A Marine-Derived Sterol, Ergosterol, Mitigates UVB-Induced Skin Photodamage via Dual Inhibition of NF-κB and MAPK Signaling
Abstract
1. Introduction
2. Results
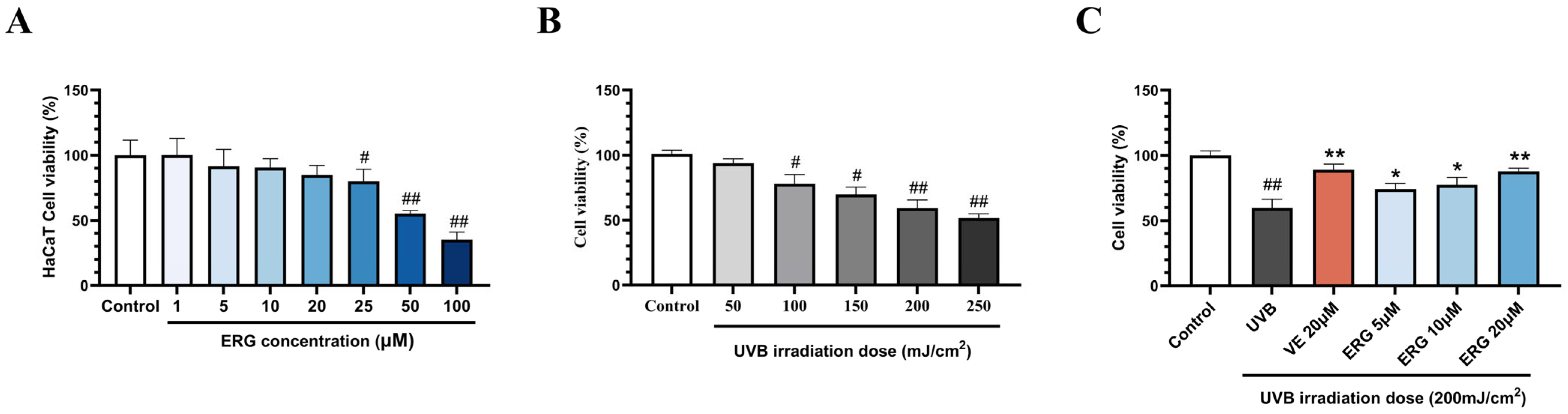
2.1. ERG Alleviates UVB-Induced Photodamage in HaCaT Cells
2.2. ERG Mitigates UVB-Induced ROS in HaCaT Cells
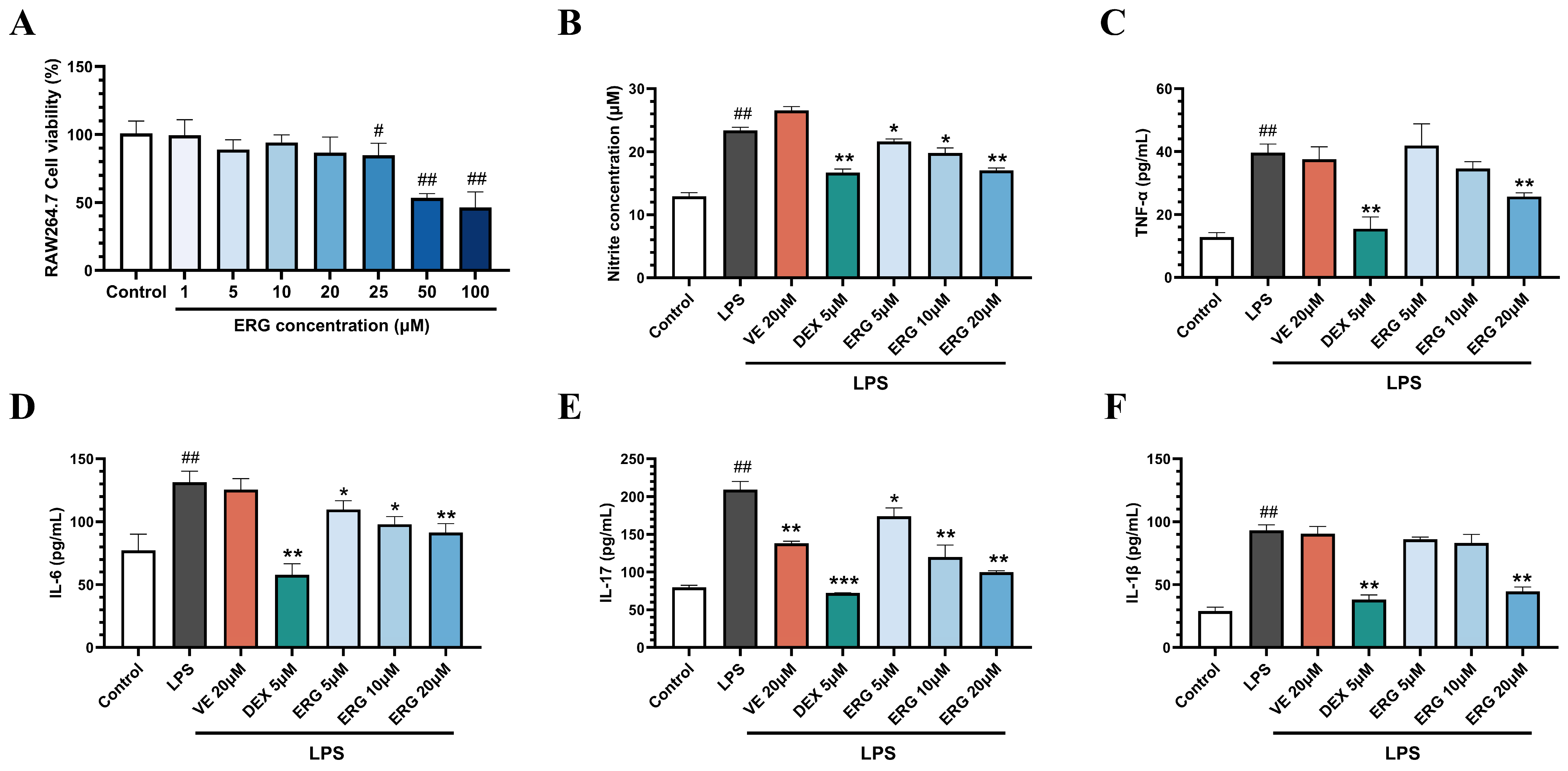
2.3. ERG Alleviates Inflammation in LPS-Induced RAW264.7 Cells
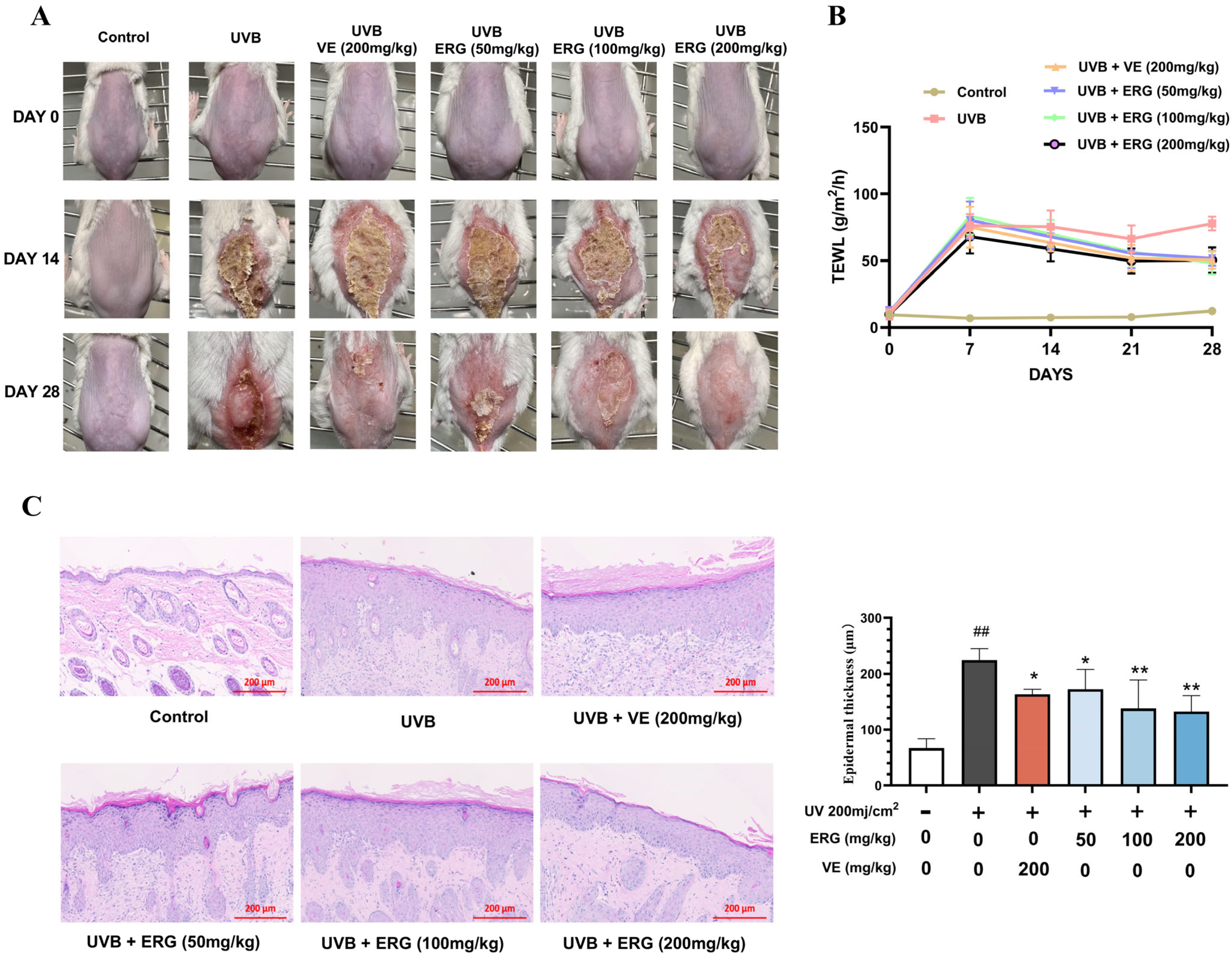
2.4. ERG Alleviates UVB-Induced Epidermal Damage and Barrier Dysfunction in Mice
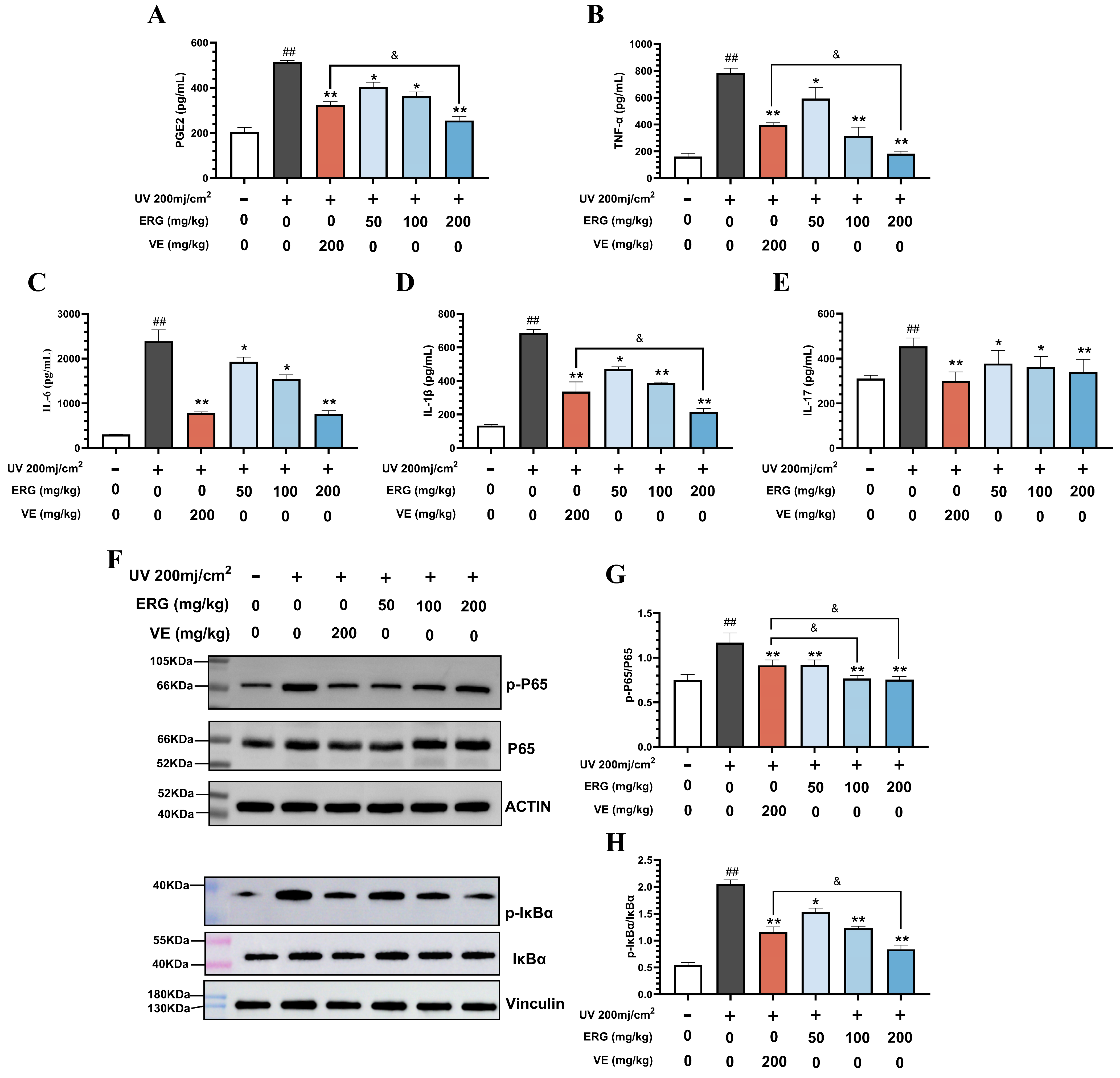
2.5. ERG Suppresses UVB-Induced Skin Inflammation in Mice by Inhibiting the NF-κB Pathway
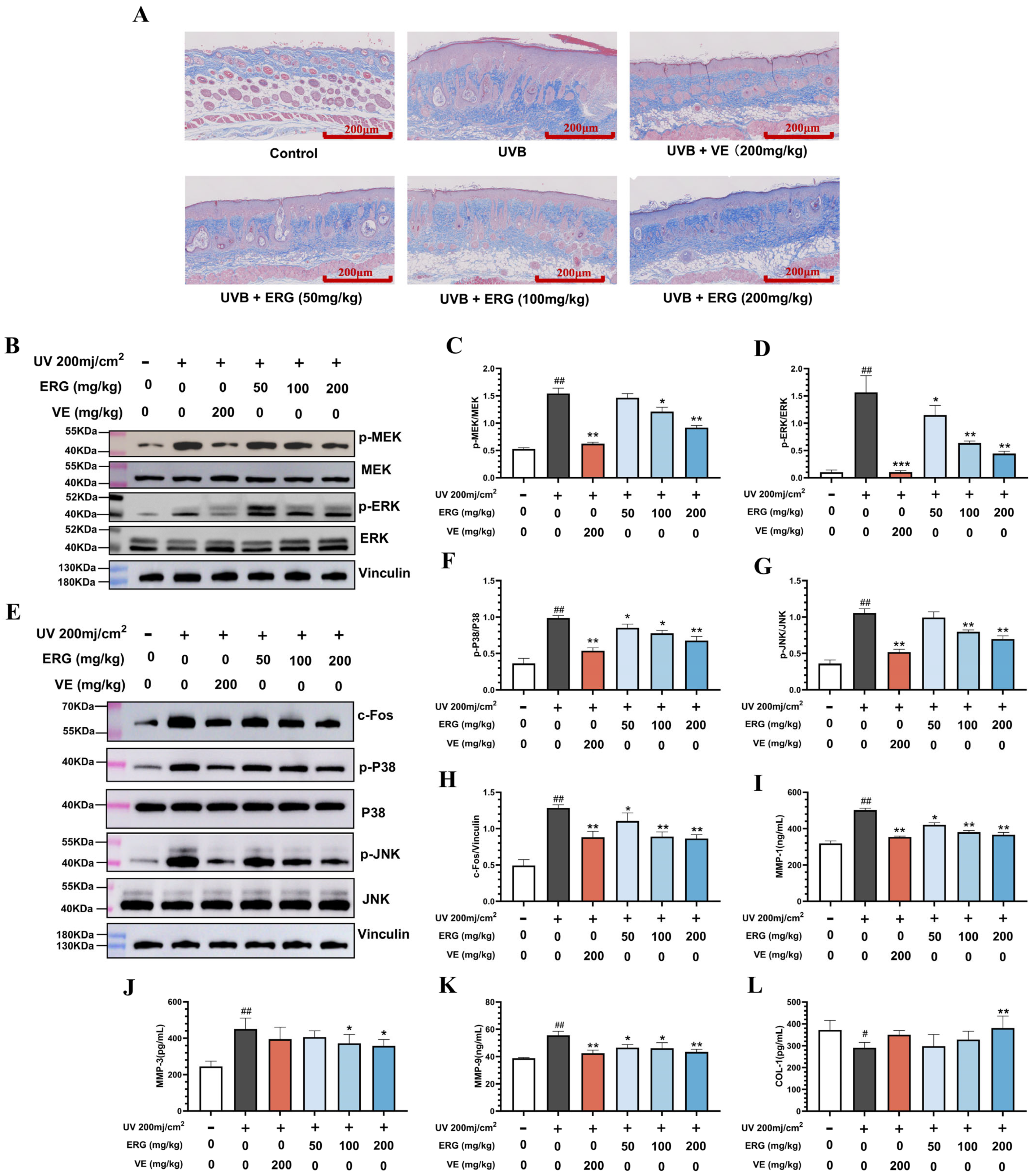
2.6. ERG Proctects the Dermal Collagen Matrix in Mouse Skin by Suppressing the MAPK Pathway
2.7. Transcriptomic Analysis Reveals ERG’s Global Regulatory Effects on UVB-Induced Skin Damage
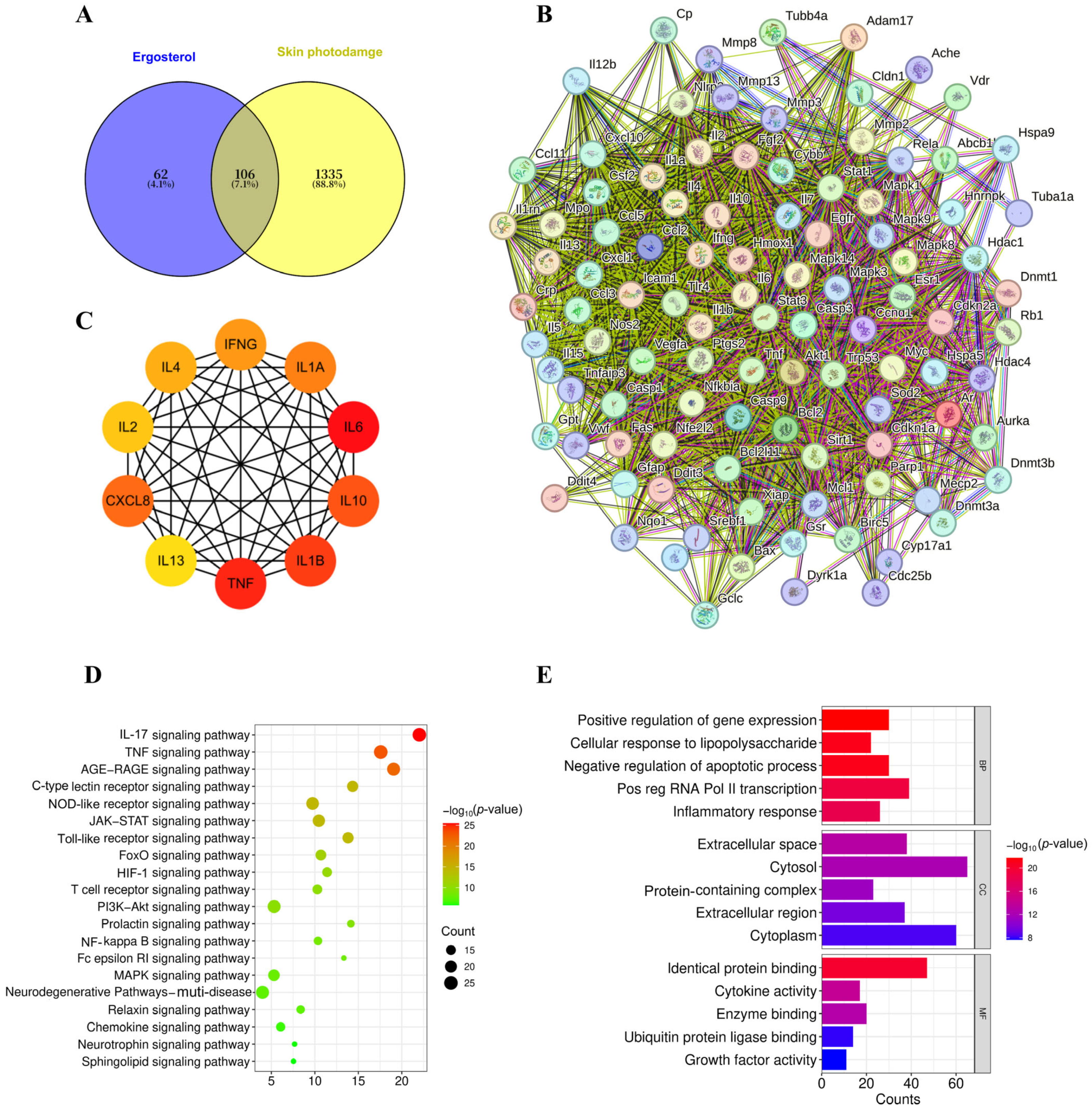
2.8. Network Pharmacological Analysis Reinforces ERG’s Multi-Target Mechanisms
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. MTT Assay for Cell Viability
4.4. Measurement of Intracellular ROS Production
4.5. Nitric Oxide Determination
4.6. Enzyme-Linked Immunosorbent Assay
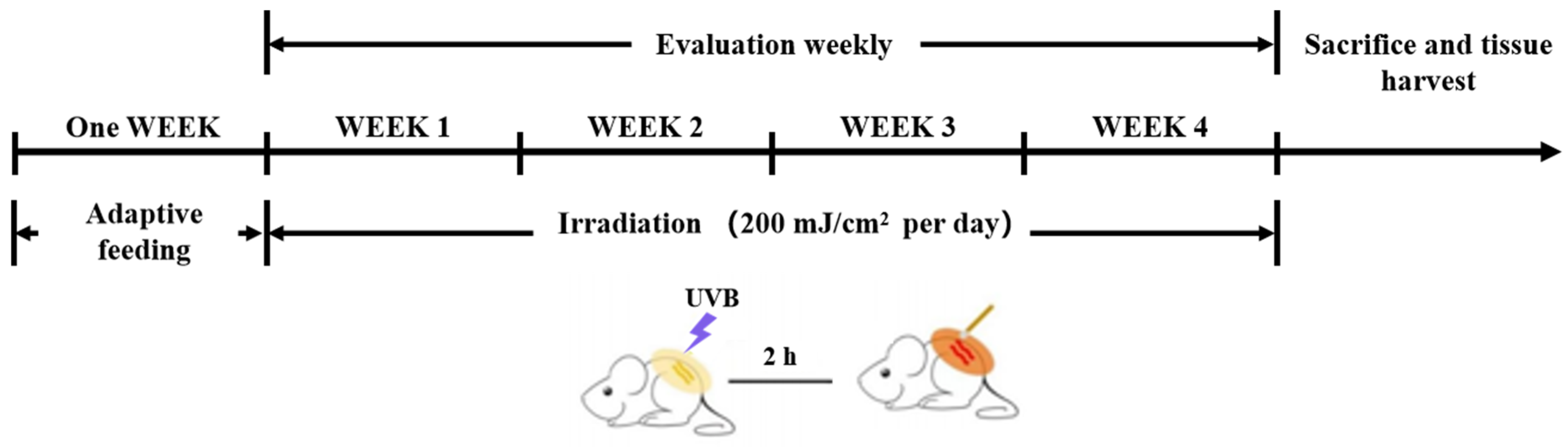
4.7. Animal Experiments
4.8. H&E Staining
4.9. Western Blot Analysis
4.10. Immunohistochemistry
4.11. Masson Staining
4.12. RNA-Seq and Transcriptome Analysis
4.13. Network Pharmacological Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) |
| AGE | advanced glycation end product |
| AG | aminoguanidine |
| Akt | protein kinase B |
| BSA | bovine serum albumin |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| Cu2+ | copper ion |
| DMSO | dimethyl sulfoxide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DEGs | differentially expressed genes |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| FDR | false discovery rate |
| FPKM | fragments per kilobase of transcript per million mapped reads |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GSH | glutathione |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GO | gene ontology |
| GSK-3β | glycogen synthase kinase-3 beta |
| IC50 | half-maximal inhibitory concentration |
| IL-18 | interleukin 18 |
| IL-33 | interleukin 33 |
| JAK-STAT | Janus kinase-signal transducer and activator of transcription |
| JNK | c- Jun N-terminal kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK | mitogen-activated protein kinase |
| MC1R | melanocortin 1 receptor |
| MGO | methylglyoxal |
| MITF | microphthalmia-associated transcription factor |
| MMP | matrix metalloproteinase |
| mRNA | messenger RNA |
| MSH | melanocyte-stimulating hormone |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PBS | phosphate-buffered saline |
| PCA | principal component analysis |
| PGE2 | prostaglandin E2 |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | protein kinase A |
| PMSF | phenylmethylsulphonyl fluoride |
| qPCR | quantitative polymerase chain reaction |
| RNA-seq | RNA sequencing |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SP | Spirulina peptides |
| TNF | tumor necrosis factor |
| TRP-1 | tyrosinase-related protein 1 |
| TRP-2 | tyrosinase-related protein 2 |
| TYR | tyrosinase |
| UV | ultraviolet |
| UVB | ultraviolet B |
References
- Pedic, L.; Pondeljak, N.; Situm, M. Recent information on photoaging mechanisms and the preventive role of topical sunscreen products. Acta Dermatovenerol. Alp. Pannonica Et Adriat. 2020, 29, 201–207. [Google Scholar]
- Li, Z.; Jiang, R.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Panax ginseng C. A. Meyer Phenolic Acid Extract Alleviates Ultraviolet B-Irradiation-Induced Photoaging in a Hairless Mouse Skin Photodamage Model. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 9962007. [Google Scholar] [CrossRef]
- Muhammad, N.; Sulong, M.S.; Bakar, M.F.A.; Latif Abu Bakar, M.A.; Mayzan, M.Z.H.; Rahim, N.F.A.; Rashidi, W.N.A.S.W.M.; Pauzi, A.N.; Hussin, N.B.; Ismail, N.I.F.N.; et al. Electronic nose investigation and antioxidant assessment of CHARMS™ skincare cosmetics toward skin tone improvement. J. Dermatol. Sci. Cosmet. Technol. 2025, 2, 100060. [Google Scholar] [CrossRef]
- Lone, A.N.; Malik, A.T.; Naikoo, H.S.; Raghu, R.S.; Tasduq, S.A. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J. Photochem. Photobiol. B Biol. 2020, 202, 111720. [Google Scholar]
- Zhang, X.J.; Yang, P.Y.; Ding, L.; Wang, J.; Li, X.L.; Xiao, W.L. Isolicoflavonol alleviates UVB-induced photodamage via protecting mitochondria and blocking the activation of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2025, 497, 117262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Han, R.; Wu, M.; Zhou, J.; Zhao, P.; Cui, B. A cutting-edge atomization-based methodology for enhancing formulation ability to resist photoaging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100045. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, W.; Tang, Y.; He, F.; Liang, B.; Chen, J.; Li, H.; Zhu, H. Curcumin targets YAP1 to enhance mitochondrial function and autophagy, protecting against UVB-induced photodamage. Front. Immunol. 2025, 16, 1566287. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, C.; Zhang, K.; Yang, X.; Feng, R.; Zong, Y.; He, Z.; Zhao, Y.; Du, R. Pilose Antler Protein Relieves UVB-Induced HaCaT Cells and Skin Damage. Molecules 2024, 29, 4060. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kong, C.; Cheng, S.; Xu, X.; Zhang, J. Succinoglycan riclin relieves UVB-induced skin injury with anti-oxidant and anti-inflammatory properties. Int. J. Biol. Macromol. 2023, 235, 123717. [Google Scholar] [CrossRef]
- Saitoh, Y.; Tanaka, A.; Hyodo, S. Protective Effects of Polyvinylpyrrolidone-Wrapped Fullerene Against Nitric Oxide/Peroxynitrite-Induced Cellular Injury in Human Skin Keratinocytes. J. Nanosci. Nanotechnol. 2021, 21, 4579–4585. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, C.; Juhasz, T.; Fidrus, E.; Janka, E.A.; Juhasz, G.; Boros, G.; Paragh, G.; Uray, K.; Emri, G.; Remenyik, E.; et al. Cyclobutane pyrimidine dimers from UVB exposure induce a hypermetabolic state in keratinocytes via mitochondrial oxidative stress. Redox Biol. 2021, 38, 101808. [Google Scholar] [CrossRef]
- Fidrus, E.; Hegedus, C.; Janka, E.A.; Paragh, G.; Emri, G.; Remenyik, E. Inhibitors of Nucleotide Excision Repair Decrease UVB-Induced Mutagenesis-An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 1638. [Google Scholar] [CrossRef]
- Togsverd-Bo, K.; Philipsen, P.A.; Haedersdal, M.; Wulf, H.C.O. Skin autofluorescence reflects individual seasonal UV exposure, skin photodamage and skin cancer development in organ transplant recipients. J. Photochem. Photobiol. B Biol. 2018, 178, 577–583. [Google Scholar] [CrossRef]
- Zhong, J.; Liang, L.; Zhao, N.; Wang, J.; Shu, P. Synergistic effects of retinol and retinyl palmitate in alleviating UVB-induced DNA damage and promoting the homologous recombination repair in keratinocytes. Front. Pharmacol. 2025, 16, 1562244. [Google Scholar] [CrossRef]
- Ma, X.Y.; Wang, H.N.; Sun, L.X.; Sun, J.; Jin, S.H.; Dai, F.X.; Sai, C.M.; Zhang, Z. Bioactive steroids from marine-derived fungi: A review (2015–2023). J. Asian Nat. Prod. Res. 2025, 27, 1236–1262. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Carreon-Palau, L.; Ozdemir, N.S.; Parrish, C.C.; Parzanini, C. Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico. Mar. Drugs 2020, 18, 598. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Qin, K.; Deng, Y.; Xue, P.; Huang, C.; Liu, S.; Hu, Z. Expression pattern, subcellular localization of Aspergillus oryzae ergosterol synthases, and their effects on ergosterol and fatty acid metabolism. Appl. Environ. Microbiol. 2025, 91, e0227324. [Google Scholar] [CrossRef]
- Zhou, X.; Hilk, A.; Solis, N.V.; Pereira De Sa, N.; Hogan, B.M.; Bierbaum, T.A.; Del Poeta, M.; Filler, S.G.; Burrack, L.S.; Selmecki, A. Erg251 has complex and pleiotropic effects on sterol composition, azole susceptibility, filamentation, and stress response phenotypes. PLoS Pathog. 2024, 20, e1012389. [Google Scholar] [CrossRef]
- Dupont, S.; Fleurat-Lessard, P.; Cruz, R.G.; Lafarge, C.; Grangeteau, C.; Yahou, F.; Gerbeau-Pissot, P.; Abrahao Junior, O.; Gervais, P.; Simon-Plas, F.; et al. Antioxidant Properties of Ergosterol and Its Role in Yeast Resistance to Oxidation. Antioxidants 2021, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Makitaipale, J.; Opsomer, H.; Steiner, R.; Riond, B.; Liesegang, A.; Clauss, M.; Hatt, J.M. Serum vitamin D concentrations in rabbits (Oryctolagus cuniculus) are more affected by UVB irradiation of food than irradiation of animals. Vet. J. (Lond. Engl. 1997) 2024, 306, 106149. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Indolfi, C.; Strisciuglio, C. Vitamin D: Immunomodulatory Aspects. J. Clin. Gastroenterol. 2018, 52 (Suppl. S1), S86–S88. [Google Scholar] [CrossRef]
- Sun, P.; Li, W.; Guo, J.; Peng, Q.; Ye, X.; Hu, S.; Liu, Y.; Liu, W.; Chen, H.; Qiao, J.; et al. Ergosterol Isolated from Antrodia camphorata Suppresses LPS-Induced Neuroinflammatory Responses in Microglia Cells and ICR Mice. Molecules 2023, 28, 2406. [Google Scholar] [CrossRef]
- Tada, H.; Kawahara, K.; Osawa, H.; Song, L.T.; Numazaki, K.; Kawai, J.; Onoue, S.; Nishioka, T.; Nemoto, E.; Matsushita, K.; et al. Hericium erinaceus ethanol extract and ergosterol exert anti-inflammatory activities by neutralizing lipopolysaccharide-induced pro-inflammatory cytokine production in human monocytes. Biochem. Biophys. Res. Commun. 2022, 636 Pt 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Mongkolpobsin, K.; Chuchawankul, S.; Tencomnao, T.; Baek, S.J. Neuroprotective effects of ergosterol against TNF-alpha-induced HT-22 hippocampal cell injury. Biomed Pharmacother. 2022, 154, 113596. [Google Scholar] [CrossRef]
- Yan, C.Y.; Zhu, Q.Q.; Guan, C.X.; Xiong, G.L.; Chen, X.X.; Gong, H.B.; Li, J.W.; Ouyang, S.H.; Kurihara, H.; Li, Y.F.; et al. Antioxidant and Anti-Inflammatory Properties of Hydrolyzed Royal Jelly Peptide in Human Dermal Fibroblasts: Implications for Skin Health and Care Applications. Bioengineering 2024, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, Y.; Liu, W.; Hayashi, T.; Xiang, W.; He, S.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Increased mitochondrial fission induces NLRP3/cGAS-STING mediated pro-inflammatory pathways and apoptosis in UVB-irradiated immortalized human keratinocyte HaCaT cells. Arch. Biochem. Biophys. 2023, 738, 109558. [Google Scholar] [CrossRef]
- Eom, J.W.; Lim, J.W.; Kim, H. Lutein Induces Reactive Oxygen Species-Mediated Apoptosis in Gastric Cancer AGS Cells via NADPH Oxidase Activation. Molecules 2023, 28, 1178. [Google Scholar] [CrossRef]
- Luo, J.; Li, C.; Zhu, Y.; Guo, R.; Huang, J.; Yu, H.; Sun, M.; Zhu, Q.; Guo, Q.; Li, Y.; et al. Deficiency of inducible nitric oxide synthase (iNOS) enhances MC903-induced atopic dermatitis-like inflammation in mice. Biochem. Biophys. Res. Commun. 2025, 771, 152028. [Google Scholar] [CrossRef]
- Wei, M.; Li, M.; Li, Y.; Wang, B.; Yan, Y.; Li, L. Upregulation of Receptor Interacting Protein 1 Induced by UVB Contributes to Photodamage of the Skin Through NF-kappaB Pathway In Vivo and In Vitro. J. Cosmet. Dermatol. 2025, 24, e70082. [Google Scholar] [CrossRef]
- Johnston, L.A.; Nagalla, R.R.; Li, M.; Whitley, S.K. IL-17 Control of Cutaneous Immune Homeostasis. J. Investig. Dermatol. 2024, 144, 1208–1216. [Google Scholar] [CrossRef]
- Qian, S.; Nagy, G.; Zolnierczuk, P.; Mamontov, E.; Standaert, R. Nonstereotypical Distribution and Effect of Ergosterol in Lipid Membranes. J. Phys. Chem. Lett. 2024, 15, 4745–4752. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging mechanisms of lipid peroxidation in regulated cell death and its physiological implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Q.; Wang, P.; Cao, S.; Williams, J.P.; An, J. Ozone rectal insufflation attenuates lung inflammation by inhibiting the TLR4/NF-kappaB pathway through upregulation of Nrf2 in mice with COPD-like pathology. Int. Immunopharmacol. 2025, 167, 115676. [Google Scholar] [CrossRef]
- Tang, H.; Xu, C.; Ge, Y.; Xu, M.; Wang, L. Multiparametric Quantitative Analysis of Photodamage to Skin Using Optical Coherence Tomography. Sensors 2023, 23, 3589. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Geng, R.; Kang, S.G.; Li, X.; Huang, K. Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics. Antioxidants 2024, 13, 168. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, M.; Lin, H.; Li, C. Theragra chalcogramma Hydrolysates, Rich in Gly-Leu-Pro-Ser-Tyr-Thr, Exerts Anti-Photoaging Potential via Targeting MAPK and NF-kappaB Pathways in SD Rats. Mar. Drugs 2022, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; Hsu, C.Y.; Hwang, E.; Wang, P.W.; Fang, J.Y. The role of cytokines/chemokines in an aging skin immune microenvironment. Mech. Ageing Dev. 2023, 210, 111761. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.A.; Tasduq, S.A. Photophagy: Unveiling a Novel Cellular Mechanism in UVB-Induced Skin Aging and Resilience. Int. J. Dermatol. 2025, 64, 1774–1777. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, Y.; Chen, G.; Mao, G.; Tang, J.; Xu, J.; Han, Y.; Chen, H.; Ding, L. Responsive nanoparticles synergize with Curcumin to break the “reactive oxygen Species-Neuroinflammation” vicious cycle, enhancing traumatic brain injury outcomes. J. Nanobiotechnology 2025, 23, 172. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, Y. Curcumin: A potential anti-photoaging agent. Front. Pharmacol. 2025, 16, 1559032. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Li, T.; Lv, C.; He, W.; Li, W.; Zhou, X.; Qin, S. Proanthocyanidins isolated from lotus seed skin mitigate glycolipid metabolism disorder through the p38/Nrf2/NF-kappaB signaling pathway. Acta Biochim. Et Biophys. Sin. 2024, 56, 1300–1310. [Google Scholar] [CrossRef]
- Lu, Z.; Xia, Q.; Cheng, Y.; Lu, Q.; Li, Y.; Zeng, N.; Luan, X.; Li, Y.; Fan, L.; Luo, D. Hesperetin attenuates UVA-induced photodamage in human dermal fibroblast cells. J. Cosmet. Dermatol. 2022, 21, 6261–6269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, S.; Luo, P.; Li, Z.; Chen, Z.; Xia, C.; Fan, L.; Li, R.; Chen, H. The regulatory effect and molecular mechanism of Epstein-Barr virus protein LMP-1 in SLE susceptibility gene expression. Immunol. Lett. 2025, 273, 106993. [Google Scholar] [CrossRef]
- Sun, X.; Cao, S.; Mao, C.; Sun, F.; Zhang, X.; Song, Y. Post-translational modifications of p65: State of the art. Front. Cell Dev. Biol. 2024, 12, 1417502. [Google Scholar] [CrossRef]
- Li, Z.; Shang, W.; Mei, T.; Fu, D.; Xi, F.; Shao, Y.; Song, X.; Wang, Z.; Qi, K.; Tu, J. Outer membrane vesicles of avian pathogenic Escherichia coli induce necroptosis and NF-kappaB activation in chicken macrophages via RIPK1 mediation. Res. Vet. Sci. 2024, 170, 105185. [Google Scholar] [CrossRef]
- Ji, L.; Shi, X.; Wang, G.; Wu, H.; Hu, Z. Overexpressing six-transmembrane protein of prostate 2 (STAMP2) alleviates sepsis-induced acute lung injury probably by hindering M1 macrophage polarization via the NF-kappaB pathway. Folia Histochem. Et Cytobiol. 2023, 61, 34–46. [Google Scholar] [CrossRef]
- Imafuku, K.; Iwata, H.; Natsuga, K.; Okumura, M.; Kobayashi, Y.; Kitahata, H.; Kubo, A.; Nagayama, M.; Ujiie, H. Zonula occludens-1 distribution and barrier functions are affected by epithelial proliferation and turnover rates. Cell Prolif. 2023, 56, e13441. [Google Scholar] [CrossRef]
- Serra, D.; Garroni, G.; Cruciani, S.; Coradduzza, D.; Pashchenko, A.; Amler, E.; Pintore, G.; Parisse, P.; Satta, R.; Martini, F.; et al. PVA and PVP nanofibers combined with Helichrysum italicum oil preserve skin cell interactions, elasticity and proliferation. Sci. Rep. 2025, 15, 10864. [Google Scholar] [CrossRef]
- Arnold, K.A.; Moran, M.C.; Shi, H.; van Vlijmen-Willems, I.; Rodijk-Olthuis, D.; Smits, J.P.H.; Brewer, M.G. CLDN1 knock out keratinocytes as a model to investigate multiple skin disorders. Exp. Dermatol. 2024, 33, e15084. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, S.J.; Kim, Y.J.; Kim, S.Y.; Jang, Y.N.; Park, A.Y.; Ho, S.H.; Kim, D.; Lee, J.O.; Yoo, K.H.; et al. Actinidia polygama Water Extract (APWE) Protects Against UVB-Induced Photoaging via MAPK/AP-1 and TGFbeta-Smad Pathway. Ann. Dermatol. 2024, 36, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef]
- Faizan, M.; Alam, P.; Rajput, V.D.; Shareen; Kaur, K.; Faraz, A.; Minkina, T.; Maqbool Ahmed, S.; Rajpal, V.R.; Hayat, S. Potential role of tocopherol in protecting crop plants against abiotic stresses. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2023, 29, 1563–1575. [Google Scholar] [CrossRef]
- Chen, J.; Tai, M.; Chen, J.; Ni, J.; Yi, H.; Chen, L.; Wang, D.; Wen, C.; Li, J.; Shen, X.; et al. Panax ginseng extract prevents UVB-induced skin photodamage by modulating VMP1-mediated ER stress. Phytomedicine 2024, 134, 156010. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, Q.; Wang, L.; Wang, B.; Chen, J.; Cai, B.; Wu, C.; Zhu, X.; Liu, F.; Han, D.; et al. Jinhua Qinggan granules attenuates acute lung injury by promotion of neutrophil apoptosis and inhibition of TLR4/MyD88/NF-kappaB pathway. J. Ethnopharmacol. 2023, 301, 115763. [Google Scholar] [CrossRef]
- Luo, R.; Yao, Y.; Chen, Z.; Sun, X. An examination of the LPS-TLR4 immune response through the analysis of molecular structures and protein-protein interactions. Cell Commun. Signal. CCS 2025, 23, 142. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, H.K. Antibacterial activity of emulsions containing unsaturated fatty acid ergosterol esters synthesized by lipase-mediated transesterification. Enzym. Microb. Technol. 2020, 139, 109581. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhong, J.; Li, Y.; Zhou, Q.; Du, Z.; Lin, L.; Shu, P.; Jiang, L.; Zhou, W. A Marine-Derived Sterol, Ergosterol, Mitigates UVB-Induced Skin Photodamage via Dual Inhibition of NF-κB and MAPK Signaling. Mar. Drugs 2025, 23, 445. https://doi.org/10.3390/md23110445
Zhang J, Zhong J, Li Y, Zhou Q, Du Z, Lin L, Shu P, Jiang L, Zhou W. A Marine-Derived Sterol, Ergosterol, Mitigates UVB-Induced Skin Photodamage via Dual Inhibition of NF-κB and MAPK Signaling. Marine Drugs. 2025; 23(11):445. https://doi.org/10.3390/md23110445
Chicago/Turabian StyleZhang, Junming, Jiangming Zhong, Yi Li, Qi Zhou, Zhiyun Du, Li Lin, Peng Shu, Ling Jiang, and Wei Zhou. 2025. "A Marine-Derived Sterol, Ergosterol, Mitigates UVB-Induced Skin Photodamage via Dual Inhibition of NF-κB and MAPK Signaling" Marine Drugs 23, no. 11: 445. https://doi.org/10.3390/md23110445
APA StyleZhang, J., Zhong, J., Li, Y., Zhou, Q., Du, Z., Lin, L., Shu, P., Jiang, L., & Zhou, W. (2025). A Marine-Derived Sterol, Ergosterol, Mitigates UVB-Induced Skin Photodamage via Dual Inhibition of NF-κB and MAPK Signaling. Marine Drugs, 23(11), 445. https://doi.org/10.3390/md23110445





