Thermal Transition Properties of Hoki (Macruronus novaezelandiae) and Ling (Genypterus blacodes) Skin Collagens: Implications for Processing
Abstract
:1. Introduction
2. Results and Discussion
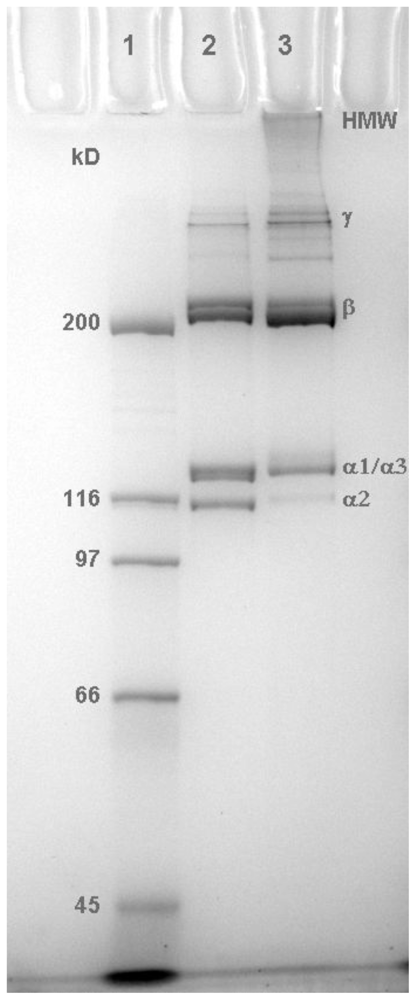
2.1. Molecular Profile of Prepared Collagens
2.2. Thermal Denaturation Temperature Measurements
2.3. Effects of Heating Rate on Thermal Denaturation of Fish Collagens
2.4. Effects of Solvents on Denaturation Temperature
3. Experimental Section
3.1. Hoki Skin Collagen Preparation
3.2. Ling Skin Collagen Preparation
3.3. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.4. Analysis of Hydroxyproline Content
3.5. Preparation of Gels for Analysis on the Rapid Visco™ Analyzer (RVA)
3.6. Determination of Viscosity with the Rapid Visco™ Analyzer
4. Conclusions
Acknowledgements
- Samples Availability: Samples are not available from the authors.
References
- Macfarlane, A. The Guide Book to New Zealand Commercial Fish Species, 2nd ed; Seafood Industry Council: Wellington, New Zealand, 2007. [Google Scholar]
- Piez, KA; Gross, J. The amino acid composition of some fish collagens: The relation between composition and structure. J. Biol. Chem 1960, 235, 995–997. [Google Scholar]
- Miller, E. Collagen types: Structure, distribution, and functions. In Collagen; Nimni, M, Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 139–155. [Google Scholar]
- Hodge, AJ; Highberger, JH; Deffner, GGJ; Schmitt, FO. The effects of proteases on the tropocollagen macromolecule and on its aggregation properties. Proc. Natl. Acad. Sci. USA 1960, 46, 197–206. [Google Scholar]
- Miles, CA; Bailey, AJ. Thermally labile domains in the collagen molecule. Micron 2001, 32, 325–332. [Google Scholar]
- Leikina, E; Metts, MV; Kuznetsova, N; Leikin, S. Type I collagen is thermally unstable at body temperature. Proc. Natl. Acad. Sci. USA 2002, 99, 1314–1318. [Google Scholar]
- Miles, CA; Burjanadze, TV; Bailey, AJ. The kinetics of the thermal denaturation of collagen in unrestrained rat tail tendon determined by differential scanning calorimetry. J. Mol. Biol 1995, 245, 437–446. [Google Scholar]
- Bella, J; Brodsky, B; Berman, HM. Hydration structure of a collagen peptide. Structure 1995, 3, 893–906. [Google Scholar]
- Miles, CA; Bailey, AJ. Studies of the collagen-like peptide (Pro-Pro-Gly)(10) confirm that the shape and position of the type I collagen denaturation endotherm is governed by the rate of helix unfolding. J. Mol. Biol 2004, 337, 917–931. [Google Scholar]
- Khoshnoodi, J; Cartailler, JP; Alvares, K; Veis, A; Hudson, BG. Molecular recognition in the assembly of collagens: Terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J. Biol. Chem 2006, 281, 38117–38121. [Google Scholar]
- Dick, YP; Nordwig, A. Effect of pH on the stability of the collagen fold. Arch. Biochem. Biophys 1966, 117, 466–468. [Google Scholar]
- Finch, A; Gardner, PJ; Ledward, DA; Menashi, S. The thermal denaturation of collagen fibres swollen in aqueous solutions of urea, hexamethylenetetramine, benzoquinone and tetra-alkylammonium salts. Biochim. Biophys. Acta 1974, 365, 400–404. [Google Scholar]
- Usha, R; Ramasami, T. Effect of pH on dimensional stability of rat tail tendon collagen fiber. J. Appl. Polym. Sci 2000, 75, 1577–1584. [Google Scholar]
- Usha, R; Ramasami, T. The effects of urea and n-propanol on collagen denaturation: Using DSC, circular dicroism and viscosity. Thermochim. Acta 2004, 409, 201–206. [Google Scholar]
- Gustavson, K. Lyotropic effects. In The Chemistry and Reactivity of Collagen; Academic Press: New York, NY, USA, 1956; pp. 171–192. [Google Scholar]
- Cowan, P; McGavin, S; North, A. The polypeptide chain configuration of collagen. Nature 1955, 176, 1062–1064. [Google Scholar]
- Rich, A; Crick, FHC. The structure of collagen. Nature 1955, 12, 915–916. [Google Scholar]
- Gustavson, KH. The function of hydroxyproline in collagens. Nature 1955, 175, 70–74. [Google Scholar]
- Ramachandran, G; Bansal, M; Bhatnagar, R. A hypothesis on the role of hydroxyproline in stabilising collagen structure. Biochim. Biophys. Acta 1973, 322, 166–171. [Google Scholar]
- Traub, W. Some stereochemical implications of the molecular conformation of collagen. Isr. J. Chem 1974, 12, 435–439. [Google Scholar]
- Gustavson, K. Some special properties of proteins. In The Chemistry and Reactivity of Collagen; Academic Press: New York, NY, USA, 1956; pp. 19–20. [Google Scholar]
- Bella, J; Eaton, M; Brodsky, B; Berman, HM. Crystal-structure and molecular-structure of a collagen-like peptide at 1.9-angstrom resolution. Science 1994, 266, 75–81. [Google Scholar]
- Shoulders, MD; Raines, RT. Collagen structure and stability. Annu. Rev. Biochem 2009, 78, 929–958. [Google Scholar]
- Laemmli, UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Hubbard, MJ. Calbindin 28 kDa and calmodulin are hyperabundant in rat dental enamel cells. Eur. J. Biochem 1995, 230, 68–79. [Google Scholar]


| Collagen source | Heating rate (°C/min) | Denaturation temperature (TD) °C |
|---|---|---|
| Hoki (Macruronus novaezelandiae) | 0.2 | 17.2 |
| 0.6 | 18.5 | |
| 2.0 | 20.7 * | |
| 2.8 | 20.9 | |
| Ling (Genypterus blacodes) | 0.6 | 16.2 * |
| 2.0 | 17.5 |
| Solvent | Concn. (M) | TD Hoki | pH | TD Ling | pH |
|---|---|---|---|---|---|
| Urea | 0.1 | 23.1 | 3.7 | 19.7 | 4.6 |
| Urea | 0.5 | 21.7 | 3.7 | 18.7 | 4.6 |
| Urea | 1 | 20.0 | 3.9 | 17.3 | 4.7 |
| Acetic acid | 0.1 | 22.2 | 3.5 | 19.4 | 3.5 |
| Acetic acid | 0.5 | 20.7 * | 3.0 | 17.5 | 3.0 |
| Citric acid | 0.1 | 20.6 | 2.3 | 17.7 | 2.4 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hofman, K.A.; Newberry, M. Thermal Transition Properties of Hoki (Macruronus novaezelandiae) and Ling (Genypterus blacodes) Skin Collagens: Implications for Processing. Mar. Drugs 2011, 9, 1176-1186. https://doi.org/10.3390/md9071176
Hofman KA, Newberry M. Thermal Transition Properties of Hoki (Macruronus novaezelandiae) and Ling (Genypterus blacodes) Skin Collagens: Implications for Processing. Marine Drugs. 2011; 9(7):1176-1186. https://doi.org/10.3390/md9071176
Chicago/Turabian StyleHofman, Kathleen Anne, and Marcus Newberry. 2011. "Thermal Transition Properties of Hoki (Macruronus novaezelandiae) and Ling (Genypterus blacodes) Skin Collagens: Implications for Processing" Marine Drugs 9, no. 7: 1176-1186. https://doi.org/10.3390/md9071176
APA StyleHofman, K. A., & Newberry, M. (2011). Thermal Transition Properties of Hoki (Macruronus novaezelandiae) and Ling (Genypterus blacodes) Skin Collagens: Implications for Processing. Marine Drugs, 9(7), 1176-1186. https://doi.org/10.3390/md9071176




