Botryocladia leptopoda Extracts Promote Wound Healing Ability via Antioxidant and Anti-Inflammatory Activities and Regulation of MMP/TIMP Expression
Abstract
1. Introduction
2. Results
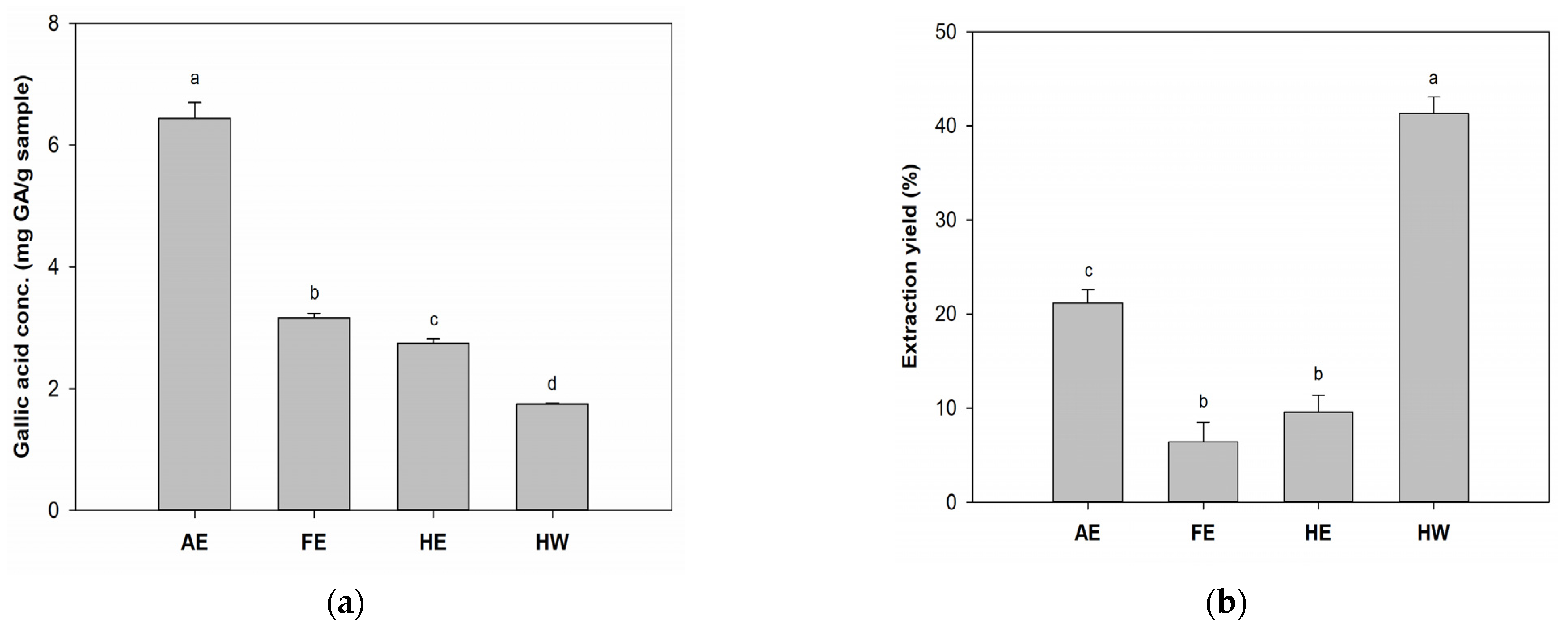
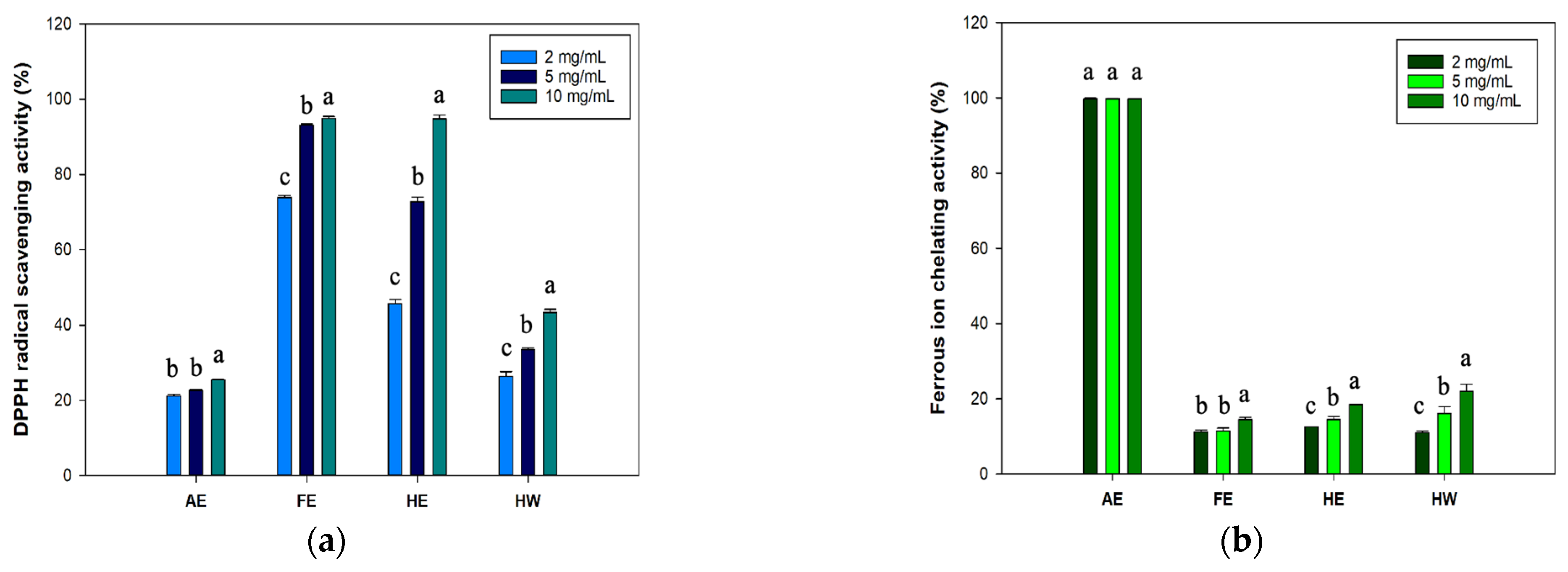
2.1. Total Phenolic Content (TPC) and Antioxidant Capacity of B. leptopoda Extracts
2.2. Anti-Inflammatory Activity of B. leptopoda Extracts in Macrophages
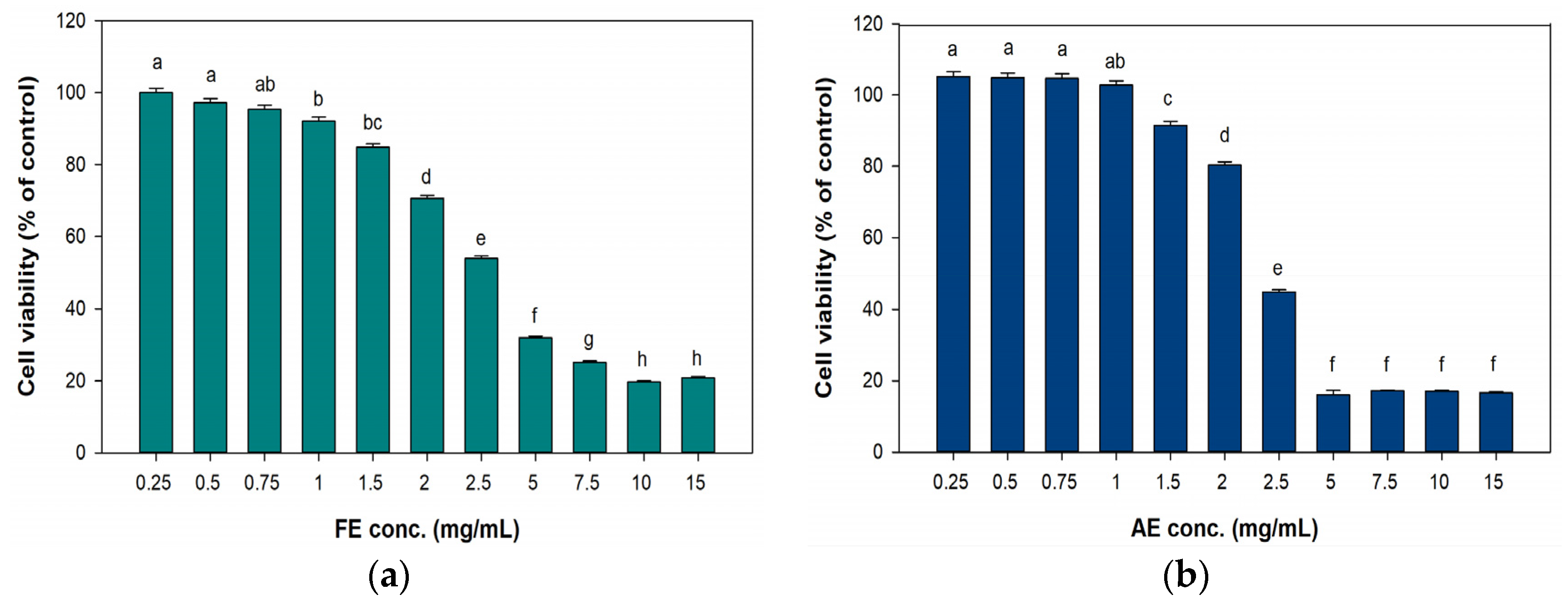
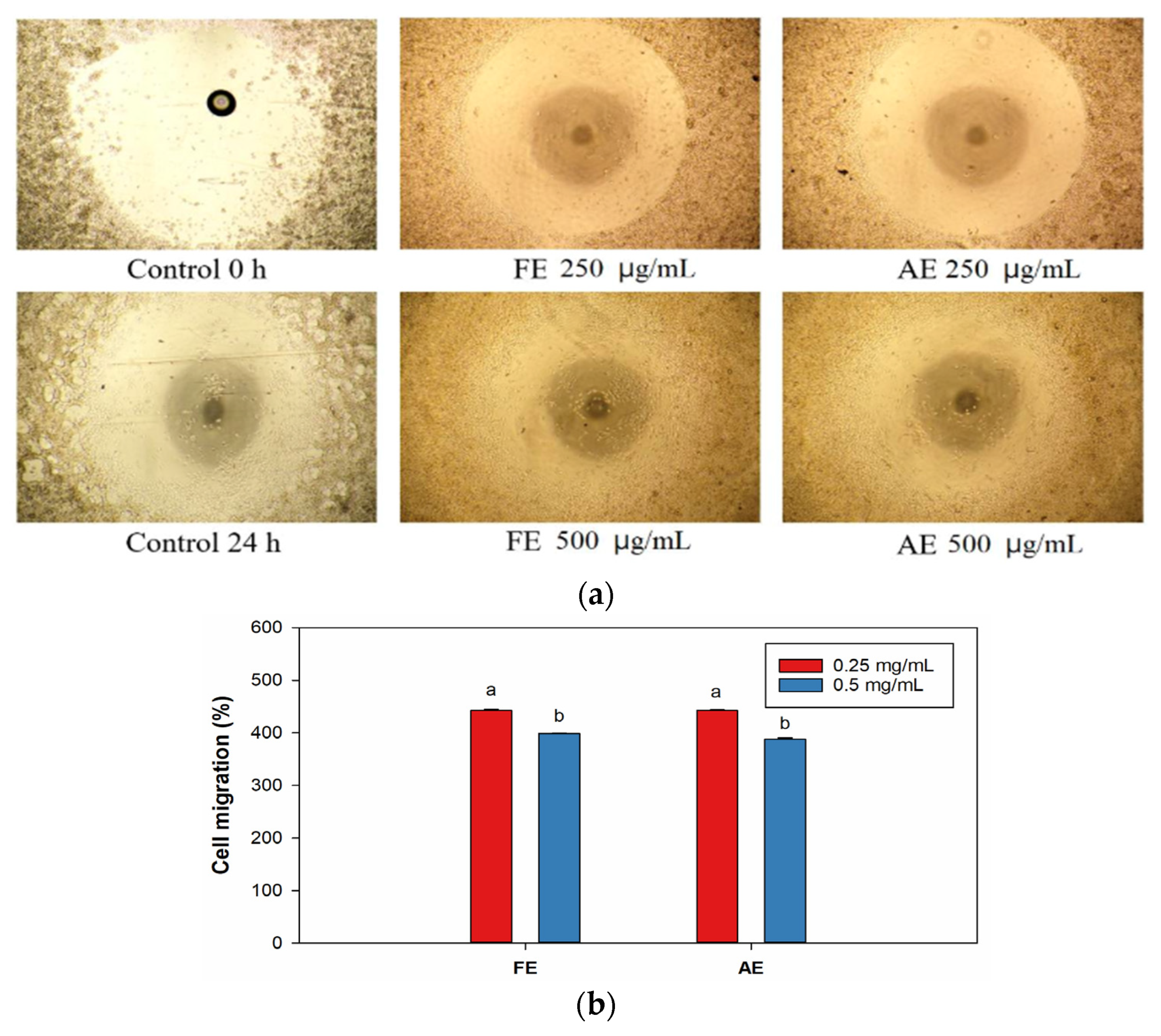
2.3. Cell Cytotoxicity, Proliferation, and Migration of Fibroblast Cells
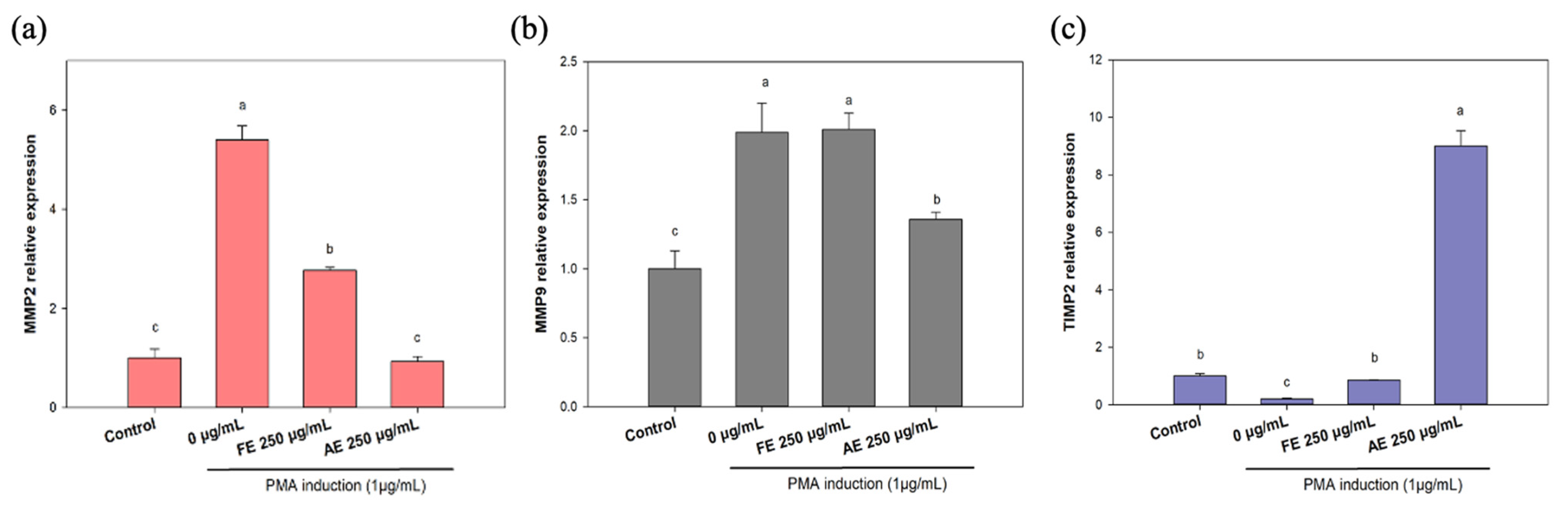
2.4. Evaluation of Scar Inhibition In Vitro
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction of B. leptopoda
4.3. Identification of B. leptopoda Extracts Using Ultra-High-Performance Liquid Chromatograph (UHPLC)
4.4. Total Phenolic Content (TPC)
4.5. Antioxidation Ability
4.6. Detection of Anti-Inflammatory Ability
4.7. Cell Cytotoxicity, Proliferation, and Migration
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouripriya, D.; Adhikari, J.; Debnath, P.; Ghosh, S.; Ghosh, P.; Thomas, S.; Ghandilyan, E.; Gorbatov, P.; Kuchukyan, E.; Gasparyan, S. 3D printing of bacterial cellulose for potential wound healing applications: Current trends and prospects. Int. J. Biol. Macromol. 2024, 279, 135213. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Bennett, G.; Abbott, J.; Sussman, G. The negative impact of medications on wound healing. Wound Pract. Res. 2024, 32, 17–24. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine algae-derived bioactive compounds: A new wave of nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Chandrasekhar, T.; Riazunnisa, K.; Vijaya Lakshmi, D.; Anu Prasanna, V.; Veera Bramhachari, P. Exploration of Bioactive Functional Molecules from Marine Algae: Challenges and Applications in Nutraceuticals. Mar. Bioact. Mol. Biomed. Pharmacother. Appl. 2024, 2024, 187–196. [Google Scholar]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The biochemistry and effectiveness of antioxidants in food, fruits, and marine algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef]
- Imchen, T.; Singh, K.S. Marine algae colorants: Antioxidant, anti-diabetic properties and applications in food industry. Algal Res. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.-S. Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Proteins and bioactive peptides from algae: Insights into antioxidant, anti-hypertensive, anti-diabetic and anti-cancer activities. Trends Food Sci. Technol. 2024, 145, 104352. [Google Scholar]
- Fernandes, F.; Martins, R.; Barbosa, M.; Valentão, P. Algae-based supplements claiming weight loss properties: Authenticity control and scientific-based evidence on their effectiveness. Mar. Drugs 2024, 22, 123. [Google Scholar] [CrossRef]
- Yurika, N.; Montuori, E.; Lauritano, C. Marine microalgal products with activities against age-related cardiovascular diseases. Mar. Drugs 2024, 22, 229. [Google Scholar] [CrossRef] [PubMed]
- Minhas, L.A.; Kaleem, M.; Farooqi, H.M.U.; Kausar, F.; Waqar, R.; Bhatti, T.; Aziz, S.; Jung, D.W.; Mumtaz, A.S. Algae-derived bioactive compounds as potential nutraceuticals for cancer therapy: A comprehensive review. Algal Res. 2024, 78, 103396. [Google Scholar] [CrossRef]
- Chénais, B. Algae and microalgae and their bioactive molecules for human health. Molecules 2021, 26, 1185. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine algae polysaccharides as basis for wound dressings, drug delivery, and tissue engineering: A review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-derived polysaccharides and their therapeutic potential in wound healing application—A review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Ko, S.-C.; Jung, W.-K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef]
- Ajayi, E.I.; Oladele, J.O.; Nkumah, A.O. Application of algae in wound healing. Next-Gener. Algae 2023, 2, 251–284. [Google Scholar]
- Ma, J.; Fang, Y.; Yu, H.; Yi, J.; Ma, Y.; Lei, P.; Yang, Q.; Jin, L.; Wu, W.; Li, H. Recent advances in living algae seeding wound dressing: Focusing on diabetic chronic wound healing. Adv. Funct. Mater. 2024, 34, 2308387. [Google Scholar] [CrossRef]
- Suthan, P. Secondary metabolites screening, in vitro antioxidant and antidiabetic activity of marine red alga Botryocladia leptopoda (J. Agardh) Kylin. Orient. J. Chem. 2022, 38, 16–27. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Yi, T.-K.; Kao, Y.-F.; Lin, S.-P.; Tu, M.-C.; Chou, Y.-C.; Lu, J.-J.; Chai, H.-J.; Cheng, K.-C. Comparative efficacy of Botryocladia leptopoda extracts in scar Inhibition and skin regeneration: A study on UV protection, collagen synthesis, and fibroblast proliferation. Molecules 2024, 29, 5688. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.-Z.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J. Surg. Res. 2020, 246, 213–223. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, Q.; Xin, P.; Yuan, Q.; Wang, Y.; Wu, J. Polydopamine/puerarin nanoparticle-incorporated hybrid hydrogels for enhanced wound healing. Biomater. Sci. 2019, 7, 4230–4236. [Google Scholar] [CrossRef]
- Salehi, M.; Vaez, A.; Naseri-Nosar, M.; Farzamfar, S.; Ai, A.; Ai, J.; Tavakol, S.; Khakbiz, M.; Ebrahimi-Barough, S. Naringin-loaded poly (ε-caprolactone)/gelatin electrospun mat as a potential wound dressing: In vitro and in vivo evaluation. Fibers Polym. 2018, 19, 125–134. [Google Scholar] [CrossRef]
- Ponrasu, T.; Veerasubramanian, P.K.; Kannan, R.; Gopika, S.; Suguna, L.; Muthuvijayan, V. Morin incorporated polysaccharide–protein (psyllium–keratin) hydrogel scaffolds accelerate diabetic wound healing in Wistar rats. RSC Adv. 2018, 8, 2305–2314. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wu, J.-Q.; Fan, R.-Y.; He, Z.-H.; Li, C.-Y.; He, M.-F. Isoliquiritin promote angiogenesis by recruiting macrophages to improve the healing of zebrafish wounds. Fish Shellfish Immunol. 2020, 100, 238–245. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xing, R.; Li, H.; Ren, J.; Chen, L.; Yu, Z.; Zhang, H.; Wu, W.; Li, C.; Zhu, L. From Terrestrial Plants to Marine Macroalgae: A Comprehensive Review of Cell Wall Component-Properties, Extraction, Modification, and Application of Algal Cellulose. J. Agric. Food Chem. 2025, 73, 23759–23782. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef]
- Utpal, B.K.; Sutradhar, B.; Zehravi, M.; Sweilam, S.H.; Panigrahy, U.P.; Urs, D.; Fatima, A.F.; Nallasivan, P.K.; Chhabra, G.S.; Sayeed, M. Polyphenols in wound healing: Unlocking prospects with clinical applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 2459–2485. [Google Scholar] [CrossRef]
- Harb, T.B.; Pereira, M.S.; Cavalcanti, M.I.L.; Fujii, M.T.; Chow, F. Antioxidant activity and related chemical composition of extracts from Brazilian beach-cast marine algae: Opportunities of turning a waste into a resource. J. Appl. Phycol. 2021, 33, 3383–3395. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Recent advances in reactive oxygen species scavenging nanomaterials for wound healing. Exploration 2024, 4, 20230066. [Google Scholar] [CrossRef]
- Li, J.; Shen, Y.; Wang, X.; Wu, T.; Huang, Q.; Shen, M.; Xu, S.; Li, Y. A multilayer hydrogel incorporating urolithin B promotes diabetic wound healing via ROS scavenging and angiogenesis. Chem. Eng. J. 2024, 496, 153661. [Google Scholar] [CrossRef]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Secor, P.R.; James, G.A.; Fleckman, P.; Olerud, J.E.; McInnerney, K.; Stewart, P.S. Staphylococcus aureus Biofilm and Planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol. 2011, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, L.; Larjava, H.; Koivisto, L. Granulation tissue formation and remodeling. Endod. Top. 2011, 24, 94–129. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kung, H.-N.; Tsai, Y.-S.; Tseng, T.-N.; Hsu, K.-D.; Cheng, K.-C. Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 2017, 24, 4927–4937. [Google Scholar] [CrossRef]
- Celik, I.; Sürücü, O.; Dietz, C.; Heymach, J.V.; Force, J.; Höschele, I.; Becker, C.M.; Folkman, J.; Kisker, O. Therapeutic efficacy of endostatin exhibits a biphasic dose-response curve. Cancer Res. 2005, 65, 11044–11050. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef]
- Tanriverdi-Akhisaroglu, S.; Menderes, A.; Oktay, G. Matrix metalloproteinase-2 and -9 activities in human keloids, hypertrophic and atrophic scars: A pilot study. Cell Biochem. Funct. 2009, 27, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, J.; Huh, S.; Lee, J.; Hwang, H.; Kim, Y.; Kim, Y.W.; Park, D.; Yo Byun, S. Matrine inhibits PMA-induced MMP-1 expression in human dermal fibroblasts. Biofactors 2008, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-T.; Bi, K.-W.; Huang, C.-C.; Wu, H.-T.; Ho, H.-Y.; Pang, J.-H.S.; Huang, S.-T. Davallia bilabiata exhibits anti-angiogenic effect with modified MMP-2/TIMP-2 secretion and inhibited VEGF ligand/receptors expression in vascular endothelial cells. J. Ethnopharmacol. 2017, 196, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Kanwal, S.; Sivaramakrishnan, R.; Katelakha, K. Therapeutic potential of microalgae-derived natural compounds in diabetic wound healing: A comprehensive review. Heliyon 2025, 11, e42723. [Google Scholar] [CrossRef]
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine algal derived phenolic compounds and their biological activities for medicinal and cosmetic applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2021; pp. 278–334. [Google Scholar]
- Fernández, S.; Arnáiz, V.; Rufo, D.; Arroyo, Y. Current status of indole-derived marine natural products: Synthetic approaches and therapeutic applications. Mar. Drugs 2024, 22, 126. [Google Scholar] [CrossRef]
- Vasilakis, G.; Marka, S.; Ntzouvaras, A.; Zografaki, M.-E.; Kyriakopoulou, E.; Kalliampakou, K.I.; Bekiaris, G.; Korakidis, E.; Papageorgiou, N.; Christofi, S. Wound healing, antioxidant, and antiviral properties of bioactive polysaccharides of microalgae strains isolated from Greek Coastal Lagoons. Mar. Drugs 2025, 23, 77. [Google Scholar] [CrossRef]
- Orhan Puskullu, M.; Tekiner, B.; Suzen, S. Recent studies of antioxidant quinoline derivatives. Mini Rev. Med. Chem. 2013, 13, 365–372. [Google Scholar]
- Chitra, G.; Selvi, M.; Franklin, D.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. pH-sensitive biopolymeric hydrogel-based on indole-3-acetic acid for wound healing and anti-cancer applications. SN Appl. Sci. 2019, 1, 1641. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu method for the estimation of (Poly)phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]



| Proportion (%) | Compound | Potential Skin Care Related Function |
|---|---|---|
| FE | ||
| 31.25 | Quinolin-3-ol | Preservatives, disinfectants and pesticides |
| 10.8 | 8-Hydroxyquinoline-5-carboxylic acid | Antioxidant |
| 10.05 | 6-Methylnicotinic acid | Anti-inflammatory |
| 8.61 | 4-Hydroxybenzaldehyde | Preservatives, skin whitening |
| 5.64 | 1H-Indole-3-carboxylic acid | Antimicrobial property |
| 3.84 | 4-Hydroxyquinoline | Dehydrogenase inhibition |
| AE | ||
| 37.2 | Indoleacetic acid | Skin moisturizing, anti-oxidation |
| 21.23 | Quinolin-3-ol | Preservatives, disinfectants and pesticides |
| 8.17 | 1H-Indole-3-carboxylic acid | Antimicrobial property, anti-inflammatory |
| 3.6 | 2-(Formylamino)benzoic acid | Preservatives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-P.; Yi, T.-K.; Kao, Y.-F.; Tu, M.-C.; Hsieh, C.-C.; Chou, Y.-C.; Lu, J.-J.; Santoso, S.P.; Chai, H.-J.; Cheng, K.-C. Botryocladia leptopoda Extracts Promote Wound Healing Ability via Antioxidant and Anti-Inflammatory Activities and Regulation of MMP/TIMP Expression. Mar. Drugs 2025, 23, 444. https://doi.org/10.3390/md23110444
Lin S-P, Yi T-K, Kao Y-F, Tu M-C, Hsieh C-C, Chou Y-C, Lu J-J, Santoso SP, Chai H-J, Cheng K-C. Botryocladia leptopoda Extracts Promote Wound Healing Ability via Antioxidant and Anti-Inflammatory Activities and Regulation of MMP/TIMP Expression. Marine Drugs. 2025; 23(11):444. https://doi.org/10.3390/md23110444
Chicago/Turabian StyleLin, Shin-Ping, Tsung-Kai Yi, Yi-Feng Kao, Ming-Chieh Tu, Chen-Che Hsieh, Yu-Chieh Chou, Jheng-Jhe Lu, Shella Permatasari Santoso, Huey-Jine Chai, and Kuan-Chen Cheng. 2025. "Botryocladia leptopoda Extracts Promote Wound Healing Ability via Antioxidant and Anti-Inflammatory Activities and Regulation of MMP/TIMP Expression" Marine Drugs 23, no. 11: 444. https://doi.org/10.3390/md23110444
APA StyleLin, S.-P., Yi, T.-K., Kao, Y.-F., Tu, M.-C., Hsieh, C.-C., Chou, Y.-C., Lu, J.-J., Santoso, S. P., Chai, H.-J., & Cheng, K.-C. (2025). Botryocladia leptopoda Extracts Promote Wound Healing Ability via Antioxidant and Anti-Inflammatory Activities and Regulation of MMP/TIMP Expression. Marine Drugs, 23(11), 444. https://doi.org/10.3390/md23110444











