bA | 95.68 ± 3.68 cC | |
| 109 human esophagus cancer cell | HAHp | – | – | – | 29.90 ± 7.18 A |
| HAHp-H | – | – | – | 55.99 ± 6.26 B | |
| Amino acid | HAHp (mg/100 mg) | Relative percent (%) | HAHp-H (mg/100 mg) | Relative percent (%) |
|---|---|---|---|---|
| Aspartic acid | 1.808 | 10.32 | 0.000 | 0.00 |
| Threonine | 0.755 | 4.31 | 1.413 | 7.70 |
| Serine | 0.859 | 4.90 | 0.780 | 4.25 |
| Glutamic acid | 2.393 | 13.66 | 2.256 | 12.29 |
| Glycine | 1.298 | 7.41 | 1.288 | 7.02 |
| Alanine | 2.327 | 13.28 | 2.535 | 13.81 |
| Cysteine | 0.157 | 0.90 | 0.000 | 0.00 |
| Valine | 1.480 | 8.45 | 1.812 | 9.87 |
| Methionine | 0.783 | 4.47 | 0.819 | 4.46 |
| Isoleucine | 0.584 | 3.33 | 0.818 | 4.46 |
| Leucine | 0.922 | 5.26 | 1.192 | 6.49 |
| Tyrosine | 0.170 | 0.97 | 0.589 | 3.21 |
| Phenylalanine | 0.303 | 1.73 | 0.326 | 1.78 |
| Lysine | 0.961 | 5.49 | 0.814 | 4.43 |
| Histidine | 0.294 | 1.68 | 1.368 | 7.45 |
| Arginine | 0.778 | 4.44 | 0.903 | 4.92 |
| Proline | 1.645 | 9.39 | 1.445 | 7.87 |
| Tryptophan | ND a | ND a | ND a | ND a |
| ΣAA b | 17.518 | 100 | 18.357 | 100 |
| ΣHAA c | 8.044 | 45.92 | 8.947 | 48.74 |
| MW (Da) | >5000 | 3000–5000 | 3000–1000 | 1000–500 | <500 |
|---|---|---|---|---|---|
| HAHp | 4.29 ± 0.35 a | 0.50 ± 0.16 a | 53.57 ± 1.82 a | 31.67 ± 0.25 a | 9.99 ± 1.22 a |
| HAHp-H | 1.63 ± 0.67 b | 38.06 ± 0.15 b | 24.74 ± 1.49 b | 17.03 ± 0.11 b | 19.00 ± 0.06 b |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, R.; Wei, R.; Zhang, B.; Yang, Z.; Wang, D. Antioxidant and Antiproliferative Activities of Heated Sterilized Pepsin Hydrolysate Derived from Half-Fin Anchovy (Setipinna taty). Mar. Drugs 2011, 9, 1142-1156. https://doi.org/10.3390/md9061142
Song R, Wei R, Zhang B, Yang Z, Wang D. Antioxidant and Antiproliferative Activities of Heated Sterilized Pepsin Hydrolysate Derived from Half-Fin Anchovy (Setipinna taty). Marine Drugs. 2011; 9(6):1142-1156. https://doi.org/10.3390/md9061142
Chicago/Turabian StyleSong, Ru, Rongbian Wei, Bin Zhang, Zuisu Yang, and Dongfeng Wang. 2011. "Antioxidant and Antiproliferative Activities of Heated Sterilized Pepsin Hydrolysate Derived from Half-Fin Anchovy (Setipinna taty)" Marine Drugs 9, no. 6: 1142-1156. https://doi.org/10.3390/md9061142
APA StyleSong, R., Wei, R., Zhang, B., Yang, Z., & Wang, D. (2011). Antioxidant and Antiproliferative Activities of Heated Sterilized Pepsin Hydrolysate Derived from Half-Fin Anchovy (Setipinna taty). Marine Drugs, 9(6), 1142-1156. https://doi.org/10.3390/md9061142




