Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties
Abstract
1. Introduction
2. Results and Discussion
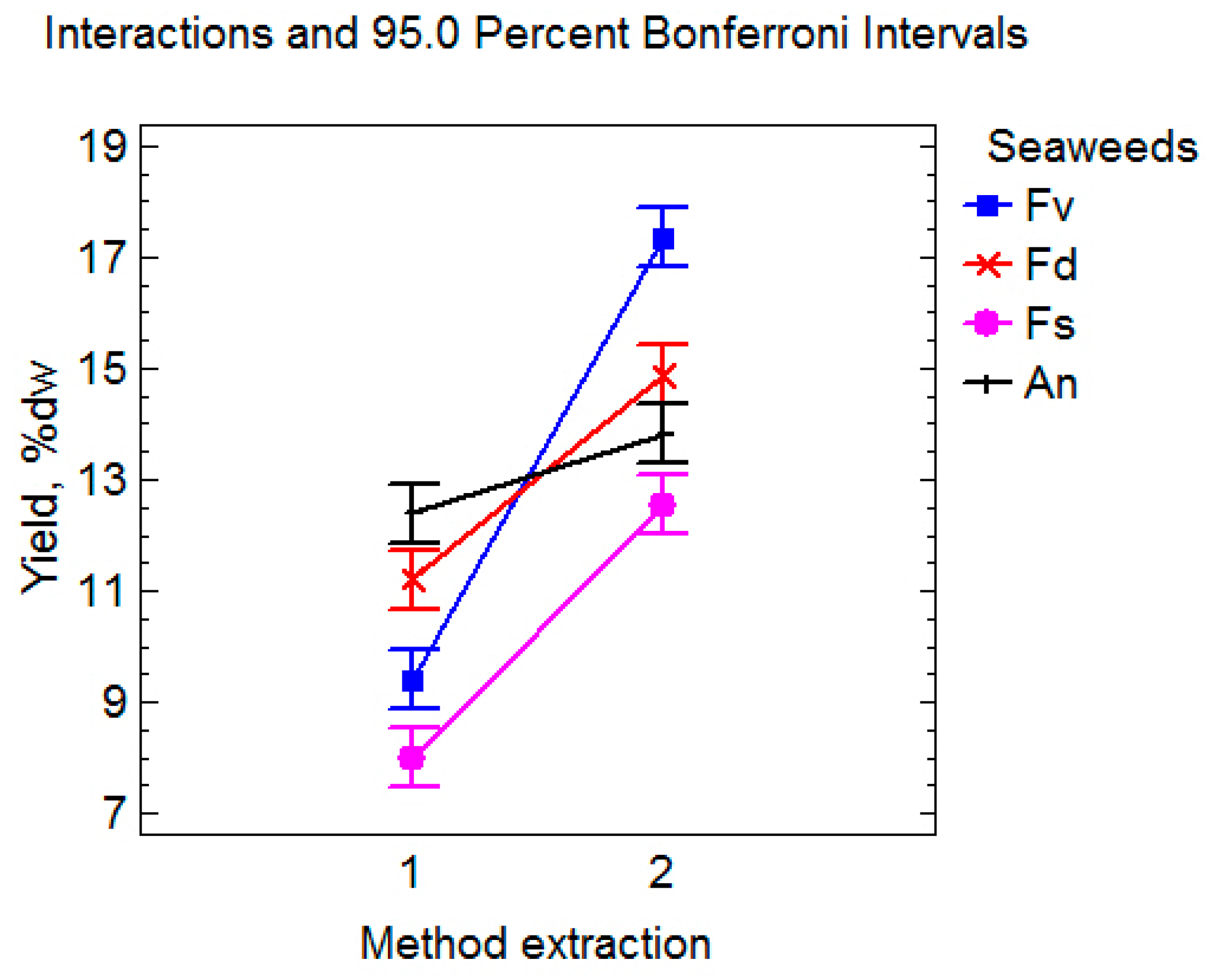
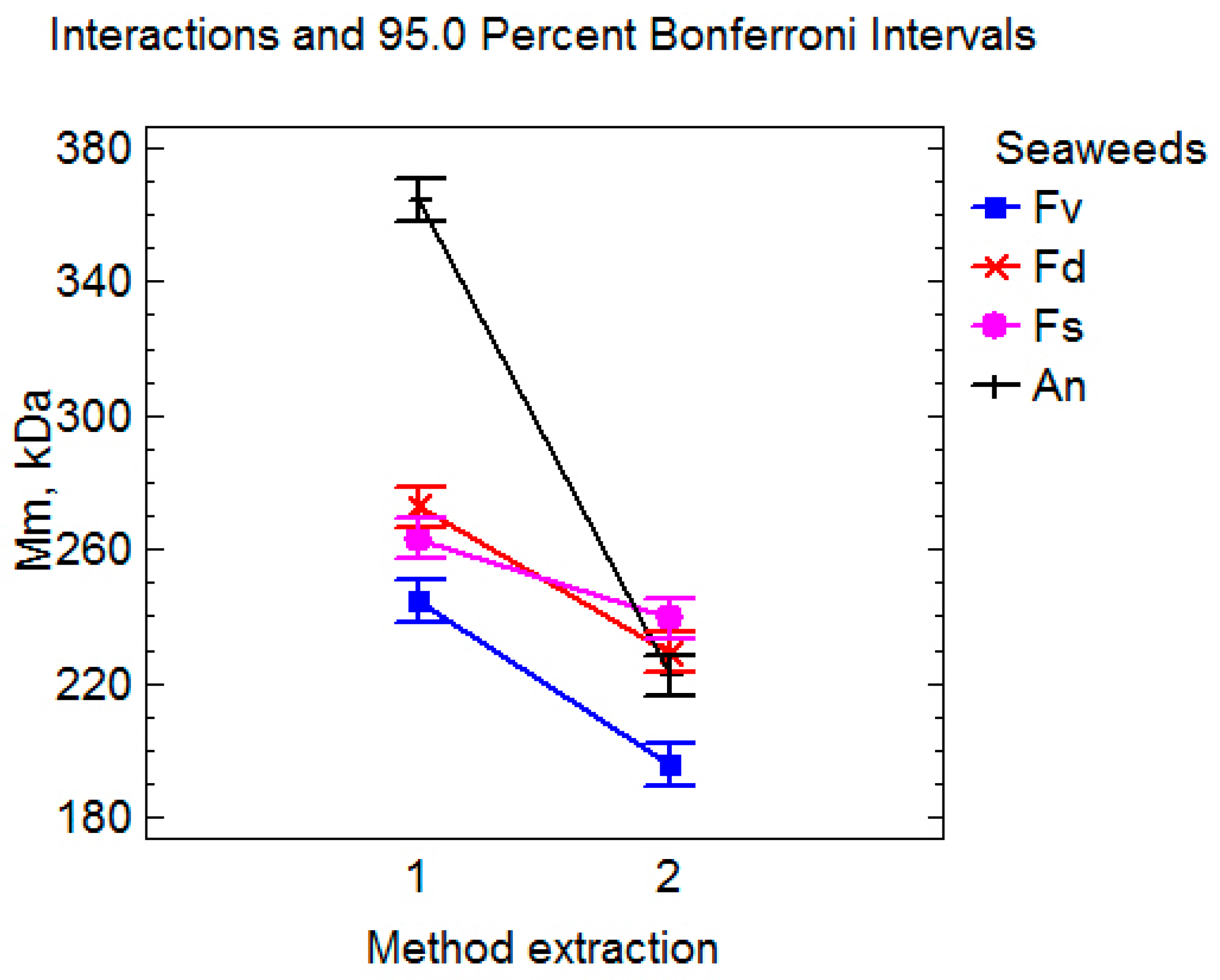
2.1. The Impact of Extraction Methods on the Yield and of Fucoidans Composition
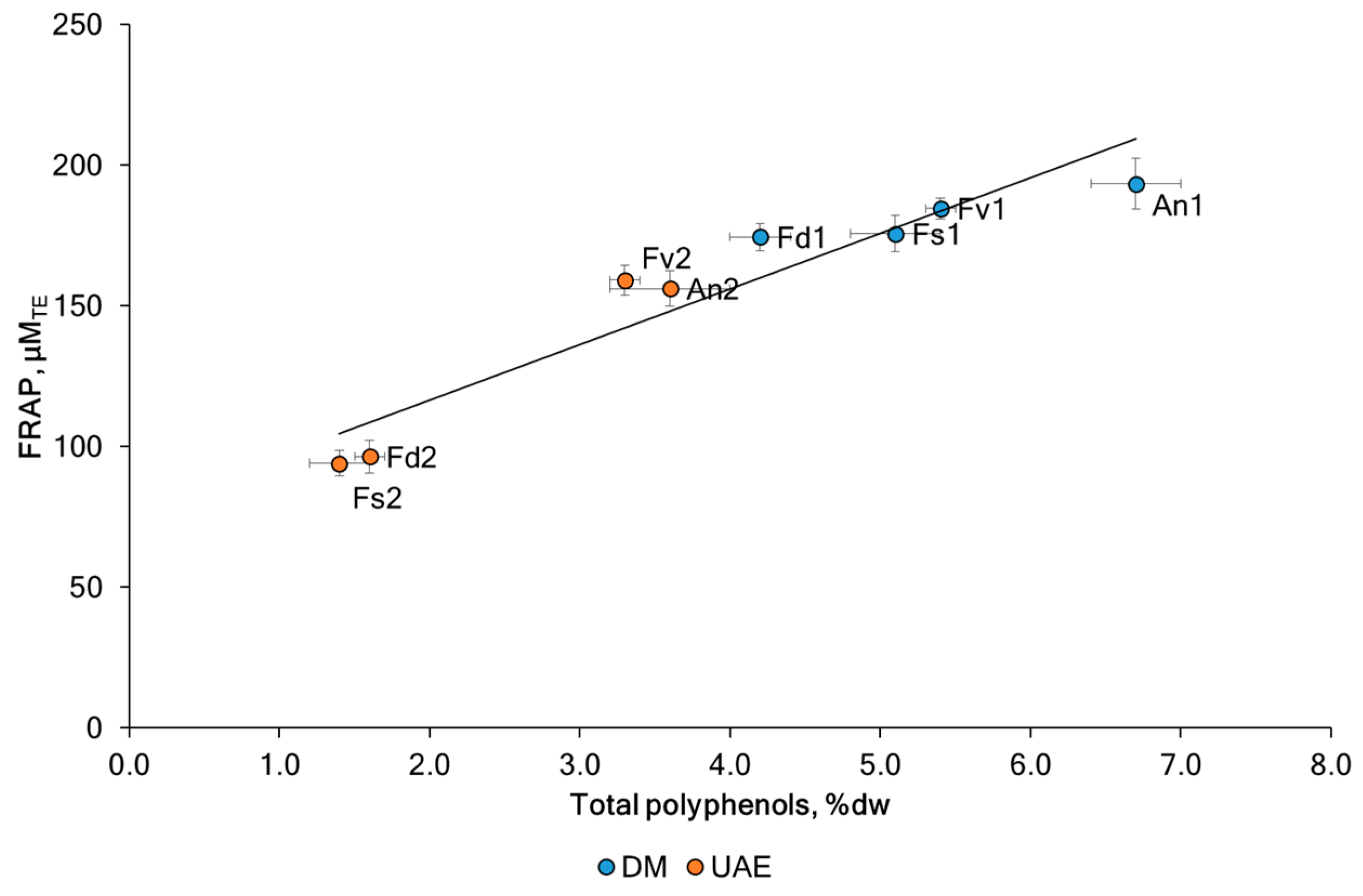
2.2. The Antioxidant Activities of Fucoidans
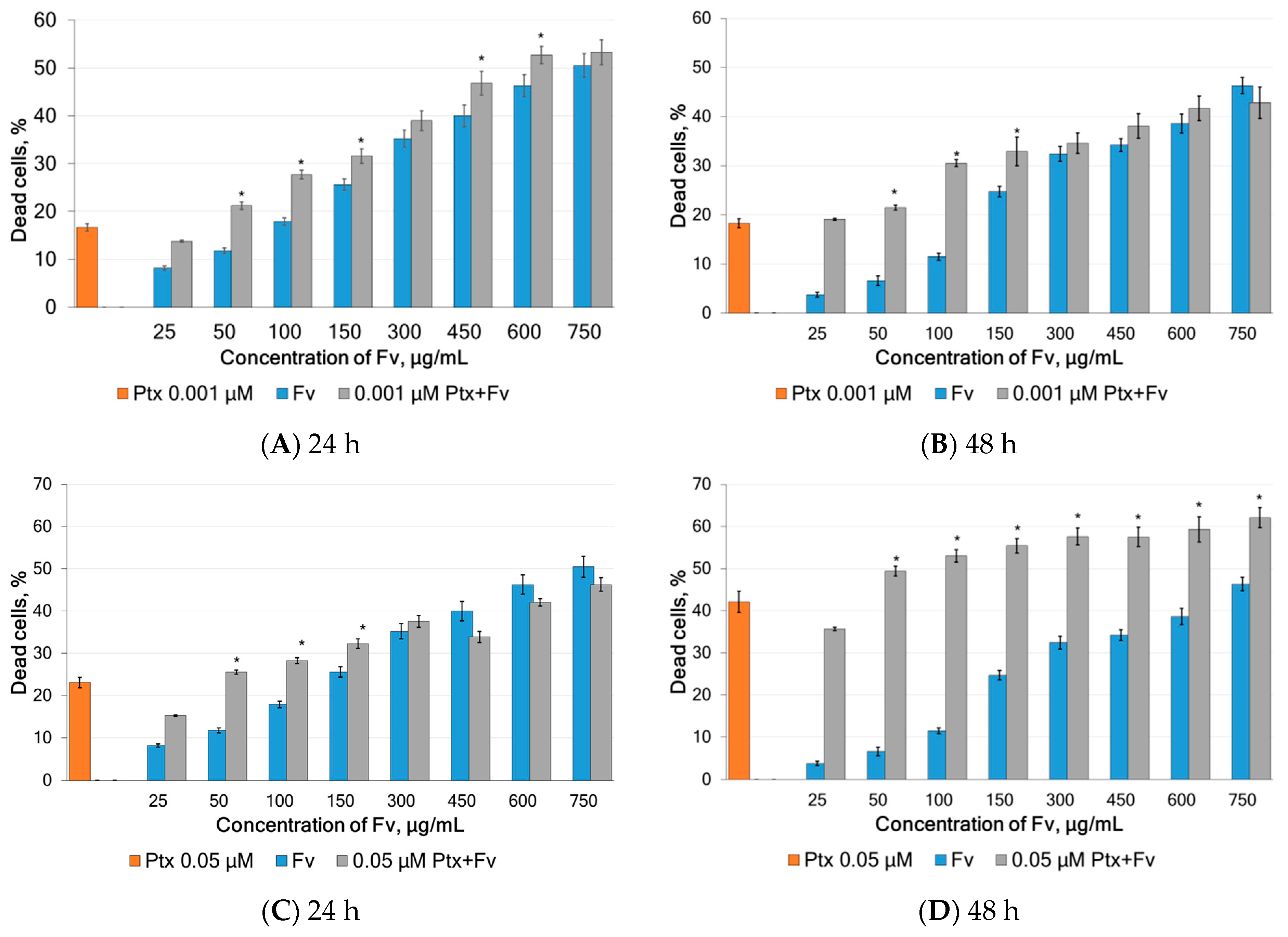
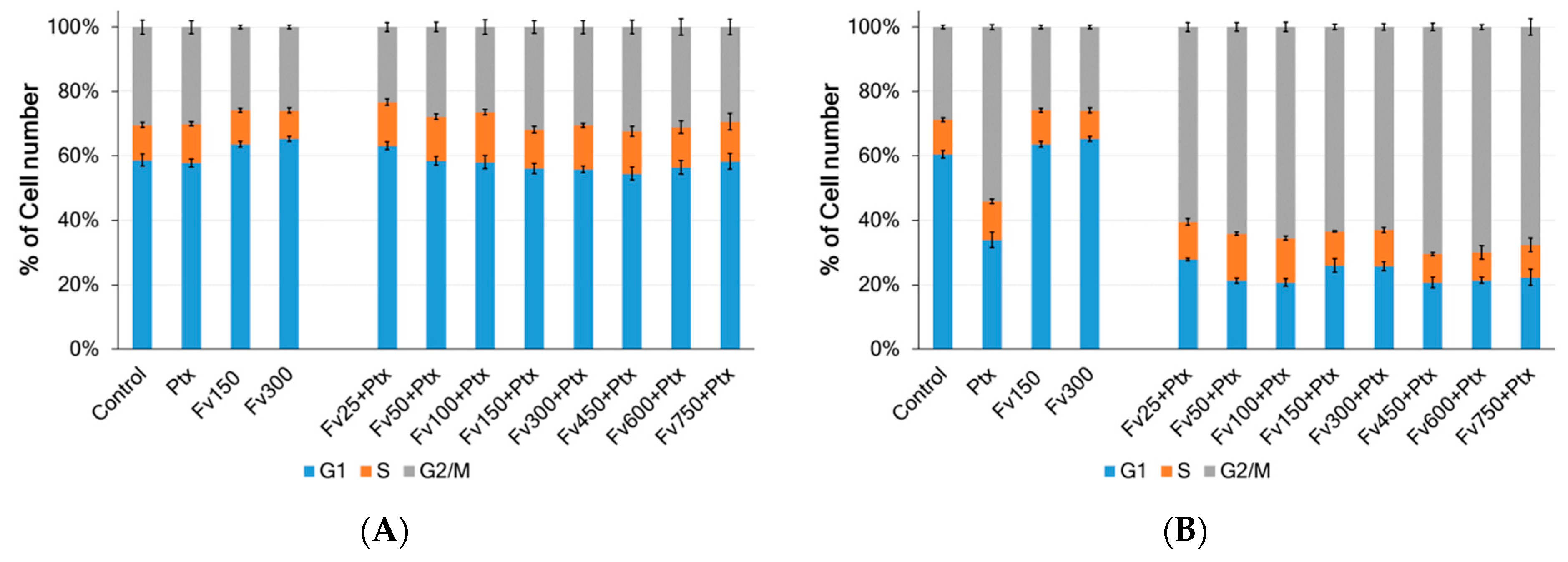
2.3. Anticancer Properties of Fucoidans
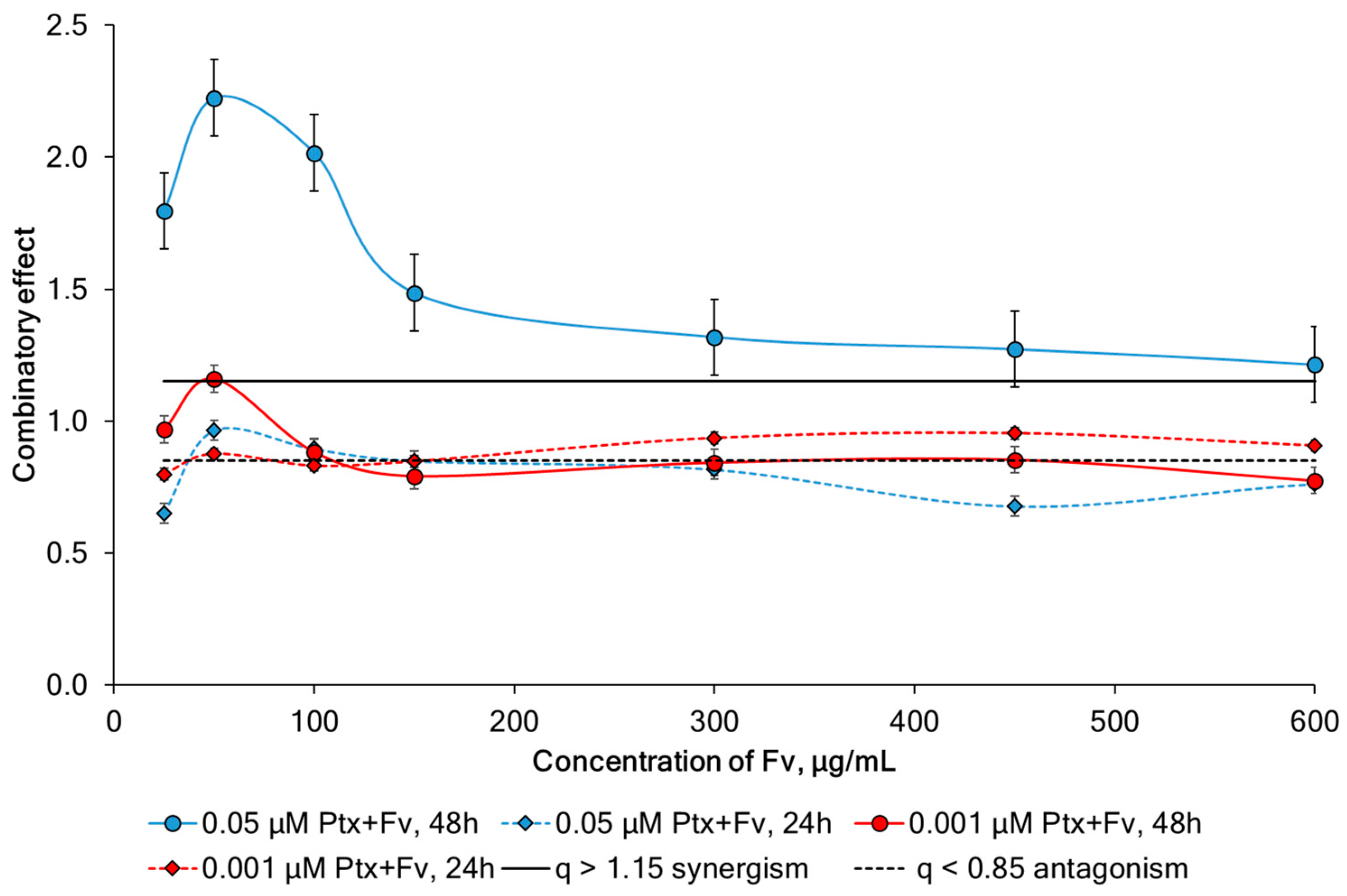
2.4. Study of Synergism
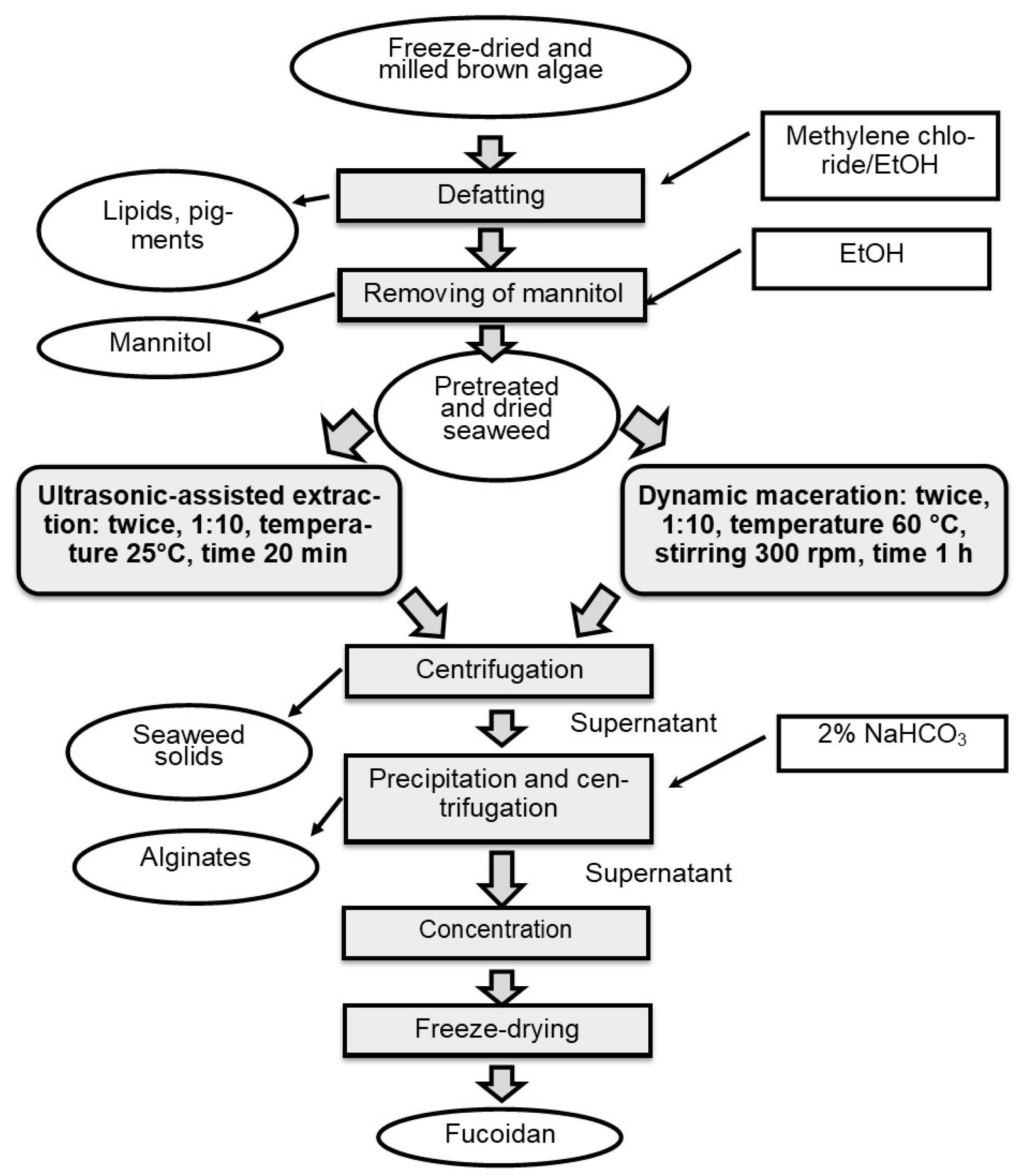
3. Materials and Methods
3.1. Sample Collection and Preparation of Alga
3.2. Materials
3.3. Fucoidan Extraction
3.3.1. Dynamic Maceration (DM)
3.3.2. Ultrasonic-Assisted Extraction (UAE)
3.4. Analysis of Fucoidan Composition
3.5. In Vitro Antioxidant Assays
3.6. Cell Culture
3.6.1. Proliferation Assay and Cell Cycle Analysis
3.6.2. Cytotoxicity Assay
3.6.3. Analysis of Drug Combinatory Effect
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Costantino, V.; Fattorusso, E.; Menna, M.; Taglialatela-Scafati, O. Chemical diversity of bioactive marine natural products: An illustrative case study. Curr. Med. Chem. 2004, 11, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2025; Available online: https://www.algaebase.org (accessed on 9 April 2025).
- WoRMS Editorial Board; World Register of Marine Species. 2025. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=145544 (accessed on 9 April 2025).
- Varzugina, M.A.; Makarchuk, R.N.; Yavorsky, A.S.; Nikolaenko, O.A.; Kuranova, L.K. Fucus algae of the Arctic region—Characteristics, areas of use. News of higher educational institutions. Arctic Region 2015, 1, 48–53. [Google Scholar]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Shepeleva, O.A.; Degteva, G.N.; Novikova, I.I.; Shevkun, I.G.; Romanenko, S.P.; Semenikhina, M.V.; Popova, O.N.; Gudkov, A.B. Seaweed as an important functional ingredient and alimentary raw material for enriching the diet of the population in the Arctic Zone of the Russian Federation (Review). J. Med. Biol. Res. 2024, 12, 99–113. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Cotas, J.; Pacheco, D.; Gonçalves, A.M.; Silva, P.; Carvalho, L.G.; Pereira, L. Seaweeds’ Nutraceutical and Biomedical Potential in Cancer Therapy: A Concise Review. J. Cancer Metastasis Treat. 2021, 7, 13. Available online: https://www.oaepublish.com/articles/2394-4722.2020.134 (accessed on 2 May 2025). [CrossRef]
- Park, G.H.; Cho, J.H.; Lee, D.; Kim, Y. Association between seafood intake and cardiovascular disease in South Korean adults: A community-based prospective cohort study. Nutrients 2022, 14, 4864. [Google Scholar] [CrossRef]
- Ren, Y.; Feng, Y.; Qing, J.; Zhang, P.; Xiao, L.; Liang, X. The correlation between nuts and algae-less diet and children’s blood pressure: From a cross-sectional study in Chongqing. Clin. Exp. Hypertens. 2023, 45, 2180024. [Google Scholar] [CrossRef]
- Kishida, R.; Yamagishi, K.; Muraki, I.; Sata, M.; Tamakoshi, A.; Iso, H.; JACC Study Group. Frequency of seaweed intake and its association with cardiovascular disease mortality: The JACC study. J. Atheroscl. Thromb. 2020, 27, 1340–1347. [Google Scholar] [CrossRef]
- Minami, Y.; Kanemura, S.; Oikawa, T.; Suzuki, S.; Hasegawa, Y.; Nishino, Y.; Fujiya, T.; Miura, K. Associations of Japanese food intake with survival of stomach and colorectal cancer: A prospective patient cohort study. Cancer Sci. 2020, 111, 2558–2569. [Google Scholar] [CrossRef]
- Senthil, S.L. A comprehensive review to assess the potential, health benefits and complications of fucoidan for developing as functional ingredient and nutraceutical. Int. J. Biol. Macromol. 2024, 277, 134226. [Google Scholar] [CrossRef]
- Lara-Hernández, G.; Ramos-Silva, J.A.; Pérez-Soto, E.; Figueroa, M.; Flores-Berrios, E.P.; Sánchez-Chapul, L.; Andrade-Cabrera, J.L.; Luna-Angulo, A.; Landa-Solís, C.; Avilés-Arnaut, H. Anticancer activity of plant tocotrienols, fucoxanthin, fucoidan, and polyphenols in dietary supplements. Nutrients 2024, 16, 4274. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In vitro anti-inflammatory activities of fucoidans from five species of brown seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs 2016, 14, 79. [Google Scholar] [CrossRef]
- Zayed, A.; Cao, H.T.T.; Trang, V.T.D.; Ulber, R. Structural tailoring of fucoidan backbones for maximizing their benefits: Enzymatic, chemical, and physical strategies. J. Appl. Phycol. 2023, 35, 2445–2462. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I. Fucoidans—Sulfated polysaccharides of brown algae. Russ. Chem. Rev. 2009, 78, 785. [Google Scholar] [CrossRef]
- Deghrigue, M.; Cherif, D.; Lajili, S.; ben Mesmia, H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Structural characterizations and bioactivities of fucoidans from Dyctyopteris membranaceae and Padina pavonica with in silico investigations. Int. J. Biol. Macromol. 2025, 307, 142133. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from fucoidan: New developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Al-Saedi, D.A.; Mensah, E.O.; Kanwugu, O.N.; Adadi, P.; Ulber, R. Fucoidan’s molecular targets: A comprehensive review of its unique and multiple targets accounting for promising bioactivities supported by in silico studies. Mar. Drugs 2023, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef]
- Kwak, J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Zhurishkina, E.V.; Lapina, I.M.; Ivanen, D.R.; Stepanov, S.I.; Shvetsova, S.V.; Shavarda, A.L.; Giliano, N.Y.; Kulminskaya, A.A. Effect of fucoidans isolated from seaweeds Laminaria digitata and Fucus vesiculosus on cell lines HeLa G-63, ECV 304 and PC 12. Tsitologya 2015, 57, 727–735. [Google Scholar] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Oh, B.; Kim, J.; Lu, W.; Rosenthal, D. Anticancer effect of fucoidan in combination with tyrosine kinase inhibitor lapatinib. Evid. Based Complement. Altern. Med. 2014, 2014, 865375. [Google Scholar] [CrossRef]
- Anjana, K.; Arunkumar, K. Brown algae biomass for fucoxanthin, fucoidan and alginate; update review on structure, biosynthesis, biological activities and extraction valorisation. Int. J. Biol. Macromol. 2014, 280, 135632. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hanjabam, M.D.; Kumar, A.; Tejpal, C.S.; Krishnamoorthy, E.; Kishore, P.; Kumar, K.A. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100200. [Google Scholar] [CrossRef]
- Zayed, A.; Hahn, T.; Finkelmeier, D.; Burger-Kentischer, A.; Rupp, S.; Krämer, R.; Ulber, R. Phenomenological investigation of the cytotoxic activity of fucoidan isolated from Fucus vesiculosus. Process Biochem. 2019, 81, 182–187. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Obluchinsksaya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N. Effects of ultrasound treatment on the chemical composition and anticoagulant properties of dry fucus extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Zayed, A.; Finkelmeier, D.; Hahn, T.; Rebers, L.; Shanmugam, A.; Burger-Kentischer, A.; Ulber, R. Characterization and cytotoxic activity of microwave-assisted extracted crude fucoidans from different brown seaweeds. Mar. Drugs 2023, 21, 48. [Google Scholar] [CrossRef]
- Manríquez-Zúñiga, A.N.; de la Torre, A.R.; Valdés-Santiago, L.; Hernández-Bustos, D.A.; Cuéllar-Sojo, S.; Hernández-Rayas, A.; Perez-Vega, S.; Molina-Guerrero, C.E. Broccoli leaves (Brassica oleracea var. italica) as a source of bioactive compounds and chemical building blocks: Optimal extraction using dynamic maceration and life cycle assessment. Sustainability 2023, 15, 16616. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Zorić, Z.; Pedisić, S.; Repajić, M.; Roje, M.; Herceg, Z.; Čož-Rakovac, R.; Dragović-Uzelac, V. Application of ultrasound-assisted extraction and non-thermal plasma for Fucus virsoides and Cystoseira barbata polysaccharides pre-treatment and extraction. Processes 2022, 10, 433. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Lee, G.; Hyun, J.; Ryu, B. Systematic characteristics of fucoidan: Intriguing features for new pharmacological interventions. Int. J. Mol. Sci. 2024, 25, 11771. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Usoltseva, R.V.; Shevchenko, N.M.; Surits, V.V.; Imbs, T.I.; Malyarenko, O.S.; Besednova, N.N.; Ivanushko, L.A.; Ermakova, S.P. Structural diversity of fucoidans and their radioprotective effect. Carbohydr. Polym. 2021, 273, 118551. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Bucciol, F.; Chaji, S.; Cravotto, G. Mechanochemical degradation of biopolymers. Molecules 2023, 28, 8031. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N. Influence of ultrasound extraction of Fucus vesiculosus on the kinetics of fucoidan degradation and its properties. Drug Dev. Regist. 2025, 14, 54–63. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N. The efficacy of two methods for extracting fucoidan from frozen Arctic algae thalli: Chemical composition, kinetic study and process optimization. J. Appl. Phycol. 2024, 36, 1413–1432. [Google Scholar] [CrossRef]
- Ivanova, J.G.; Toshkova-Yotova, T.S.; Toshkova, R.A.; Deleva, V.R.; Georgieva, A.K.; Gigova, L.G. Antioxidant and anticancer potential of extracellular polysaccharide from Porphyridium aerugineum (Rhodophyta). Fermentation 2024, 10, 259. [Google Scholar] [CrossRef]
- Lahrsen, E.; Schoenfeld, A.K.; Alban, S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydr. Polym. 2018, 189, 162–168. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Purification and molecular characterization of fucoidan isolated from Ascophyllum nodosum brown seaweed grown in Ireland. Mar. Drugs 2023, 21, 315. [Google Scholar] [CrossRef]
- Hans, N.; Pattnaik, F.; Malik, A.; Naik, S. Comparison of different green extraction techniques and their influence on chemical characteristics of sulfated polysaccharide (fucoidan) from Padina tetrastromatica and Turbinaria conoides. Algal Res. 2023, 74, 103199. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant content and activity of the seaweed Saccharina latissima: A seasonal perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Zhurishkina, E.V.; Stepanov, S.I.; Ayrapetyan, O.N.; Skorik, Y.A.; Vlasova, E.N.; Kruchina-Bogdanov, I.V.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. The effect of polydisperse fucoidans from Fucus vesiculosus on Hep G2 and Chang liver cells. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100209. [Google Scholar] [CrossRef]
- Cabral, E.M.; Mondala, J.R.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- Mathew, L.; Burney, M.; Gaikwad, A.; Nyshadham, P.; Nugent, E.K.; Gonzalez, A.; Smith, J.A. Preclinical evaluation of safety of fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus for use in cancer treatment. Integr. Cancer Ther. 2017, 16, 572–584. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Shao, Y.; Xu, J.; Liang, B.; Liu, W.; Zheng, J.; Li, C.; Guan, H.; Wang, S.; et al. Marine sulfated polysaccharide PMGS synergizes with paclitaxel in inhibiting cervical cancer in vitro. Mar. Drugs 2023, 21, 259. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Bioactivities of Nizamuddinia zanardinii sulfated polysaccharides extracted by enzyme, ultrasound and enzyme-ultrasound methods. J. Food Sci. Technol. 2019, 56, 1212–1220. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, J.; Wu, Z.G.; Yang, J.M.; Li, W.; Shen, L.X. Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2016, 93, 136–144. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT-Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ravindran, R.; Walsh, O.; O’Doherty, J.; Jaiswal, A.K.; Tiwari, B.K.; Rajauria, G. Evaluation of ultrasound, microwave, ultrasound–microwave, hydrothermal and high pressure assisted extraction technologies for the recovery of phytochemicals and antioxidants from brown macroalgae. Mar. Drugs 2021, 19, 309. [Google Scholar] [CrossRef]
- Athukorala, Y.; Ahn, G.N.; Jee, Y.H.; Kim, G.Y.; Kim, S.H.; Ha, J.H.; Kang, J.S.; Lee, K.W.; Jeon, Y.J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J. Appl. Phycol. 2009, 21, 307–314. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.M.; Kurup, G.M. Sulfated polysaccharides from Padina tetrastromatica arrest cell cycle, prevent metastasis and downregulate angiogenic mediators in HeLa cells. Bioact. Carbohydr. Diet. Fibre 2017, 12, 7–13. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Y.; Wang, R.; Najafi, M. Mechanisms of cancer cell death induction by paclitaxel: An updated review. Apoptosis 2022, 27, 647–667. [Google Scholar] [CrossRef]
- Asnaashari, S.; Amjad, E.; Sokouti, B. Synergistic effects of flavonoids and paclitaxel in cancer treatment: A systematic review. Cancer Cell Int. 2023, 23, 211. [Google Scholar] [CrossRef]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour Dizaj, T.; Safaeian, A.; Bakhshi, A.; Abazari, O.; et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene 2021, 787, 145638. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Abudabbus, A.; Badmus, J.A.; Shalaweh, S.; Bauer, R.; Hiss, D. Effects of fucoidan and chemotherapeutic agent combinations on malignant and non-malignant breast cell lines. Curr. Pharm. Biotechnol. 2017, 18, 748–757. [Google Scholar] [CrossRef]
- Thakur, V.; Lu, J.; Roscilli, G.; Aurisicchio, L.; Cappelletti, M.; Pavoni, E.; White, W.L.; Bedogni, B. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget 2017, 8, 17887. [Google Scholar] [CrossRef]
- Blaszczak, W.; Lach, M.S.; Barczak, W.; Suchorska, W.M. Fucoidan exerts anticancer effects against head and neck squamous cell carcinoma in vitro. Molecules 2018, 23, 3302. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Smirnova, G.P.; Klochkova, N.G. Polysaccharides of algae: 55. Polysaccharide composition of several brown algae from Kamchatka. Russ. J. Bioorg. Chem. 2001, 27, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Dische, Z.; Shettles, L.B.; Osnos, M. New specific color reactions of hexoses and spectrophotometric micro-metods for their determination. Arch. Biochem. 1949, 22, 169–184. [Google Scholar] [PubMed]
- Silvestri, L.J.; Hurst, R.E.; Simpson, L.; Settine, J.M. Analysis of sulfate in complex carbohydrates. Anal. Biochem. 1982, 123, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W. Colorimetric determination of hexuronic acids in plant materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Voskoboinikov, G.M.; Galynkin, V.A. Contents of alginic acid and fuccidan in Fucus algae of the Barents Sea. Appl. Biochem. Microbiol. 2002, 38, 186–188. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown alga Durvillaea antarctica. Dry. Technol. 2020, 38, 1915–1928. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Molecular weight distribution of polysaccharides from edible seaweeds by high-performance size-exclusion chromatography (HPSEC). Talanta 2012, 93, 153–159. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.; Henderson, T.; Nigam, P.; Owusu-Apenten, R. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Johnson, S.; Nguyen, V.; Coder, D. Assessment of cell viability. Curr. Protoc. Cytom. 2013, 64, 9.2.1–9.2.26. [Google Scholar] [CrossRef] [PubMed]
- Giliano, N.Y.; Noskin, L.A.; Zhurishkina, E.V.; Stepanov, S.I.; Ibatullin, F.M.; Torshin, B.I.; Yakunina, E.B.; Alchinova, I.B. The combination of low doses of glucosamine D and 2-DG enhances the cytotoxic effect in human tumor cells in culture. Pathol. Physiol. Exp. Ther. 2019, 63, 41–49. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture; Methods in Molecular Biology; Cree, I., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 237–245. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH• and ABTS• assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2018, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Boehm, A.; Reiche, A.; Diet, A.; Mozet, C.; Wichmann, G. Combined effects of lapatinib and cisplatin on colony formation of head and neck squamous cell carcinoma. Anticancer Res. 2012, 32, 3191–3199. [Google Scholar]



| Species | Extraction Method | Sample | Fucoidan Yield, %dw | Fucoidan, %dw | Mw, kDa | IC50, μg/mL | TPC, %dw | FRAP, μMTE |
|---|---|---|---|---|---|---|---|---|
| F. vesiculosus | DM | Fv1 | 9.4 ± 0.4 * | 59.4 ± 0.2 * | 244 ± 8 * | 368 ± 13 * | 5.4 ± 0.1 * | 185 ± 4 * |
| UAE | Fv2 | 17.4 ± 0.3 | 64.1 ± 0.4 | 195 ± 11 | 640 ± 12 | 3.3 ± 0.1 | 159 ± 5 | |
| F. distichus | DM | Fd1 | 11.2 ± 0.3 * | 58.8 ± 0.3 * | 273 ± 7 * | 626 ± 20 * | 4.2 ± 0.2 * | 175 ± 5 * |
| UAE | Fd2 | 14.9 ± 0.7 | 63.5 ± 0.2 | 229 ± 9 | 1212 ± 34 | 1.6 ± 0.1 | 96 ± 6 | |
| F. serratus | DM | Fs1 | 8.0 ± 0.3 * | 53.1 ± 0.2 * | 263 ± 8 * | 301 ± 17 * | 5.1 ± 0.3 * | 176 ± 6 * |
| UAE | Fs2 | 12.6 ± 0.5 | 57.7 ± 0.5 | 239 ± 6 | 1498 ± 54 | 1.4 ± 0.2 | 94 ± 4 | |
| A. nodosum | DM | An1 | 12.4 ± 0.4 * | 58.8 ± 0.6 * | 364 ± 10 * | 667 ± 20 * | 6.7 ± 0.3 * | 194 ± 9 * |
| UAE | An2 | 13.8 ± 0.3 | 60.7 ± 0.1 | 222 ± 7 | 1042 ± 30 | 3.6 ± 0.4 | 156 ± 6 |
| Sample | Monosaccharide Composition, mol.% | Uronic Acid, % | Sulfate, % | ||||
|---|---|---|---|---|---|---|---|
| Fucose | Xylose | Mannose | Glucose | Galactose | |||
| Fv1 | 71.9 ± 0.9 * | 9.4 ± 0.4 | 6.0 ± 0.2 | 8.3 ± 0.3 | 4.4 ± 0.1 * | 5.3 ± 0.4 * | 25.4 ± 0.3 * |
| Fv2 | 75.7 ± 1.1 | 7.6 ± 0.5 | 4.3 ± 0.1 | 5.8 ± 0.4 | 6.6 ± 0.1 | 6.4 ± 0.3 | 23.8 ± 0.2 |
| Fd1 | 63.6 ± 1.2 * | 4.2 ± 0.3 | 4.8 ± 0.2 | 21.6 ± 0.7 | 5.8 ± 0.2 * | 5.9 ± 0.5 * | 26.6 ± 0.5 * |
| Fd2 | 68.2 ± 0.8 | 5.8 ± 0.3 | 4.7 ± 0.2 | 17.9 ± 0.9 | 3.4 ± 0.1 | 7.0 ± 0.5 | 25.4 ± 0.3 |
| Fs1 | 69.7 ± 0.7 * | 5.9 ± 0.5 | 8.1 ± 0.3 | 3.0 ± 0.2 | 13.3 ± 0.3 * | 4.1 ± 0.4 * | 22.4 ± 0.4 * |
| Fs2 | 72.8 ± 1.0 | 4.5 ± 0.4 | 7.5 ± 0.2 | 4.4 ± 0.3 | 10.8 ± 0.4 | 4.8 ± 0.2 | 20.9 ± 0.2 |
| An1 | 47.8 ± 1.2 * | 12.4 ± 0.3 | 14.3 ± 0.4 | 13.3 ± 0.4 | 12.2 ± 0.5 | 3.3 ± 0.6 * | 17.6 ± 0.2 * |
| An2 | 49.3 ± 0.9 | 11.7 ± 0.5 | 14.0 ± 0.5 | 13.4 ± 0.3 | 11.6 ± 0.4 | 4.5 ± 0.5 | 16.4 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obluchinskaya, E.D.; Pozharitskaya, O.N.; Lapina, I.M.; Kulminskaya, A.A.; Zhurishkina, E.V.; Shikov, A.N. Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties. Mar. Drugs 2025, 23, 230. https://doi.org/10.3390/md23060230
Obluchinskaya ED, Pozharitskaya ON, Lapina IM, Kulminskaya AA, Zhurishkina EV, Shikov AN. Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties. Marine Drugs. 2025; 23(6):230. https://doi.org/10.3390/md23060230
Chicago/Turabian StyleObluchinskaya, Ekaterina D., Olga N. Pozharitskaya, Irina M. Lapina, Anna A. Kulminskaya, Elena V. Zhurishkina, and Alexander N. Shikov. 2025. "Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties" Marine Drugs 23, no. 6: 230. https://doi.org/10.3390/md23060230
APA StyleObluchinskaya, E. D., Pozharitskaya, O. N., Lapina, I. M., Kulminskaya, A. A., Zhurishkina, E. V., & Shikov, A. N. (2025). Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties. Marine Drugs, 23(6), 230. https://doi.org/10.3390/md23060230










