Marine-Derived Natural Substances with Anticholinesterase Activity
Abstract
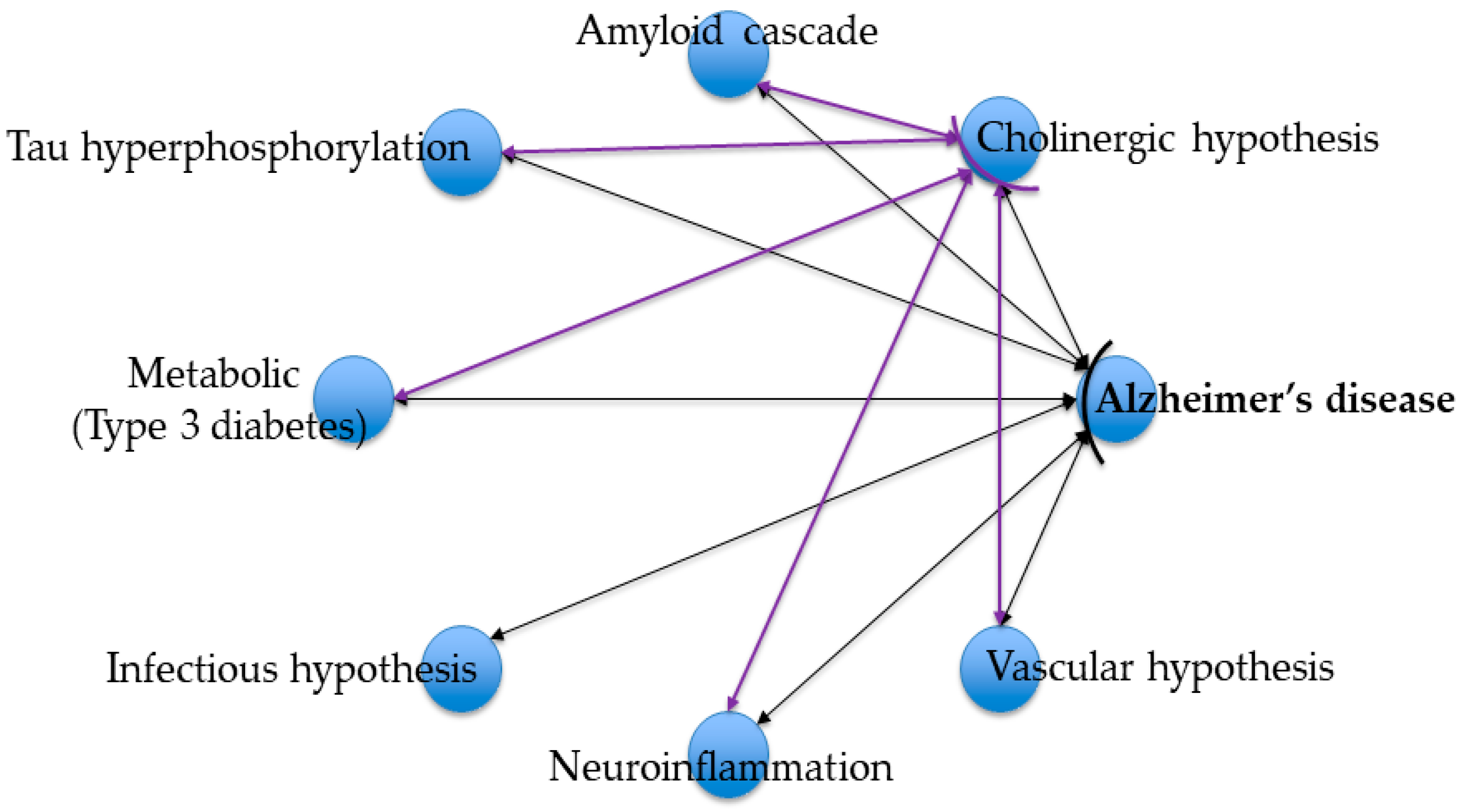
1. Introduction
2. Results
2.1. Marine Bacteria as a Source of Anticholinesterase Compounds
2.2. Marine Fungi as a Source of Anticholinesterase Compounds
2.3. Marine Sponges as a Source of Anticholinesterase Compounds
2.4. Marine Algae as a Source of Anticholinesterase Compounds
2.5. Compounds with Anticholinesterase Activity Derived from Other Marine Organisms
2.6. Mechanistic Insights into the Multi-Target Potential of Marine-Sourced Bioactive Compounds
- Amyloid-β aggregation. Certain compounds derived from marine sources function as dual inhibitors, simultaneously targeting both AChE and the aggregation of Aβ, which proves advantageous in the treatment of AD. This dual mechanism may have the potential to decelerate the progression of the disease more effectively. A research focuses on the docking analysis of bioactive compounds exhibiting anticholinesterase properties, evaluating their potential impact on Aβ aggregation [72]. Several compounds and their derivatives that influence both targets—such as sesquiterpene acetate, pyrrole derivatives, plastoquinones, and farnesylacetones—are discussed.
- Modulation of neuronal survival pathways. A recent investigation into peptides sourced from Litopenaeus vannamei shrimp has identified two hexapeptides, QMDDQ and KMDDQ, as effective modulators of the cholinergic system [70]. In PC12 cells subjected to scopolamine-induced neurotoxicity, both peptides significantly inhibited AChE activity and enhanced ACh levels in a dose-dependent manner, with QMDDQ demonstrating superior efficacy. At a concentration of 0.5 mg/mL, QMDDQ significantly reduced AChE activity and increased ACh to 4.98 ± 0.51 μg/mg protein in hippocampal tissue in vivo. This effect was associated with its structural characteristics: two-dimensional correlation–nuclear overhauser effect spectroscopy NMR analysis indicated that QMDDQ adopts an extended spatial conformation with the N-terminal glutamine residue exposed, thereby enhancing its interaction with the AChE active site. Electrostatic considerations further imply that the lack of a positively charged lysine at the N-terminus (which is present in KMDDQ) provides greater conformational stability and bioactivity. Mechanistically, QMDDQ not only directly inhibited AChE but also activated the PKA/CREB/BDNF and protein kinase B (AKT) signaling pathways, leading to a reduction in pro-apoptotic proteins (Bcl-2-associated X protein, Caspase-3) and an increase in anti-apoptotic protein B-cell lymphoma-extra large (BCL-XL) levels. In vivo, the intraperitoneal administration of QMDDQ (30 mg/kg) to scopolamine-treated C57BL/6 mice resulted in improved spatial learning and memory in the Morris water maze, thereby confirming its neuroprotective potential through both enzymatic inhibition and modulation of neuronal survival pathways.
- Antioxidant properties. Specific marine compounds, particularly those obtained from the seaweed E. bicyclis, demonstrate both cholinesterase inhibitory effects and antioxidant properties. The ethyl acetate fraction of E. bicyclis exhibited potent inhibitory effects on AChE and BuChE, in addition to notable antioxidant capabilities, which may assist in alleviating oxidative stress linked to neurodegenerative disorders [56]. Fungi sourced from marine environments, including Aspergillus unguis, generate secondary metabolites that demonstrate considerable inhibitory activity against AChE. Among these metabolites are diverse bioactive compounds, such as benzazepin-2-one and derivatives of cinnamic acid, which possess further antioxidant characteristics that enhance their anticholinesterase effects [46].
- Proteinaceous Venoms. Venoms derived from marine echinoderms, including sea urchins, possess proteinaceous compounds that impede the activity of both AChE and BuChE. Additionally, these venoms include alkaloids, terpenes, and steroids, which play a role in enhancing their enzyme inhibitory properties [73,74].
2.7. Structural Insights and Drug Development
3. Discussion
4. Materials and Methods
- (1)
- Mechanistic studies that investigate various marine sources of substances with AChE-inhibitory activity—specifically their effects on the neurodegeneration process;
- (2)
- Research and meta-analyses that assess the efficacy of marine-derived substances.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AChEI(s) | Acetylcholinesterase inhibitor(s) |
| AD | Alzheimer’s disease |
| AgNPs | Silver nanoparticles |
| APP | Amyloid precursor protein |
| BDNF | Brain-derived neurotrophic factor |
| BuChE | Butyrylcholinesterase |
| CREB | cAMP response element-binding protein |
| DHA | Docosahexaenoic acid |
| EC50 | Half maximal effective concentration |
| IC50 | Half maximal inhibitory concentration |
| EEAChE | Electric eel acetylcholinesterase |
| EPA | Eicosapentaenoic acid |
| EqBuChE | Butyrylcholinesterase from equine serum |
| GC-MS | Gas chromatography–mass spectrometry |
| HAChE | Human recombinant acetylcholinesterase |
| KMDDQ / QMDDQ | Hexapeptides derived from shrimp |
| MUFA(s) | Monounsaturated fatty acid(s) |
| PC12 cells | Rat pheochromocytoma cell line 12 |
| PKA | Protein kinase A |
| PUFA(s) | Polyunsaturated fatty acid(s) |
| ROS | Reactive oxygen species |
| WHO | World Health Organization |
References
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Mieling, M.; Meier, H.; Bunzeck, N. Structural degeneration of the nucleus basalis of Meynert in mild cognitive impairment and Alzheimer’s disease—Evidence from an MRI-based meta-analysis. Neurosci. Biobehav. Rev. 2023, 154, 105393. [Google Scholar] [CrossRef]
- Berry, A.S.; Harrison, T.M. New perspectives on the basal forebrain cholinergic system in Alzheimer’s disease. Neurosci. Biobehav. Rev. 2023, 150, 105192. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the cholinergic hypothesis in Alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prev. Alzheimers Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: Therapeutic relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Silveyra, M.X.; Sáez-Valero, J. Association between acetylcholinesterase and β-amyloid peptide in Alzheimer’s cerebrospinal fluid. Chem.-Biol. Interact. 2008, 175, 209–215. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Bilgel, M.; Wong, D.F.; Moghekar, A.R.; Ferrucci, L.; Resnick, S.M. Alzheimer’s Disease Neuroimaging Initiative. Causal links among amyloid, tau, and neurodegeneration. Brain Commun. 2022, 4, fcac193. [Google Scholar] [CrossRef]
- Nam, Y.; Shin, S.J.; Kumar, V.; Won, J.; Kim, S.; Moon, M. Dual modulation of amyloid beta and tau aggregation and dissociation in Alzheimer’s disease: A comprehensive review of the characteristics and therapeutic strategies. Transl. Neurodegener. 2025, 14, 15. [Google Scholar] [CrossRef]
- Teitsdottir, U.D.; Darreh-Shori, T.; Lund, S.H.; Jonsdottir, M.K.; Sneadal, J.; Petersen, P.H. Phenotypic displays of cholinergic enzymes associate with markers of inflammation, neurofibrillary tangles, and neurodegeneration in pre- and early symptomatic dementia subjects. Front. Aging Neurosci. 2022, 14, 876019. [Google Scholar] [CrossRef]
- Biswas, K. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory processes in Alzheimer’s disease—Pathomechanism, diagnosis and treatment: A review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Atabi, F.; Moassesfar, M.; Nakhaie, T.; Bagherian, M.; Hosseinpour, N.; Hashemi, M. A systematic review on type 3 diabetes: Bridging the gap between metabolic dysfunction and Alzheimer’s disease. Diabetol. Metab. Syndr. 2025, 17, 356. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, G.; Zhao, Y. Dysregulation of energy metabolism in Alzheimer’s disease. J. Neurol. 2025, 272, 2. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Insulin resistance as the molecular link between diabetes and Alzheimer’s disease. World J. Diabetes 2024, 15, 1430–1447. [Google Scholar] [CrossRef]
- Andrade, L.J.O.; de Oliveira, L.M.; Bittencourt, A.M.V.; Lourenço, L.G.C.; de Oliveira, G.C.M. Brain insulin resistance and Alzheimer’s disease: A systematic review. Dement. Neuropsychol. 2024, 18, e20230032. [Google Scholar] [CrossRef]
- Vojtechova, I.; Machacek, T.; Kristofikova, Z.; Stuchlik, A.; Petrasek, T. Infectious origin of Alzheimer’s disease: Amyloid beta as a component of brain antimicrobial immunity. PLoS Pathog. 2022, 18, e1010929. [Google Scholar] [CrossRef]
- Onisiforou, A.; Charalambous, E.G.; Zanos, P. Shattering the amyloid illusion: The microbial enigma of Alzheimer’s disease pathogenesis—From gut microbiota and viruses to brain biofilms. Microorganisms 2025, 13, 90. [Google Scholar] [CrossRef]
- Scheffer, S.; Hermkens, D.M.A.; van der Weend, L.; de Vries, H.D.; Daemen, M.J.A.P. Vascular hypothesis of Alzheimer disease: Topical review of mouse models. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 4. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Meng, F.; He, F.; Ni, J.; Fu, Y. The vascular contribution of apolipoprotein E to Alzheimer’s disease. Brain 2024, 147, 2946–2965. [Google Scholar] [CrossRef]
- Eisenmenger, L.B.; Peret, A.; Famakin, B.M.; Spahic, A.; Roberts, G.S.; Bockholt, J.H.; Johnson, K.M.; Paulsen, J.S. Vascular contributions to Alzheimer’s disease. Transl. Res. 2023, 254, 41–53. [Google Scholar] [CrossRef]
- Mehta, R.I.; Mehta, R.I. The vascular-immune hypothesis of Alzheimer’s disease. Biomedicines 2023, 11, 408. [Google Scholar] [CrossRef]
- Battle, C.E.; Abdul-Rahim, A.H.; Shenkin, S.D.; Hewitt, J.; Quinn, T.J. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 2, CD013306. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, L.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Pandey, S.; Sree, A.; Sethi, D.; Kumar, C.; Kakollu, S.; Chowdhury, L.; Dash, S.S. A marine sponge associated strain of Bacillus subtilis and other marine bacteria can produce anticholinesterase compounds. Microb. Cell Factories 2014, 13, 24. [Google Scholar] [CrossRef]
- Almasi, F.; Mohammadipanah, F.; Adhami, H.; Hamedi, J. Introduction of marine-derived Streptomyces sp. UTMC 1334 as a source of pyrrole derivatives with anti-acetylcholinesterase activity. J. Appl. Microbiol. 2018, 125, 1370–1382. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, L.; Liu, D.; Huang, J.; Lin, W. Circumdatin D exerts neuroprotective effects by attenuating LPS-induced pro-inflammatory responses and downregulating acetylcholinesterase activity in vitro and in vivo. Front. Pharmacol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Olivo-Flores, K.G.; Couttolenc, A.; Suárez-Medellín, J.; Trigos, Á.; Espinoza, C. Acetylcholinesterase inhibition exerted by the extract of Daldinia eschscholtzii, a marine fungus associated with the coral Siderastrea siderea: GC-MS analysis and molecular docking of identified compounds. Electron. J. Biotechnol. 2024, 72, 12–19. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, W.; Liu, Y.; Yang, J.; Lei, X.; Gerwick, W.H.; Zhang, Y. Acetylcholinesterase inhibitors and antioxidants mining from marine fungi: Bioassays, bioactivity coupled LC–MS/MS analyses and molecular networking. Mar. Life Sci. Technol. 2020, 2, 386–397. [Google Scholar] [CrossRef]
- El-Hady, F.K.A.; Abdel-Aziz, M.S.; Shaker, K.H.; El-Shahid, Z.A. Tyrosinase, acetylcholinesterase inhibitory potential, antioxidant and antimicrobial activities of sponge derived fungi with correlation to their GC/MS analysis. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 338–345. [Google Scholar]
- El-Sayed, H.; Hamada, M.A.; Elhenawy, A.A.; Sonbol, H.; Abdelsalam, A. Acetylcholine esterase inhibitory effect, antimicrobial, antioxidant, metabolomic profiling, and an in silico study of non-polar extract of the halotolerant marine fungus Penicillium chrysogenum MZ945518. Microorganisms 2023, 11, 769. [Google Scholar] [CrossRef]
- Kuno, F.; Otoguro, K.; Shiomi, K.; Iwai, Y.; Omura, S. Arisugacins A and B, novel and selective acetylcholinesterase inhibitors from Penicillium sp. FO-4259. I. Screening, taxonomy, fermentation, isolation and biological activity. J. Antibiot. 1996, 49, 742–747. [Google Scholar] [CrossRef]
- Otoguro, K.; Kuno, F.; Ōmura, S. Arisugacins, selective acetylcholinesterase inhibitors of microbial origin. Pharmacol. Ther. 1997, 76, 45–54. [Google Scholar] [CrossRef]
- Dai, W.; Sandoval, I.T.; Cai, S.; Smith, K.A.; Delacruz, R.G.C.; Boyd, K.A.; Mills, J.J.; Jones, D.A.; Cichewicz, R.H. Cholinesterase inhibitory arisugacins L–Q from a Penicillium sp. isolate obtained through a citizen science initiative and their activities in a phenotype-based zebrafish assay. J. Nat. Prod. 2019, 82, 2627–2637. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Kumla, D.; Kijjoa, A. Chemical diversity and biological activities of meroterpenoids from marine-derived fungi: A comprehensive update. Mar. Drugs 2020, 18, 317. [Google Scholar] [CrossRef]
- Long, Y.; Cui, H.; Liu, X.; Xiao, Z.; Wen, S.; She, Z.; Huang, X. Acetylcholinesterase inhibitory meroterpenoid from a mangrove endophytic fungus Aspergillus sp. 16-5c. Molecules 2017, 22, 727. [Google Scholar] [CrossRef]
- Ding, B.; Wang, Z.; Huang, X.; Liu, Y.; Chen, W.; She, Z. Bioactive α-pyrone meroterpenoids from mangrove endophytic fungus Penicillium sp. Nat. Prod. Res. 2016, 30, 2805–2812. [Google Scholar] [CrossRef]
- Kim, W.; Cho, K.; Lee, C.; Yoo, I. Terreulactone A, a novel meroterpenoid with anti-acetylcholinesterase activity from Aspergillus terreus. Tetrahedron Lett. 2002, 43, 3197–3198. [Google Scholar] [CrossRef]
- Yoo, I.; Cho, K.; Lee, C.; Kim, W. Isoterreulactone A, a novel meroterpenoid with anti-acetylcholinesterase activity produced by Aspergillus terreus. Bioorganic Med. Chem. Lett. 2005, 15, 353–356. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, H.; Wu, Q.; Yuan, S.; Liu, L. Two new picoline-derived meroterpenoids with anti-acetylcholinesterase activity from ascidian-derived fungus Amphichorda felina. Molecules 2022, 27, 5076. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Deng, M.; Qi, C.; Chen, C.; Zhu, H.; Luo, Z.; Wang, J.; Xue, Y.; Zhang, Y. Asperversins A and B, two novel meroterpenoids with an unusual 5/6/6/6 ring from the marine-derived fungus Aspergillus versicolor. Mar. Drugs 2018, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cao, W.; Wu, Z.; Wang, S.; Cheng, Y. Zizhines G–O, AChE inhibitory meroterpenoids from Ganoderma sinensis. Fitoterapia 2019, 134, 411–416. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Dramae, A.; Komwijit, S.; Laorob, T.; Khamsaeng, S.; Pittayakhajonwut, P. Antimicrobial, antimalarial and anticholinesterase substances from the marine-derived fungus Aspergillus terreus BCC51799. Tetrahedron 2020, 76, 131496. [Google Scholar] [CrossRef]
- Dimitrova, D.; Dimitrova, S.; Kehayova, G.; Dragomanova, S. Meroterpenoids from terrestrial and marine fungi: Promising agents for neurodegenerative disorders—An updated review. Curr. Issues Mol. Biol. 2025, 47, 96. [Google Scholar] [CrossRef]
- Abdelwahab, G.M.; Mira, A.; Cheng, Y.; Abdelaziz, T.A.; Lahloub, M.F.I.; Khalil, A.T. Acetylcholine esterase inhibitory activity of green synthesized nanosilver by naphthopyrones isolated from marine-derived Aspergillus niger. PLoS ONE 2021, 16, e0257071. [Google Scholar] [CrossRef]
- Olsen, E.K.; Hansen, E.; Moodie, L.W.K.; Isaksson, J.; Sepčić, K.; Cergolj, M.; Svenson, J.; Andersen, J.H. Marine AChE inhibitors isolated from Geodia barretti: Natural compounds and their synthetic analogs. Org. Biomol. Chem. 2016, 14, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Aristyawan, A.D.; Setyaningtyas, V.F.; Wahyuni, T.S.; Widyawaruyanti, A.; Ingkaninan, K.; Suciati, S. In vitro acetylcholinesterase inhibitory activities of fractions and iso-agelasine C isolated from the marine sponge Agelas nakamurai. J. Res. Pharm. 2025, 26, 279–286. [Google Scholar] [CrossRef]
- Defant, A.; Carloni, G.; Innocenti, N.; Trobec, T.; Frangež, R.; Sepčić, K.; Mancini, I. Structural insights into the marine alkaloid discorhabdin G as a scaffold towards new acetylcholinesterase inhibitors. Mar. Drugs 2024, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Ryu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: Lead compounds for Alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lu, Y.; Je, J.; Jayawardena, T.U.; Kang, M.; Lee, S.; Kim, T.H.; Lee, D.S.; Lee, J.M.; Yim, M.J.; et al. Effects of ethanol extracts from Grateloupia elliptica, a red seaweed, and its chlorophyll derivative on 3T3-L1 adipocytes: Suppression of lipid accumulation through down-regulation of adipogenic protein expression. Mar. Drugs 2021, 19, 91. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.S. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch. Pharmacal Res. 2015, 38, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.P.; Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Centeno, D.C.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Rev. Bras. Farmacogn. 2015, 25, 657–662. [Google Scholar] [CrossRef]
- Kannan, R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Van Staden, J. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2012, 54, 1250–1254. [Google Scholar] [CrossRef]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef]
- Athitya, L.S.; Bharath, V.M.V.; Nellore, J.; Prakash, P. Screening of Gracilaria corticata extracts for acetylcholinesterase-inhibitory activity. Res. J. Pharm. Technol. 2018, 11, 3848. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.; Rahman, M.A.; Uddin, J.; Alam, M.; Moon, I.S. Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Khairinisa, M.A.; Latarissa, I.R.; Athaya, N.S.; Charlie, V.; Musyaffa, H.A.; Prasedya, E.S.; Puspitasari, I.M. Potential application of marine algae and their bioactive metabolites in brain disease treatment: Pharmacognosy and pharmacology insights for therapeutic advances. Brain Sci. 2023, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; How, S.; Wong, K.; Lim, S.; Phang, S.; Yow, Y. Cholinesterase inhibitory activities of neuroprotective fraction derived from red alga Gracilaria manilaensis. Fish. Aquat. Sci. 2022, 25, 49–63. [Google Scholar] [CrossRef]
- Dhanabalan, A.K.; Kumar, P.; Vasudevan, S.; Chworos, A.; Velmurugan, D. Identification of a novel drug molecule for neurodegenerative disease from marine algae through in-silico analysis. J. Biomol. Struct. Dyn 2024, 1–10. [Google Scholar] [CrossRef]
- Hu, D.; Jin, Y.; Hou, X.; Zhu, Y.; Chen, D.; Tai, J.; Chen, Q.; Shi, C.; Ye, J.; Wu, M.; et al. Application of marine natural products against Alzheimer’s disease: Past, present and future. Mar. Drugs 2023, 21, 43. [Google Scholar] [CrossRef]
- Ghoran, H.S.; Kijjoa, A. Marine-derived compounds with anti-Alzheimer’s disease activities. Mar. Drugs 2021, 19, 410. [Google Scholar] [CrossRef]
- Mohebbi, G.; Nabipour, I.; Vazirizadeh, A.; Vatanpour, H.; Farrokhnia, M.; Maryamabadi, A.; Bargahi, A. Acetylcholinesterase inhibitory activity of a neurosteroidal alkaloid from the upside-down jellyfish Cassiopea andromeda venom. Rev. Bras. Farmacogn. 2018, 28, 568–574. [Google Scholar] [CrossRef]
- Silva, M.; Seijas, P.; Otero, P. Exploitation of marine molecules to manage Alzheimer’s disease. Mar. Drugs 2021, 19, 373. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, S.; Sun, N.; Zhu, B.; Lin, S. Neuroprotective function of a novel hexapeptide QMDDQ from shrimp via activation of the PKA/CREB/BNDF signaling pathway and its structure–activity relationship. J. Agric. Food Chem. 2020, 68, 6759–6769. [Google Scholar] [CrossRef]
- Moura, S.M.; de Morais, S.M.; Carioca, J.O.B.; Rodrigues, A.L.M.; Alves, D.R.; da Silva, F.F.M.; Silva, A.C.S.; Amaral, S.M.B.; Silva, Y.Y.V. Fatty acids profile and anti-cholinesterase activity of fish lipids from Brazilian Northeast. Res. Soc. Dev. 2021, 10, 18968. [Google Scholar] [CrossRef]
- Stoddard, S.V.; Hamann, M.T.; Wadkins, R.M. Insights and ideas garnered from marine metabolites for development of dual-function acetylcholinesterase and amyloid-β aggregation inhibitors. Mar. Drugs 2014, 12, 2114–2131. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Rashedinia, M.; Mohebbi, G.; Vazirizadeh, A.; Baghban, N. Antioxidant and anticholinesterase properties of Echinometra mathaei and Ophiocoma erinaceus venoms from the Persian Gulf. Front. Chem. 2023, 11, 1332921. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Rashedinia, M.; Mohebbi, G.; Vazirizadeh, A. Studies on secondary metabolites and in vitro and in silico anticholinesterases activities of the sea urchin Echinometra mathaei crude venoms from the Persian Gulf-Bushehr. Nat. Prod. J. 2024, 14, 14–31. [Google Scholar] [CrossRef]
- Elumalai, V.; Trobec, T.; Grundner, M.; Labriere, C.; Frangež, R.; Sepčić, K.; Hansen, J.H.; Svenson, J. Development of potent cholinesterase inhibitors based on a marine pharmacophore. Org. Biomol. Chem. 2022, 20, 5589–5601. [Google Scholar] [CrossRef]
- Gade, A.C.; Murahari, M.; Pavadai, P.; Kumar, M.S. Virtual screening of a marine natural product database for in silico identification of a potential acetylcholinesterase inhibitor. Life 2023, 13, 1298. [Google Scholar] [CrossRef]

| Compound/Extract | IC50 (AChE) | IC50 (BuChE) | Ref. |
|---|---|---|---|
| 6,6′-Bieckol (Grateloupia elliptica) | 44.5 µM | 27.4 µM | [54] |
| Amphichoterpenoid D (Amphichorda feline) | 12.5 µM | n/a | [44] |
| Amphichoterpenoid E (Amphichorda feline) | 11.6 µM | n/a | |
| Arisugacin A (Penicillium sp.) | 1.0 nM | >21,000 nM | [38,39] |
| Arisugacin B (Penicillium sp.) | 25.8 nM | >516,000 nM | |
| Arisugacin C (Penicillium sp.) | 2.5 µM (2500 nM) | 30,000 nM | [39] |
| Arisugacin D (Penicillium sp.) | 3.5 µM (3500 nM) | 30,000 nM | |
| Arisugacin O (Penicillium sp.) | 191 nM | n/a | |
| Aspergillus versicolor metabolite | 13.6 µM | n/a | [45] |
| Aspernigrin A (Aspergillus niger) | Active (no value) | n/a | [49] |
| Aurasperone A (Aspergillus niger) | Active (no value) | n/a | |
| Barettin (Geodia barretti) | Active (non-competitive) | n/a | [50] |
| Cynerine A (Gracilaria manilaensis) | Active | Active | [64] |
| Dayaolingzhiol D (Ganoderma lucidum) | 8.52 µM | n/a | [47] |
| Dayaolingzhiol E (Ganoderma lucidum) | 7.37 µM | n/a | |
| Dehydroaustin (Aspergillus spp.) | 0.40 µM | n/a | [40] |
| Dehydroaustinol (Aspergillus spp.) | 3.0 µM | n/a | |
| Dieckol (Ecklonia stolonifera) | 17.11 µM | Active | [55] |
| Diphlorethohydroxycarmalol (brown seaweeds) | Predicted active | Predicted active | [65] |
| Discorhabdin G (Latrunculia) | Active (no value) | Selective for AChE | [52] |
| Ecklonia maxima phlorotannins | 62.6–150.8 µg/mL | n/a | [58] |
| Phlorofucofuroeckol-A (Ecklonia stolonifera) | 4.89 µM | Active | [55] |
| Eckol (Ecklonia maxima; Ecklonia stolonifera) | 20.56 µM | Active | |
| Eisenia bicyclis extract | Active | Active | [56] |
| Fish oil (Haemulon plumieri) | 4.81 µg/mL | n/a | [71] |
| Fish oil (Lutjanus synagris) | 2.84 µg/mL | n/a | |
| Fish oil (Scomberomorus cavalla) | 2.60 µg/mL | n/a | |
| Fonsecinone A (A. niger, AgNPs) | 0.089 µM | n/a | [49] |
| Graveolinine (Gracilaria manilaensis) | Active | Active | [64] |
| Iso-agelasine C (Agelas nakamurai) | 30.68 µg/mL | n/a | [51] |
| Isoaustinol | 2.50 µM | n/a | [40] |
| Ochtodes secundiramea extract | 400 µg/mL | n/a | [57] |
| Penicillium chrysogenum extract | 60.87 µg/mL | n/a | [35] |
| Peptide KMDDQ (shrimp) | Active (dose-dependent) | n/a | [70] |
| Peptide QMDDQ (shrimp) | Active (dose-dependent) | n/a | |
| Phytol (Gelidiella acerosa) | 2.704 µg/mL | 5.798 µg/mL | [62] |
| Sargaquinoic acid | 23.2 µM | 26 nM | [54] |
| Territrem B (Aspergillus terreus) | 0.071 µM (71 nM) | n/a | [47] |
| Compound Group | Representative Compounds | Source Organisms | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Meroterpenoids | Territrem B, Arisugacin A–Q, Terreulactone A, Dehydroaustinol, Amphichoterpenoid D/E | Marine and endophytic fungi (Aspergillus, Penicillium, Amphichorda) | Potent AChE inhibition (nM–µM), selective for AChE; antioxidant; anti-amyloid | [36,37,38,39,40,41,42,43,44] |
| Alkaloids | Fascaplysin, Iso-agelasine C, Androtoxin B, Petrosamine, Pulmonarins, Marinoquinoline A | Marine sponges, corals, bacteria, jellyfish | Reversible/noncompetitive AChE inhibition; dual AChE/BuChE inhibition; BACE-1 inhibition; antioxidant | [50,51,67,68,69] |
| Phlorotannins | Eckol, Dieckol, Phlorofucofuroeckol, Eckstolonol | Brown algae (Ecklonia, Eisenia, Sargassum) | AChE and BuChE inhibition (noncompetitive); antioxidant; anti-amyloid; BACE-1 inhibition | [53,54,55,56,57,62,63,64,65] |
| Peptides | QMDDQ, KMDDQ | Shrimp (Litopenaeus vannamei) | Direct AChE inhibition; activation of PKA/CREB/BDNF and AKT pathways; anti-apoptotic and neurogenic | [70] |
| Polysaccharides/Oligosaccharides | Fucoidans, Chitosan oligosaccharides (COS, DEAE-COS), Sodium oligomannate | Marine algae, crustaceans | Mild AChE inhibition; anti-amyloid and anti-apoptotic; modulation of gut microbiota; anti-inflammatory | [63,66,70,72] |
| Sterols/Lipids | Fucosterol, Cholesterol derivatives, EPA, DHA | Marine algae and fish (Scomberomorus cavalla, Lutjanus synagris) | Noncompetitive AChE inhibition; membrane stabilization; antioxidant; anti-inflammatory; anti-tau | [56,66,68] |
| Carotenoids | Fucoxanthin, Astaxanthin | Brown and red algae, marine invertebrates | Mild AChE inhibition; antioxidant; anti-inflammatory | [66] |
| Phenolics/Halogenated metabolites | Bromophenols, Halogenated monoterpenes (Ochtodes secundiramea, Rhodomela spp.) | Red algae | AChE inhibition; antioxidant | [57,60] |
| Proteinaceous/Venom compounds | Neurosteroidal alkaloids, proteinaceous venoms (Cassiopea andromeda, Echinometra mathaei) | Marine invertebrates (jellyfish, sea urchins) | AChE and BuChE inhibition; modulation of Ca2+ channels and synaptic function; antioxidant and antiapoptotic | [69,71,72,73,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrova, D.; Kehayova, G.; Dimitrova, S.; Dragomanova, S. Marine-Derived Natural Substances with Anticholinesterase Activity. Mar. Drugs 2025, 23, 439. https://doi.org/10.3390/md23110439
Dimitrova D, Kehayova G, Dimitrova S, Dragomanova S. Marine-Derived Natural Substances with Anticholinesterase Activity. Marine Drugs. 2025; 23(11):439. https://doi.org/10.3390/md23110439
Chicago/Turabian StyleDimitrova, Daniela, Gabriela Kehayova, Simeonka Dimitrova, and Stela Dragomanova. 2025. "Marine-Derived Natural Substances with Anticholinesterase Activity" Marine Drugs 23, no. 11: 439. https://doi.org/10.3390/md23110439
APA StyleDimitrova, D., Kehayova, G., Dimitrova, S., & Dragomanova, S. (2025). Marine-Derived Natural Substances with Anticholinesterase Activity. Marine Drugs, 23(11), 439. https://doi.org/10.3390/md23110439








