Abstract
Global agricultural production is challenging due to climate change and a number of phyto-pathogenic organisms and pests that pose a significant threat to both crop growth and productivity. The growing resistance of pests and diseases to synthetic chemicals makes crop production even more difficult, which highlights the urgent need for alternative solutions. From this perspective, marine microorganisms have emerged as a significant natural product source for their distinctive bioactive compounds and environmentally sustainable potential pesticidal activity. The unique microbial resources and structurally diverse metabolites of the marine ecosystem have been proven to have strong antagonistic effects against a broad spectrum of agricultural diseases and pests, making them a valuable candidate for the development of novel pesticides. This review highlights 126 marine natural products from marine microorganisms with diverse metabolic pathways and bioactivities against agricultural pests, pathogens, and weeds. The findings underscore the potential of marine-derived compounds in addressing the growing challenges of crop protection and offering an appealing strategy for future agrochemical research and development.
1. Introduction
Crop cultivation has long been an integral part of human civilization that has contributed to both economic development and population growth. The world’s population is expected to increase substantially from 8 billion to 9.7 billion people by 2050, posing a significant planning challenge for food security [1]. Therefore, sufficient crop production must be ensured to sustain human survival and social stability. The United Nations (UN) has made ending world hunger and poverty a priority in its 2030 sustainable development goals (SDGs) agenda. Unfortunately, global crop growth and production has been extensively influenced by several biotic and abiotic factors. Among biotic factors, disease causative agents such as fungi [2], bacteria [3], and viruses, as well as weeds and pests, have become the potential threats towards successful agricultural production. No doubt, the use of synthetic pesticide is the most powerful approach in controlling plant disease and achieving high-quality yields. However, the excessive use of these chemical substances has led to serious problems for the environment, human health, water, soil fertility, emergence of pathogen resistance, and targeting several beneficial species [4,5]. Therefore, scientists have diverted their attention towards several ecofriendly management strategies, such as safer pesticides, while keeping in view rising resistance levels and the growing demand for safer and more effective drugs and pesticides. The discovery of new lead compounds with high activity, good selectivity, and novel targets or mechanisms of action presents a major opportunity and challenge in technological innovation. The search for new pesticide lead compounds with novel structural frameworks has become a key focus of current research.
Natural products refer to compounds synthesized or extracted from organisms in nature, typically secondary metabolites that exist in plants, microorganisms, animals, or their secretions [6]. Researchers worldwide have screened and isolated natural products from plants, microorganisms, and marine organisms, and evaluated the bioactivities of these compounds. This has led to the discovery of valuable compounds, including some of the most effective agrochemicals in history [7], such as antifungal agents (e.g., Nisin), antiparasitic agents [8,9,10] (e.g., avermectins), insecticides [11] (e.g., spinosad), and herbicides (e.g., glyphosate). The discovery of new active ingredients heavily relies on natural products, particularly in the field of insecticides, where 70% of the insecticides available worldwide are natural products or their derivatives. Similarly, approximately 60% of herbicides and fungicides are based on natural compounds [12]. The marine environment is the largest and most biodiverse ecosystem on Earth, with abundant microbial resources. These species exhibit unique growth environments and are rich sources of novel bioactivities and metabolic pathways, with diversified structures that distinguish them from terrestrial microbes. Researchers are increasingly interested because of their capability of producing structurally unique compounds [13].
Marine natural products have potential inhibitory effects towards several harmful organisms. These microorganisms have emerged as promising sources for discovering pesticides, with their strong antagonistic activity against several agricultural pathogens, pests and weeds. There is strong evidence from advances in science and technology that microorganisms once again play a key role. In the future, several interesting and novel compounds with potential effects will likely to be discovered from the ocean.
This review introduces 126 natural products from marine microorganisms with various structural types and bioactivity against agricultural pathogens, pests, or weeds and a total of 105 references are cited.
2. Results
Several types of phyto-pathogens such as Fusarium sp., Alternaria sp., Colletotrichum sp., Phytophthora sp., and many others cause significant losses to agricultural production [12,13]. For instance, Fusarium sp. is associated with severe pathogenic diseases like Fusarium head blight, Fusarium ear rot, and Fusarium wilt, whereas Alternaria sp. is responsible for Alternaria wilt, leaf spot, early blight, and leaf blight [14,15]. The natural products from marine microorganisms including fungi and bacteria from marine and mangrove sources have distinct structural characteristics and have shown inhibitory effects against a wide range of pathogens. These antimicrobial compounds have the potential to serve as leading compounds in the development of new pesticides, providing effective, environmentally friendly, and long-lasting solutions for controlling and preventing agricultural pathogens.
2.1. Agricultural Antimicrobial Metabolites
2.1.1. Marine-Sourced Fungi (Excluding Those from Mangroves)
Cladosporium sp., was isolated from the brown algae Feldmannia elachistaeformis collected from Haikou Bay, Hainan, China. The strain produced a novel isochromanone, cladosporinisochromanone (1), together with 15 known compounds. Cytochalasin H (2) exhibited excellent antifungal activity against Colletotrichum sp., with a minimum inhibitory concentration (MIC) of 16 μg/mL, surpassing the MIC value of 64 μg/mL of thiophanate methyl fungicide [16].
Zou et al., conducted a bioassay-guided investigation of a fermentation culture of Scheffersomyces spartinae W9, which was isolated from a tropical marine sea. This leads to the production of volatile organic compounds (VOCs), which inhibited the mycelial growth of Botrytis cinerea and spore germination, with inhibition rates of 77.8% and 58.3% as compared to a control. Additionally, these VOCs reduced the incidence of disease of Botrytis cinerea on the surface of strawberries and the diameter of lesions by 20.7% and 67.4%, respectively, as compared to CK. A series of compounds were isolated and their structures were characterized in order to describe the components of these VOCs. Among them, 2-phenylethanol (3) was found to have the strongest antifungal activity for controlling Botrytis cinerea in strawberries [17].
In another study, chemical investigations of secondary metabolites from Aspergillus sydowii LW09 resulted to the discovery of a wide range of compounds which showed a potential response against agricultural plant pathogens. (7S,11S)-(+)-12-hydroxysydonic acid (5) exhibited antagonistic activity against Pseudomonas syringae, with an MIC of 32 μg/mL. Aspergillusene B (4) and expansol G (6) also showed antifungal activity against Ralstonia solanacarum, both with MIC values of 32 μg/mL. Additionally, (S)-sydonic acid (7) demonstrated excellent inhibitory effects against Fusarium oxysporum, with an EC50 value of 1.85 μg/mL. Furthermore, compounds 4–7 and (−)-(R)-cyclo-hydroxysydonic acid (8) were able to inhibit the germination of Alternaria alternata [18].
Penicillium sp. CRM1540, isolated from Antarctic marine sediments, yielded a bioactive secondary metabolite called cyclopaldic acid. This compound showed a maximum inhibition rate of 90% against Macrophomina phaseolina, Rhizoctonia solani, and Sclerotinia sclerotiorum, with a concentration of 100 μg/mL. Even at a concentration of 50 μg/mL, the inhibition rate against these pathogenic fungi was over 70% [19]. Bin Liu isolated an actinomycete strain from the intertidal zone that was identified as Streptomyces cinereoruber. A metabolite named as aurone (9) was discovered from the fermentation product of this strain. Greenhouse experiments revealed that herboxidiene exhibited high fungicidal activity against powdery mildew (Botrytis cinerea), cucumber powdery mildew (Sphaerotheca fuliginea), and rice blast (Pyricularia oryzae) disease [20].
Oppong-Danquah et al. reported that the marine-derived fungus Cosmospora sp. exhibits inhibitory activity against plant pathogens through microbial co-culture techniques. Further research on culture extracts of this fungus led to the discovery of sudanones A, E, and D (10–12) and pseudoanguillosporins (13). Soudanone E (11) and soudanone D (12) exhibited antifungal activity against M. oryzae and Phytophthora infestans, while compound 13 demonstrated broad-spectrum antimicrobial activity against Pseudomonas syringae, Xanthomonas campestris, M. oryzae, and P. infestans, with MIC values of 23.4, 7.4, 3.2, and 0.8 μg/mL, respectively [21].
The marine-derived fungus Aspergillus tabacinus yielded a number of benzyl ether compounds. MIC values for violaceol I (14), violaceol II (15), and diorcinol (16) ranging from 6.3 to 200 μg/mL have demonstrated broad-spectrum antibacterial activity. Above all, diorcinol (16) showed excellent antifungal potency against rice blast (M. oryzae), tomato late blight (P. infestans), and pepper anthracnose (C. coccodes) [22].
Similarly, compounds were obtained from ethyl acetate and n-butanol extracts of the fermentation broth of the marine-derived fungus Trichoderma longibrachiatum, which effectively inhibited the growth of tomato gray mold (B. cinerea), rice blast (M. oryzae), and tomato late blight (P. infestans). In vitro tests revealed that all compounds successfully inhibited the fungal growth of P. infestans, while bisvertinolone (17) showed the strongest inhibitory activity, with an MIC value of 6.3 μg/mL. Moreover, compound 17 exhibited broad-spectrum antimicrobial activity against C. coccodes, Cylindrocarpon destructans, and M. oryzae at concentrations of 12.5, 25, and 50 μg/mL, respectively [23].
The endophytic marine-derived fungus Aspergillus sp. SCAU150 provided unsaturated acid derivatives and polyketide compounds, while the structures of these compounds were determined through chemical analysis techniques. Experimental tests revealed that 8-ethyl 2-methyleneoctanedioate (18) exhibited inhibitory effects against Fusarium solani bio-80814, with a 6 mm diameter inhibitory zone under 5 µg/disc [24].
Similarly, marine-derived fungus Penicillium sp. N-5 was the source of three andrastin-type meroterpenoids hemiacetalmeroterpenoids A-C (19–21), and a drimane sesquiterpenoid astellolide Q (22), with eleven known compounds. Parasiticolide A (23) was created for the first time from a natural source. An in vitro study revealed that hemiacetalmeroterpenoids A (19), citreohybridone A (24), and andrastins B (25) exhibited significant activity against Penicillium italicum and Colletrichum gloeosporioides, with MIC values ranging from 1.56 to 6.25 μg/mL [25]. The structures of compounds 1–25 are shown in Figure 1.
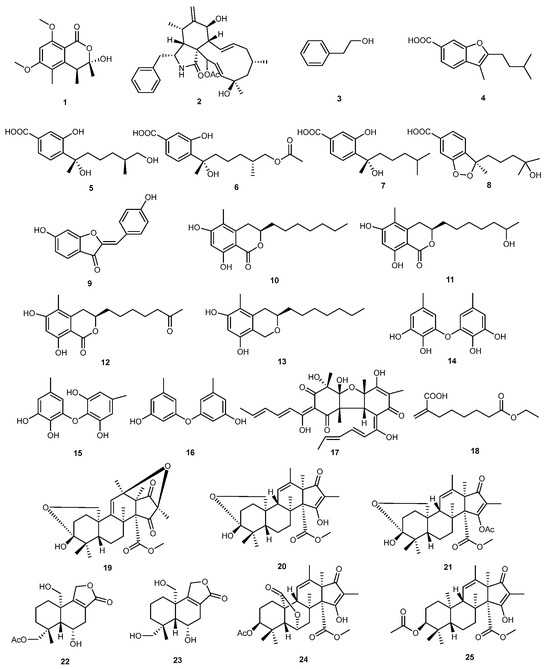
Figure 1.
Chemical structures of compounds 1–25.
Saad et al. discovered a growth-inhibitory compound from the secondary metabolites of the marine-derived fungus Eurotium rubrum that is effective against agricultural plant pathogens. The compound was identified as methyleurotinone (26). It exhibited strong antibacterial activity against Pectobacterium carotovorum subsp. carotovorum, Pseudomonas syringae pv. syringae, Rhizobium radiobacter, and Ralstonia solanacearum, with MIC values of 31.3, 125, 31.3, and 125 mg/L, respectively [26].
Four new compounds were discovered from the strain Aspergillus terreus BCC5799, which was isolated from soil endophytic sources in a marine environment. These compounds were identified as asperteramide (27), aspulvinone O (28), luteoride E (29), and asterriquinone CT5 (30), with significant antagonistic properties. A bioassay study showed that these compounds exhibited potential inhibitory activity against Alternaria brassicicola with MIC values of 6.3, 50.0, 50.0, and 50.0 μg/mL, respectively [27].
Trichoderma sp. Z43 isolated from marine brown algae Dictyopteris was the source of six new lipids along with a known compound triharzianin B (33). These seven compounds were further evaluated for their activity against three plant pathogens. Results revealed that trichoderols B (31), trichoderols E (32), and triharzianin B (33) exhibited inhibitory activity against Fusarium graminearum, Gaeumannomyces graminis, and Glomerella cingulata, with MIC values of 64 μg/mL. Additionally, these compounds showed low toxicity to the brine shrimp Artermia salina, indicating their potential as environmentally friendly fungicides [28].
A novel polyketide-terpenoid hybrid natural product, tennessenoid A (34), was obtained from Asperigills sp. strain 1022LEF, which was derived from marine red alga Grateloupia turuturu in Qingdao, China. This compound significantly responds against the growth of several phyto-pathogens, with inhibition zone diameters ranging from 2 to 7 mm [29].
Penicillium chrysogenum LD-201810 yielded a pair of new nor-bisabolane enantiomers methylsulfinyl-1-hydroxyboivinianin A (35) and two new hydroxyphenylacetic acid derivatives chrysoalide A-B (37 and 38), along with four known compounds which were isolated from the culture filtrate. Among them, hydroxysydonic acid (36) exhibited excellent antifungal effects against Botrytis cinerea, with an EC50 value of 13.6 μg/mL [30].
Similarly, five different fungal strains were isolated from sea anemones and these were assessed for their antagonistic properties. Emericella sp. SMA01 showed strong activity among five strains of fungi and analysis from the ethyl acetate extract of the fermentation broth revealed its main metabolite as phenazine-1-carboxylic acid (39). This compound exhibited inhibitory activity against Phytophthora capsici, Gibberella zeae, and Verticillium dahliae, with EC50 values ranging from 23.26 to 53.89 μg/mL [31].
Investigation of red algae endophytic fungi Trichoderma brevicompactum A-DL-9-2 resulted in the isolation of a series of trichothecene derivatives. Testing the growth inhibition effects of these compounds on common plant pathogenic fungi revealed that trichodermin (40) exhibited the highest activity. The MIC values for compound 40 were 4.0 μg/mL against both Botrytis cinerea and Fusarium oxysporum, and 8.0 μg/mL against Phomopsis asparagi [32].
Three new compounds, polyhydroxy steroid (41), tricyclic diterpenoid (42) and isaridin, were obtained from a marine-derived entomopathogenic fungus Beauveria feline. Biological activity evaluation showed that compounds 41 and 42 exhibited antifungal activity against drug-resistant Botrytis cinerea, with MIC values ranging from 16 to 32 μg/mL, significantly outperforming carbendazim (MIC value of 256 μg/mL) [33].
A new meroterpenoid-type alkaloid, oxalicine C (43), and two new erythritol derivatives, penicierythritols A (44) and B (45), were produced by Penicillium chrysogenum XNM-12, which was isolated from the marine algae. Among them, compounds 43 and 45 exhibited moderate activity against Ralstonia solanacearum, with MIC values of 8 and 4 μg/mL, respectively. Additionally, penicierythritols B (45) also showed moderate activity against Alternaria alternata, with an MIC value of 8 μg/mL [34].
A co-culture of marine red algal-derived endophytic fungi Aspergillus terreus EN-539 and Paecilomyces lilacinus EN-531 resulted in the production of a new terrein derivative, asperterrein (46) with two known compounds dihydroterrein (47) and terrein (48). These compounds were not detected when the strains were cultured separately. Antibacterial activity tests revealed that compounds 46–48 exhibited inhibitory effects against Alternaria brassicae, with MIC values of 64, 64, and 8 μg/mL, respectively, in comparison to control (chloramphenicol) (4 μg/mL) [35].
Three new sesquiterpenoid compounds, chermesiterpenoids A–C (49–51), were isolated and identified from the secondary metabolites of the endophytic fungus Penicillium chermesinum EN-480, derived from red algae. Biological activity tests showed that these compounds exhibited modest inhibitory activity against Colletottichum gloeosporioides, with concentrations of 64, 32, and 16 μg/mL, respectively [36]. The structures of compounds 26–51 are shown in Figure 2.
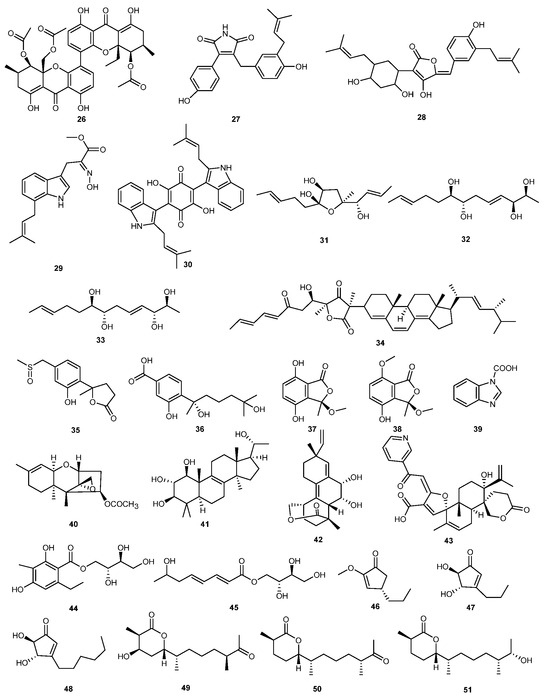
Figure 2.
Chemical structures of compounds 26–51.
2.1.2. Marine-Sourced Bacteria
The ethyl acetate extracts of the fermentation broth of two bacterial strains (Bacillus velezensis GP521A and Pseudoalteromonas caenipelagi GP3R5) from the marine soil successfully inhibited the mycelial growth of Colletotrichum camelliae, C. fructicola CF-1, and Pyricularia oryzae P131 at concentrations of 100 and 200 μg/mL. Moreover, the extracts also suppressed the conidial germination and appressorial formation of Colletotrichum spp., with 50 μg/mL of concentration. Interestingly, the detached oil tea leaves treated with the application of these extracts at 100 μg/mL results in decreasing the infection area significantly by 98.0% and 97.5% [37]. Two new pyrrolizine alkaloids phenopyrrolizins A (52) and B (53) were identified from the fermentation broth of marine bacterium Micromonospora sp. HU138. These two compounds were found inhibiting the fungal growth of Botrytis cinerea by 18.9% and 35.9%, respectively, under in vitro conditions [38].
According to NMR and mass spectrometry results, another compound was identified as 2, 4-di-tert butyl phenol (2, 4-DTBP) (54), which was isolated from marine bacterial strain Serratia marcescens BKACT. This compound potentially inhibited the spore germination growth of Fusarium foetens NCIM 1330 at 0.53 mM of concentration. However, it totally suppressed the germination of Fusarium sp. spores on wheat seeds without any toxic effects under greenhouse conditions, proving to be a promising candidate for anti-Fusarium applications [39].
Similarly, another study revealed that ethyl acetate extract fermentation broth boasts a compound that inhibits the growth and spore germination of plant pathogenic fungus Alternaria alternata. It was isolated from plant disease suppressive compost containing residues from the fishing industry and peat moss. Chemical structure elucidation revealed it to be a newly reported compound known as arthropeptide B (55) [40].
The secondary metabolites of the marine bacterium Subtilis subsp. spizizenii MC6B-22 revealed the presence of lipopeptide compounds. Subsequently, these compounds were recognized as mycosubtilin upon further identification, which exhibited broad-spectrum antifungal activity against ten plant pathogenic fungi of tropical crops, with MIC values ranging from 25 to 400 μg/mL. The authors believe that these findings hold potential for applications in agricultural biocontrol [41]. Lipopeptide compounds, including fengycin A and surfactin were discovered from the secondary metabolites of the marine strain Bacillus amyloliquefaciens HY2-1. These compounds exhibited a broad-spectrum antifungal activity against seven fungal phyto-pathogens. Electron microscopy revealed that these compounds exert their antifungal effects by disrupting the cell membranes of the pathogenic fungi, thereby inhibiting the formation of fungal mycelia and spores [42]. Kocuria palustris 19C38A from marine organism was the source of a class of compounds, benzimidazole (56), with antifungal activity against Fusarium oxysporum. Further studies on the antifungal mechanism showed that these compounds disrupted the cell integrity of Fusarium oxysporum and inhibited spore germination [43].
The culture supernatant of B. amyloliquefaciens was collected from the Arctic Ocean and yielded an antifungal peptide W1 with a molecular weight of 2.4 kDa. It was considered as a new antifungal peptide derived from a fragment of preprotein translocase subunit YajC. It prevents the growth of Sclerotinia sclerotiorum and Fusarium oxysporum at concentrations of 140 and 58 μg/mL [44]. A cyclic lipopeptide, C17-fenngycin B, was identified from the metabolites of the deep-sea-derived bacterium Bacillus subtilis 2H11. This compound exhibited an 89.8% inhibition rate against Fusarium solani growth at a concentration of 0.2 mg/mL through pot experiments [45]. A new strain of Streptomyces yongxingensis sp. nov. (JCM 34965) was obtained from a marine soft coral to check its bioactivity. A bioactive compound, niphimycin C (57), was isolated from this strain and exhibited strong antifungal activity against banana wilt disease caused by Fusarium oxysporum f. sp. cubense pathogen, with an EC50 value of 1.2 μg/mL. This compound significantly inhibited the growth of pathogenic mycelia and spore germination. Further studies revealed that niphimycin C reduced the activity of key enzymes in the tricarboxylic acid cycle and electron transport chain. The authors suggest that niphimycin C is a promising agrochemical fungicide for controlling fungal diseases [46]. The structures of compounds 52–57 are shown in Figure 3.
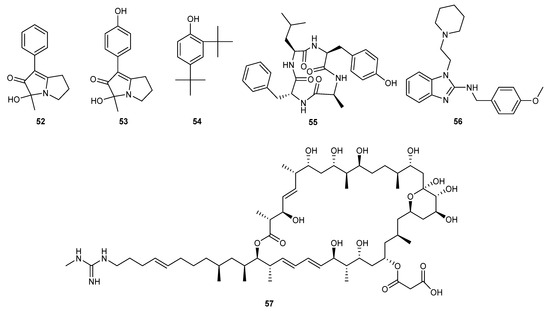
Figure 3.
Chemical structures of compounds 52–57.
2.1.3. Fungi from Mangroves
A variety of compounds were isolated from the secondary metabolites of an endophytic fungus, Alternaria iridiaustralis, which was isolated from halophytic plants in coastal areas. A series of compounds was discovered, among which alternanone A, B, D, and C (58–61) exhibited antifungal activity against benomyl-resistant strains of Botrytis cinerea, with MIC values ranging from 32 to 128 μg/mL, which were superior compared to the MIC value of 256 μg/mL with benomyl fungicide. This indicated the potential of these compounds as microbial pesticides for disease control [47].
The solid rice culture of the mangrove-derived fungus Nigrospora sp. QQYB1 produced 12 new griseofulvin derivatives. Out of these, compounds 62 and 63 demonstrated inhibitory effects against Colletotrichum truncatum, Microsporum gypseum, and Trichophyton mentagrophyte [48].
Similarly, an endophytic mangrove-derived fungus (Trichoderma lentiforme ML-P8-2) strain, was the source of a tandyukusin derivative and polyketide compounds. From the culture broth of this strain, compounds 64 and 65 both exhibited antifungal activity against Penicillium italicum, with an MIC value of 6.25 μM. Furthermore, biological activity tests indicated that tandyukisin C and G (66 and 67) had shown inhibitory action against the Penicillium italicum pathogen, both with 12.5 μg/mL concentrations [49].
Two strains of Penicillium javanicum HK1-23 as well as P. janthinellum HK1-6 were obtained from mangrove rhizosphere soil of the South China Sea. Activity-guided isolation from these two strains led to the discovery of the antibacterial secondary metabolites brefeldin A (68) and penicillic acid (69). At the same concentration (50 μg/mL), penicillic acid inhibited Rhizoctonia solani and R. cerealis by 67.5% and 76%, respectively. In contrast, brefeldin A showed a 56.4% inhibition rate against R. cerealis [50].
Two novel heterodimeric tetrahydroxanthones (aflaxanthones A (70) and B (71)), dimerized via an unprecedented 7,7′-linkage, have been obtained from the mangrove endophytic fungus Aspergillus flavus QQYZ. Aflaxanthones A (70) had an MIC value of 12.5 μM against Colletotrichum gloeosporioides and 3.13 μM against Fusarium oxysporum. Aflaxanthones B (71) also showed MIC values of 12.5 μM against both Fusarium oxysporum and Collettrichum musae indicating broad-spectrum and potential antifungal activity under in vitro conditions [51].
Investigation of Cladosporium cladosporioides MA-299 identified a bicyclic 5/9 ring system, macrolide cladocladosin A (72), along with two new sulfur-containing macrolides, thiocladospolides F (73) and G (74). The antifungal activity tests against Fusarium oxysporum f. sp. momodicae revealed that these compounds exhibited MIC values of 32, 16, and 32 μg/mL, respectively, as compared to a positive control (amphotericin B) value of 0.5 μg/mL [52].
Several new compounds were isolated from the secondary metabolites of the fungus Penicillium javanicum HK1-23, which was sourced from mangrove rhizosphere soil. The inhibitory activity of emindole SB (75) and penialidin A (76) against Alternaria alternata at gradient concentrations of 50.0, 10.0, and 2.0 μg/mL was evaluated. The results showed that emindole SB (75) exhibited a higher inhibitory activity at 50 μg/mL, with an inhibition rate of 77.3%, whereas penialidin A (76) had an inhibition rate of 56.8% at the same concentration [53].
The endophytic fungus Penicillium coffeae MA-314, isolated from the mangrove plant Laguncularia racemosa, has produced a new δ-lactone penicoffeazine A (77) and pairs of new isocoumarin derivatives (78 and 79). Penicoffeazine A (77) showed strong antifungal potency against Fusarium oxysporum f. sp. momordicae nov. f. and Colletotrichum gloeosporioides, with an MIC value of 5 μM, which is comparable to the positive control amphotericin B [54].
Four new isocoumarin derivatives, botryospyrones A, B, C, and D (80–83), as well as a new natural tryptamine derivative compound 84, were isolated through spectroscopic structure elucidation. These were purified from the culture broth of a novel endophytic fungus, Botryosphaeria ramose L29, which was derived from the leaf of Myoporum bontioides. These were further assessed for their antagonistic properties and showed MIC values of 25.0, 25.0, 50.0, and 6.2 μg/mL, respectively. Surprisingly, compound 84 was 16 times more potent than the positive control, triadimefon (MIC = 100 μg/mL). Additionally, all compounds, except for compound 80 (MIC = 199 μg/mL), exhibited significant antifungal activity as compared to triadimefon (MIC = 150 μg/mL), whereas compound 84 (MIC = 6.2 μg/mL) was found to be 18 times more active than triadimefon [55].
Study on the secondary metabolites of the marine-derived fungus Alternaria sp. (P8) led to the discovery of a new benzopyranone (+)-(2S,3R,4aR)-altenuene (85). This compound exhibited inhibitory effects on the growth of Alternaria brassicicola [56]. The structures of compounds 58–85 are shown in Figure 4.
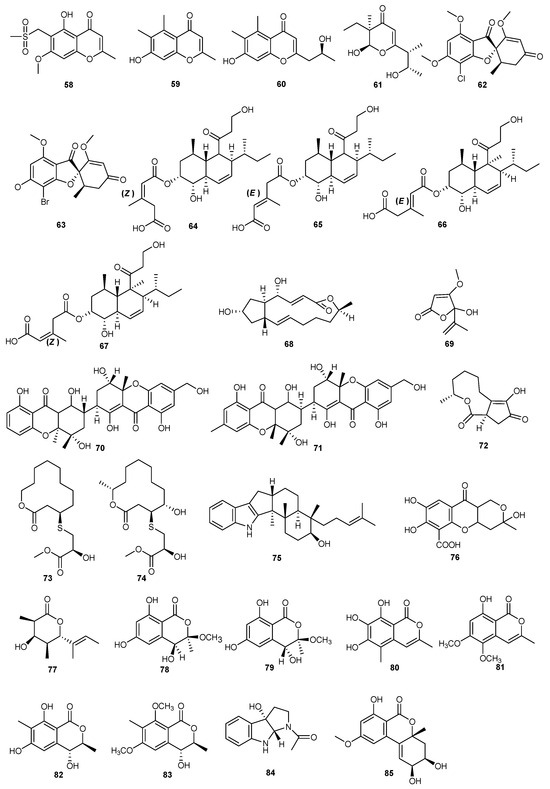
Figure 4.
Chemical structures of compounds 58–85.
2.2. Agricultural Antiviral Metabolites
Plant viral diseases, often referred to as “plant cancer” [57], lead to significant yield losses once crops are infected. These diseases pose a major threat to agricultural production worldwide due to their complex transmission mechanisms and the challenging management strategies required. The prevention and control of plant viruses mainly rely on preventive and indirect methods [58]. Currently, commercial antiviral agents such as amino-oligosaccharide, ribavirin, and ningnanmycin are available [59,60], but their field control efficacy is less than 60% [61]. Many natural products derived from marine microorganisms have been found to express antiviral activities (including activities against animal viruses). Some researchers have used natural products with antiviral activity as a scaffold to design and synthesize a series of derivatives with enhanced antiviral properties [62].
EI-Gendy et al. discovered the structure of essramycin (86), produced by Streptomyces Merv 8102. However, its antiviral properties were not examined at that time [63]. As of 2020, this compound was found to have significant inhibitory effects on the tobacco mosaic virus (TMV). Using essramycin (86) as the main compound, a series of its derivatives were designed and synthesized. Most of these derivatives exhibited stronger antiviral activity than ribavirin. Furthermore, compounds 87 and 88 even surpassed ningnanmycin in efficacy. Therefore, essramycin (86) holds the potential to be the main compound in the development of novel antiviral treatments [64].
A compound named acterophenone A (89) was identified from the secondary metabolites of the marine-derived Streptomyces sp. KCB-132. Antiviral experiments revealed that acterophenone A (89) expressed significant antiviral activity against plant viruses, including TMV, Tomato Mosaic Virus (ToMV), and Cucumber Mosaic Virus (CMV), with more than 70% inhibition rates. This efficacy even surpasses the positive control agent ningnanmycin, highlighting its potential as an effective antiviral agent [65]. The structures of compounds 86–89 are shown in Figure 5.
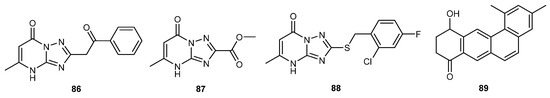
Figure 5.
Chemical structures of compounds 86–89.
2.3. Agricultural Herbicidal Metabolites
Weeds have a significant negative impact on crop growth and productivity in agricultural production. These significantly interfere with crops for light, water, and nutrients, affecting their normal growth and development. Additionally, weeds are the major source providing habitats for plant pathogens and pests, indirectly harming crop health. Moreover, some weeds can secrete allelopathic substances, which directly inhibit crop growth and even lead to crop death [66]. Currently, there are few studies on the herbicidal activity of marine natural products (MNPs), with only a handful of publications available. However, MNPs hold great potential and research value as herbicides.
A study has highlighted 37 strains of Alternaria sp. From marine habitats (P8), which underwent further identification and chemical investigation. It provided one new benzopyranone, along with seven known compounds. Activity tests revealed that (+)-(2S,3R,4aR)-altenuene (90), (+)-isoaltenuene (91), stemphyperylenol (92), and alterperylenol (93) exhibited significant phytotoxicity, markedly inhibiting the growth of amaranth seeds and leaves [56].
Alkalodi (94) was isolated from the secondary metabolites of marine-derived Alternaria iridiaustralis, which exhibited potential growth inhibitory effects on Echinochloa crusgalli seedlings. At concentrations of 20 and 40 μg/mL, the inhibitory activity reached 90%, surpassing the herbicide acetochlor. Additionally, alkalodi (94) also showed inhibitory effects on other weeds such as Digitaria sanguinalis, Portulaca oleracea, and Descurainia sophia [47].
Penicillum sclerotiorum HY5 from mangroves yielded seven pairs of azaphilones E/Z isomers and the structures of these compounds were determined using various analytical techniques. Isochromophilone H (95), ochlephilone (96), and isochromophilone I (97) exhibited strong inhibitory effects on the growth of radicle and plumule on Amaranthus retroflexus L., with EC50 values ranging from 234.87 to 320.84 μM, compared to the positive control glufosinate-ammonium, with EC50 values of 555.11 μM (radicle inhibition) and 656.04 μM (plumule inhibition). Additionally, ochlephilone (96) and isochromophilone I (97) also inhibited the growth of velvetleaf (Abutilon theophrasti Medikus), with EC50 values ranging from 768.97 to 1201.52 μM. The authors suggest that these compounds provide new leading compounds for the development of marine-derived bioherbicides [67].
Seed germination tests were conducted with the secondary metabolites of the fungus Eurotium rubrum. The tested compounds significantly reduced the germination of Echinochloa crusgalli seeds at a concentration of 2 mM. Notably, integric acid A (98) and brifeldin A (99) completely inhibited seed germination. Additionally, compounds 100–104 significantly inhibited the root and shoot growth of E. crusgalli at the same concentration [26].
Another study showed 449 marine-derived fungal strains that were screened in order to find compounds inhibiting pyruvate phosphate dikinase (PPDK), a potential herbicidal target in C4 plants. Several isolates were found selectively inhibiting PPDK activity. However, during experimental tests, one isolate was purified and identified as unguinol (105). This compound inhibited PPDK via a novel mode of action, with an EC50 value of 42.3 ± 0.8 μM [68]. The structures of compounds 90–105 are shown Figure 6.
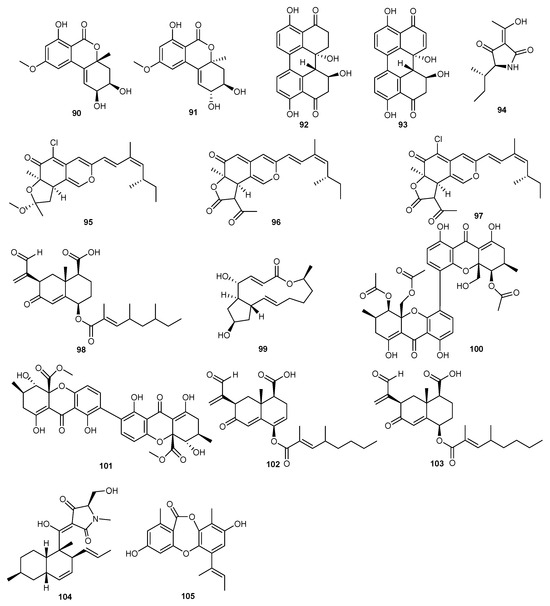
Figure 6.
Chemical structures of compounds 90–105.
2.4. Agricultural Insecticidal Metabolites
Pests can directly reduce crop yield and quality by feeding on leaves, stems, fruits, and other parts of plants. For example, aphids suck plant sap, causing slow growth or even the death of the plant. Additionally, some pests serve as vectors for plant pathogens such as powdery mildew, viruses, and bacteria. These pests transmit diseases from one plant to another, leading to outbreaks and spread of plant diseases [69]. This indirect damage is often more severe than the direct feeding damage. Moreover, controlling these pest-borne diseases is more challenging, which can lead to even greater losses. On the other hand, extensive use of chemical pesticides to control pests has harmful effects on non-target organisms, including beneficial insects, soil microorganisms, and birds, disrupting the balance of agroecosystems [70]. Some pests have developed resistance to synthetic chemicals, making future control efforts more difficult. Currently, many highly effective commercial insecticides are primarily composed of chemically synthesized halides, sulfides, and nitrides. These elements are abundant in marine natural products but are rare in terrestrial natural products [71,72]. As natural sources of chemicals, marine natural products offer a competitive advantage in “green chemistry”.
Four new xanthene derivatives, penicixanthenes A–D (106–109), were isolated from the secondary metabolites of the mangrove endophytic fungus Pencillium sp. JY246. The insecticidal activity of these compounds was tested, revealing that penicixanthenes B and C (107 and 108) showed inhibitory activity against the growth of Hubner larvae (Helicoverpa armigera), with LC50 values of 100 and 200 μg/mL, respectively. Additionally, compounds 106, 108, and 109 exhibited insecticidal activity against Culex quinquefasciatus larvae, with LC50 values of 38.5, 11.6, and 20.5 μg/mL, respectively. These xanthene derivatives show potential for development as insecticides [73].
The marine alga-derived endophytic fungus Acremonium vitellinum provided three chloramphenicol derivatives compounds, 110–112. Synthomycin (110) had the most significant activity against Helicoverpa armigera, with an LC50 value of 0.56 μg/mL (compared to the positive control, matrine, with an LC50 value of 0.24 μg/mL). Compound 111 was developed as a novel, eco-friendly, and safe biopesticide with an improved profile in agrochemicals [74].
The ethyl acetate extract of the fermentation of Aspergillus fumigatus JRJ11048, isolated from the leaves of Hainan-endemic mangrove plant Acrostichum specioum, exhibited insecticidal activity against Spodoptera litura. Seven compounds were extracted from the secondary metabolites of fungus among which aspergide (113) and 11-methyl-11-hydroxyldodecanoic acid amide (114) were newly identified. Further studies on these individual compounds revealed that aspergide (113) demonstrated significant insecticidal activity against Spodoptera litura larvae [75].
Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergia, yielded four new indole alkaloids, cristatumins A–D (115–118) and six known compounds. The insecticidal activity of the isolated compounds was evaluated and showed that cristatumins A (115) exhibited moderate lethality against brine shrimp [76].
Aspergillus oryzae was the source of two new indoloditerpene derivatives, asporyzin A (119) and asporyzin B (120), and one new indoloditerpene, asporyzin C (121), along with three known indoloditerpenes. Asporyzin C (121) exhibited potent activities against E. coli, with an inhibition diameter of 8.3 mm. The authors suggest that the presence of the indole and tetrahydrofuran units in JBIR-03 (122) likely enhances its insecticidal activity. This indole unit structure offers a new avenue for developing novel indoloditerpene insecticides [77]. The structures of compounds 106–122 are shown Figure 7. All the mentioned compounds are listed in Table 1.
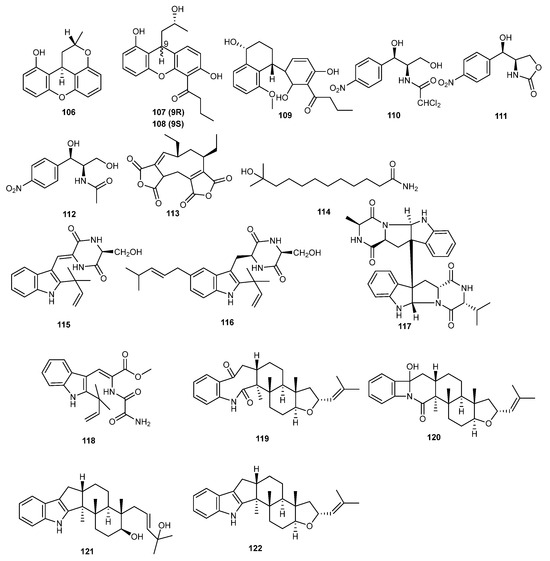
Figure 7.
Chemical structures of compounds 106–122.

Table 1.
Secondary metabolites associated with marine origin.
3. Discussion
This paper summarizes a total of 122 microbial secondary metabolites with agricultural activities. Among these, 106 compounds exhibit agriculturally active properties, while 16 compounds show no agricultural activity. There are 71 compounds with antibacterial activity, 4 compounds with antiviral activity, 16 compounds related to herbicidal activity, and 15 compounds with insecticidal activity. These compounds demonstrate a broad spectrum of activity against multiple genera and species of agricultural pathogenic microorganisms, as well as weeds and pests (Figure 8).
Figure 8.
The antagonistic activity of secondary metabolites.
The results indicate that among the compounds with antibacterial activity, those derived from marine fungi, marine bacteria, and mangrove-derived fungi account for 51 (59%), 6 (7%), and 30 (34%), respectively. Among the fungal-derived compounds, the majority of active compounds originate from the genera Penicillium sp. (25%) and Aspergillus sp. (17%) (Figure 9).
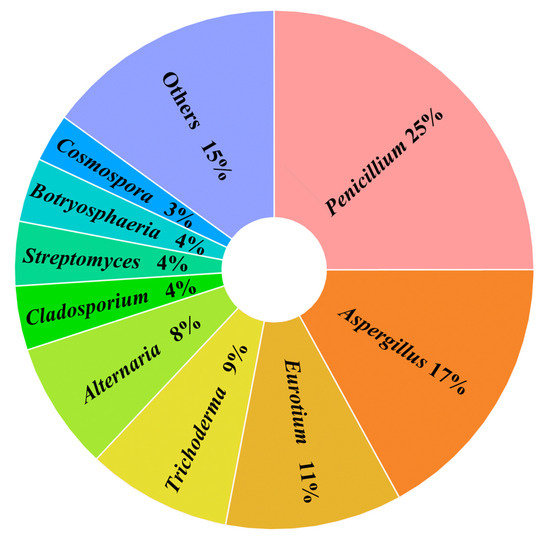
Figure 9.
The proportion of secondary metabolites from fungal origin.
Mechanism of Action of MBCs
The mechanism of marine bioactive compounds is complex (Figure 10). Plant pathogenic bacteria and fungi rely on their cell walls for cellular stability, osmotic pressure, ion exchange mechanism, and metabolism [78]. Marine-derived bioactive compounds target key component cell walls of fungi, such as glucosamine to prevent cell wall growth and synthesis. For example, the microalgal phenolic extracts (MPEs) derived from the microalgae Spirulina sp. and Nanochloropsis have been reported to degrade the glucosamine structure of Fusarium graminearum while reducing its production by 15% [79]. Similarly, chitinase enzyme from marine bacterium B. pumilus hydrolyzed chitin in F. oxysporum and inhibited its growth [80].
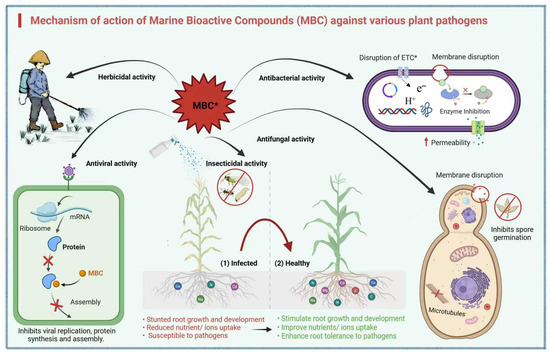
Figure 10.
Mechanism of action of MBCs against plant pathogens and their beneficial effects on plant health. The figure illustrates the multifaceted roles of MBCs in combating various plant pathogens, including herbicides, viruses, insects, and fungi, through mechanisms such as disruption of the electron transport chain (ETC*), membrane disruption, enzyme inhibition, and inhibition of viral replication, protein synthesis, and assembly. Additionally, MBCs promote plant health by stimulating root growth and development, improving nutrient uptake, and enhancing root tolerance to pathogens. Key processes, such as microtubule interference, spore germination inhibition, and modulation of ribosomal activity, are also highlighted. The schematic provides a comprehensive overview of the dual functionality of MBCs in pathogen suppression and plant growth enhancement.
The cell membrane constitutes a lipid bilayer that exhibits semi-permeability, which regulates the bidirectional movement of molecules within and outside the cells of plant pathogens. The ethyl acetate extract from marine Streptomyces sp. AMA49 disrupts the cell membrane of M. oryzae and suppresses pathogen growth and spore germination [56,81]. Similarly, marine bioactive compounds have been observed inhibiting bacterial cell membrane integrity against several pathogens [82,83,84,85,86]. Marine natural products also target vital metabolic pathways. Fatty acid metabolism, which is vital for appressorium formation and host penetration were also disrupted to prevent infection [87]. Compounds such as haliangicin isolated from marine myxobacteria impede fungal respiration by inhibiting cytochrome bc-1 complex while blocking the electron transport chain, resulting in cell death [88]. MBCs also disrupt bacterial quorum sensing (QS), a cell density-controlled system that is necessary for gene virulence. Compounds such as 2-methyl-N-(2′-phenylethyl)-butanamide inhibit QS in Burkholderia glumae, suppressing production of virulence factors and preventing host infection [89,90,91]. In addition, certain marine natural products induce plant immunity, controlling bacterial pathogens indirectly through stimulating host defense mechanisms as opposes to direct antimicrobial activity [92,93,94].
MBCs also exhibited strong antiviral properties against plant viruses through inhibition of multiple stages of the viral cycle, including attachment, penetration, replication, and viral assembly. The essramycin compound from Streptomyces sp. inhibits TMV while disrupting viral assembly and interfering with 20S coat protein disk aggregation [64]. Aldidine derivative and laurene sesquiterpenes similarly inhibited TMV disease [62]. Marine actinomycetes like Streptomyces yielded secondary metabolites that can directly inactivate plant viruses like TMV and cucumber mosaic virus (CMV) or induce systemic resistance in plants. Sulfated polysaccharides of marine algae prevent viral adsorption and entry by binding to viral particles or host receptors, blocking infection [95]. Similarly, Euphorbia factor L-1, a marine plant-derived terpenoid from Euphorbia tirucalli, has shown anti-viral activity against citrus tristeza virus (CTV) by inhibiting viral RNA synthesis and replication [96]. Seaweed phenolics enhance plant growth and stress resistance as well as seed germination, elongation of roots and shoots, and photosynthesis, and protect against drought, salt, and heavy metal stresses through triggering antioxidants while inhibiting pathogens and pests. These also contribute to cell wall strengthening, hormone modification, and improved plant health and yield [97].
MBCs have been found disrupting the number of physiological and biochemical functions of insect pests. Alkaloid and flavonoid derivatives primarily exert their insecticidal effect by inducing cytotoxicity, which directly damages insect cells. Fatty acids and peptides prevent larval growth, feeding behavior, and transformation by disrupting the protein system and nervous system regulation. Moreover, organosulfur compounds and marine-derived steroids cause muscular and cardiac impairment, leading to paralysis or death of insects. Marine insecticidal proteins, such as cry toxins from marine Bacillus, cause cell lysis and larval death by binding to specific receptors in the insect midgut [72]. Manzamine alkaloids isolated from marine sponges exhibited the significant insecticidal activity through disrupting the essential physiological processes of insects such as western corn rootworm [98].
Marine sources exert a herbicidal mode of action by inhibiting photosystem II, disrupting the electron transport chain and interfering with chlorophyll activity, thereby preventing the energy production required for growth and resulting in the death of weeds [99]. Similarly, secondary metabolites such as Asparagopsis armata interfere with chloroplast electron transport, inducing oxidative stress and cellular damage in weeds. They also suppress carotenoid metabolism, impairing plant resistance against oxidative damage, which weakens weed defense [100]. Inhibition of acetolactate synthase (ALS), essential for branched-chain amino acid production, additionally impedes protein synthesis and inhibits weed development [101]. These targeted mechanisms of action reduce damage to non-target and beneficial microorganisms, offering sustainable alternatives to synthetic herbicides.
Author Contributions
Investigation and data curation, M.Y., H.M.U.S., C.Z. and Y.X.; writing—review and editing, M.Y., H.M.U.S., B.H., Y.Y. and W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (2021JJLH0073) and the Jiangsu Agriculture Science and Technology Innovation Fund (CX(23)3018).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jackson, V.; Sherer, C.; Jordan, L.; Clohessy, T. Unveiling the potential: Exploring the efficacy of complex III inhibitors in fungal disease control. Pest Manag. Sci. 2025, 81, 2450–2456. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Shaheen, H.; Rajput, N.; Atiq, M.; Kachelo, G.; Ahmad, H.; Wahab, M.; Tahir, M.; Hasnain, A. Antifungal potential of medicinal plant extracts against brown leaf spot (BLS) disease of rice caused by Bipolaris oryzae. Sarhad J. Agric. 2024, 40, 603–614. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.; Monaghan, R.L.; Olson, G.; Putter, I.; et al. Avermectins, New Family of Potent Anthelmintic Agents: Producing Organism and Fermentation. Antimicrob. Agents Chemother. 1979, 15, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mukohara, T.; Nagai, S.; Mukai, H.; Namiki, M.; Minami, H. Eribulin Mesylate in Patients with Refractory Cancers: A Phase I Study. Investig. New Drugs 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, X.M.; Zhang, Y.R.; Cai, Y.Y.; Wang, J.Y.; Liu, M.Y.; Wang, J.Y.; Bao, J.D.; Lin, F.C. Research on the Molecular Interaction Mechanism between Plants and Pathogenic Fungi. Int. J. Mol. Sci. 2022, 23, 4658. [Google Scholar] [CrossRef] [PubMed]
- Ncube, E.; Flett, B.C.; Van den Berg, J.; Erasmus, A.; Viljoen, A. The Effect of Busseola fusca Infestation, Fungal Inoculation and Mechanical Wounding on Fusarium Ear Rot Development and Fumonisin Production in Maize. Crop Prot. 2017, 99, 177–183. [Google Scholar] [CrossRef]
- Thakur, O.; Prasad, R. Engineering Resistance to Alternaria cyamopsidis by RNAi Mediated Gene Silencing of Chitin Synthase Export Chaperone CHS7 in Guar. Physiol. Mol. Plant Pathol. 2020, 112, 101541. [Google Scholar] [CrossRef]
- Dai, L.; Xie, Q.; Guo, J.; Ma, Q.; Yang, L.; Yuan, J.; Dai, H.; Yu, Z.; Zhao, Y. Bioactive Chemical Constituents from the Marine-Derived Fungus Cladosporium sp. DLT-5. J. Oceanol. Limnol. 2024, 42, 905–914. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Zhu, J.; Sun, J.; Shao, X. Volatile Organic Compounds of Scheffersomyces spartinae W9 Have Antifungal Effect against Botrytis cinerea on Strawberry Fruit. Foods 2023, 12, 3619. [Google Scholar] [CrossRef]
- Yang, X.; Yu, H.; Ren, J.; Cai, L.; Xu, L.; Liu, L. Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09. J. Fungi 2023, 9, 347. [Google Scholar] [CrossRef]
- Vieira, G.; Sette, L.D.; de Angelis, D.A.; Sass, D.C. Antifungal Activity of Cyclopaldic Acid from Antarctic penicillium against Phytopathogenic Fungi. Biotech 2023, 13, 374. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Wang, W.; Wang, X.; Aihaiti, P.; Lin, T.; Fu, Z.; Xu, R.; Wu, M.; Li, Z.; et al. A New Method of Preparing Aurone by Marine Actinomycetes and Its Potential Application in Agricultural Fungicides. Molecules 2023, 28, 17. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Bluemel, M.; Scarpato, S.; Mangoni, A.; Tasdemir, D. Induction of Isochromanones by Co-Cultivation of the Marine Fungus Cosmospora sp. and the Phytopathogen Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 782. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.; Han, J.W.; Kim, H.; Choi, G.J. Phenyl Ethers from the Marine-Derived Fungus Aspergillus tabacinus and Their Antimicrobial Activity Against Plant Pathogenic Fungi and Bacteria. ACS Omega 2022, 7, 33273–33279. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.T.; Van Nguyen, M.; Han, J.W.; Park, M.S.; Kim, H.; Choi, G.J. In Vitro and In Vivo Antifungal Activity of Sorbicillinoids Produced by Trichoderma longibrachiatum. J. Fungi 2021, 7, 428. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.Y.; Deng, R.S.; Tan, Z.; Chen, G.Y.; Nong, X.H. Three New Unsaturated Fatty Acids from Marine-Derived Fungus Aspergillus sp. SCAU150. Nat. Prod. Res. 2022, 36, 3965–3971. [Google Scholar] [CrossRef]
- Chen, T.; Yang, W.; Li, T.; Yin, Y.; Liu, Y.; Wang, B.; She, Z. Hemiacetalmeroterpenoids A-C and Astellolide Q with Antimicrobial Activity from the Marine-Derived Fungus Penicillium sp. N-5. Mar. Drugs 2022, 20, 514. [Google Scholar] [CrossRef]
- Saad, M.M.G.; Abdelgaleil, S.A.M.; Shiono, Y. Antibacterial and Herbicidal Properties of Secondary Metabolites from Fungi. Nat. Prod. Res. 2021, 35, 5446–5451. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Dramae, A.; Komwijit, S.; Laorob, T.; Khamsaeng, S.; Pittayakhajonwut, P. Antimicrobial, Antimalarial and Anticholinesterase Substances from the Marine-Derived Fungus Aspergillus terreus BCC51799. Tetrahedron 2020, 76, 131496. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Yin, X.-L.; Ji, N.-Y. Trichoderols B-G, Six New Lipids from the Marine Algicolous Fungus Trichoderma sp. Z43. Mar. Drugs 2023, 21, 453. [Google Scholar] [CrossRef]
- Zhao, D.L.; Wang, H.S.; Gao, L.W.; Zhang, P. Tennessenoid A, an Unprecedented Steroid-Sorbicillinoid Adduct from the Marine-Derived Endophyte of Aspergillus sp. Strain 1022LEF. Front. Mar. Sci. 2022, 9, 923128. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, W.L.; Huang, Q.R.; Wei, M.L.; Li, Y.Z.; Jiang, L.L.; Li, C.L.; Yu, X.; Zhu, H.W.; Chen, G.Z.; et al. New Enantiomers of a Nor-Bisabolane Derivative and Two New Phthalides Produced by the Marine-Derived Fungus Penicillium chrysogenum LD-201810. Front. Microbiol. 2021, 12, 727670. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Hu, L.; Liu, S.; Xing, R.; Li, P. Isolation and Identification of Antimicrobial Metabolites from Sea Anemone-Derived Fungus Emericella sp. SMA01. J. Oceanol. Limnol. 2021, 39, 1010–1019. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Liu, X.H.; Li, X.N.; Ji, N.Y. Antifungal and Antimicroalgal Trichothecene Sesquiterpenes from the Marine Algicolous Fungus Trichoderma brevicompactum A-DL-9-2. J. Agric. Food Chem. 2020, 68, 15440–15448. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Sun, Z.C.; Meng, L.H.; Wang, B.G. Secondary Metabolites with Agricultural Antagonistic Potentials from Beauveria felina, a Marine-Derived Entomopathogenic Fungus. J. Agric. Food Chem. 2020, 68, 14824–14831. [Google Scholar] [CrossRef]
- Xu, K.; Wei, X.L.; Xue, L.; Zhang, Z.F.; Zhang, P. Antimicrobial Meroterpenoids and Erythritol Derivatives Isolated from the Marine-Algal-Derived Endophytic Fungus Penicillium chrysogenum XNM-12. Mar. Drugs 2020, 18, 578. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Yang, S.Q.; Cao, J.; Li, Y.H.; Wang, B.G. Induced Terreins Production from Marine Red Algal-Derived Endophytic Fungus Aspergillus terreus EN-539 Co-Cultured with Symbiotic Fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2020, 73, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Li, X.M.; Yang, S.Q.; Liu, H.; Meng, L.H.; Wang, B.G. Three New Sesquiterpenoids from the Algal-Derived Fungus Penicillium chermesinum EN-480. Mar. Drugs 2020, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Xu, X.; Feng, J.; Hao, L. Inhibition of Oil Tea Anthracnose by Natural Product Extracts from Bacillus and Pseudoalteromonas Isolates from Mangrove Soil. Front. Mar. Sci. 2023, 10, 1299118. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, X.; Xu, H.; Qi, H.; Du, S.; Huang, J.; Zhang, J.; Wang, J. Phenopyrrolizins A and B, Two Novel Pyrrolizine Alkaloids from Marine-Derived Actinomycetes Micromonospora sp. HU138. Molecules 2023, 28, 7672. [Google Scholar] [CrossRef]
- Kharat, B.A.; Said, M.S.; Dastager, S.G. Antifungal Compound from Marine Serratia Marcescens BKACT and Its Potential Activity against Fusarium sp. Int. Microbiol. 2022, 25, 851–862. [Google Scholar] [CrossRef]
- Gomez, J.S.; Shaikhet, M.; Loganathan, A.K.; Darnowski, M.G.G.; Boddy, C.N.N.; McMullin, D.R.R.; Avis, T.J.J. Characterization of Arthropeptide B, an Antifungal Cyclic Tetrapeptide from Arthrobacter humicola. J. Chem. Ecol. 2023, 49, 528–536. [Google Scholar] [CrossRef]
- Guillén-Navarro, K.; López-Gutiérrez, T.; García-Fajardo, V.; Gómez-Cornelio, S.; Zarza, E.; De la Rosa-García, S.; Chan-Bacab, M. Broad-Spectrum Antifungal, Biosurfactants and Bioemulsifier Activity of Bacillus subtilis subsp. spizizenii—A Potential Biocontrol and Bioremediation Agent in Agriculture. Plants 2023, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ling, X.; Peng, S.; Tan, M.; Yan, L.; Liang, Y.; Li, G.; Li, K. A Marine Lipopeptides-Producing Bacillus amyloliquefaciens HY2-1 with a Broad-Spectrum Antifungal and Antibacterial Activity and Its Fermentation Kinetics Study. World J. Microbiol. Biotechnol. 2023, 39, 196. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, A.; Setiawan, F.; Juliasih, N.L.G.R.; Widyastuti, W.; Laila, A.; Setiawan, W.A.; Djailani, F.M.; Mulyono, M.; Hendri, J.; Arai, M. Fungicide Activity of Culture Extract from Kocuria palustris 19C38A1 against Fusarium oxysporum. J. Fungi 2022, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, R.; Ouyang, Z.; He, T.; Zhang, W.; Chen, X. Identification of a New Antifungal Peptide W1 from a Marine Bacillus amyloliquefaciens Reveals Its Potential in Controlling Fungal Plant Diseases. Front. Microbiol. 2022, 13, 922454. [Google Scholar] [CrossRef]
- Liu, W.; Sun, C. C17-Fengycin B, Produced by Deep-Sea-Derived Bacillus subtilis, Possessing a Strong Antifungal Activity against Fusarium solani. J. Oceanol. Limnol. 2021, 39, 1938–1947. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Y.; Cai, B.; Zhou, D.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; Wedge, D.E.; Pan, Z.; et al. Discovery of Niphimycin C from Streptomyces yongxingensis sp. Nov. as a Promising Agrochemical Fungicide for Controlling Banana Fusarium Wilt by Destroying the Mitochondrial Structure and Function. J. Agric. Food Chem. 2022, 70, 12784–12795. [Google Scholar] [CrossRef]
- Fan, J.; Guo, F.; Zhao, C.; Li, H.; Qu, T.; Xiao, L.; Du, F. Secondary Metabolites with Herbicidal and Antifungal Activities from Marine-Derived Fungus Alternaria iridiaustralis. J. Fungi 2023, 9, 716. [Google Scholar] [CrossRef]
- Zou, G.; Yang, W.; Chen, T.; Liu, Z.; Chen, Y.; Li, T.; Said, G.; Sun, B.; Wang, B.; She, Z. Griseofulvin Enantiomers and Bromine-Containing Griseofulvin Derivatives with Antifungal Activity Produced by the Mangrove Endophytic Fungus Nigrospora sp. QQYB1. Mar. Life Sci. Technol. 2023, 6, 102–114. [Google Scholar] [CrossRef]
- Yin, Y.; Tan, Q.; Wu, J.; Chen, T.; Yang, W.; She, Z.; Wang, B. The Polyketides with Antimicrobial Activities from a Mangrove Endophytic Fungus Trichoderma lentiforme ML-P8-2. Mar. Drugs 2023, 21, 566. [Google Scholar] [CrossRef]
- Shen, N.; Liang, Z.; Liu, Q.; Tu, C.; Dong, K.; Wang, C.; Chen, M. Antifungal Secondary Metabolites Isolated from Mangrove Rhizosphere Soil-Derived Penicillium Fungi. J. Ocean. Univ. China 2020, 19, 717–721. [Google Scholar] [CrossRef]
- Zang, Z.; Yang, W.; Cui, H.; Cai, R.; Li, C.; Zou, G.; Wang, B.; She, Z. Two Antimicrobial Heterodimeric Tetrahydroxanthones with a 7,7′-Linkage from Mangrove Endophytic Fungus Aspergillus flavus QQYZ. Molecules 2022, 27, 2691. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Meng, L.H.; Wang, B.G. Cladocladosin A, an Unusual Macrolide with Bicyclo 5/9 Ring System, and Two Thiomacrolides from the Marine Mangrove-Derived Endophytic Fungus, Cladosporium cladosporioides MA-299. Bioorg. Chem. 2020, 101, 103950. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Y.; Shen, N.X.; Zhou, X.J.; Zheng, Y.Y.; Chen, M.; Wang, C.Y. Bioactive Indole Diterpenoids and Polyketides from the Marine-Derived Fungus Penicillium javanicum. Chem. Nat. Compd. 2020, 56, 379–382. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New Lactone and Isocoumarin Derivatives from the Marine Mangrove-Derived Endophytic Fungus Penicillium coffeae MA-314. Phytochem. Lett. 2019, 32, 1–5. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Zhang, X.; Chen, Z.; Li, T.; She, Z.; Ding, W.; Li, C. Four New Isocoumarins and a New Natural Tryptamine with Antifungal Activities from a Mangrove Endophytic Fungus Botryosphaeria ramosa L29. Mar. Drugs 2019, 17, 88. [Google Scholar] [CrossRef]
- Huang, R.H.; Gou, J.Y.; Zhao, D.L.; Wang, D.; Liu, J.; Ma, G.Y.; Li, Y.Q.; Zhang, C.S. Phytotoxicity and Anti-Phytopathogenic Activities of Marine-Derived Fungi and Their Secondary Metabolites. Rsc Adv. 2018, 8, 37573–37580. [Google Scholar] [CrossRef]
- Jones, R.A.C. Disease Pandemics and Major Epidemics Arising from New Encounters between Indigenous Viruses and Introduced Crops. Viruses 2020, 12, 1388. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, A.; Wang, Q. Chapter 39—Marine Natural Products and Plant Virus Control. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Academic Press: Cambridge, MA, USA, 2021; pp. 563–569. [Google Scholar]
- Liu, Y.; Song, H.; Huang, Y.; Li, J.; Zhao, S.; Song, Y.; Yang, P.; Xiao, Z.; Liu, Y.; Li, Y.; et al. Design, Synthesis, and Antiviral, Fungicidal, and Insecticidal Activities of Tetrahydro-β-Carboline-3-Carbohydrazide Derivatives. J. Agric. Food Chem. 2014, 62, 9987–9999. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Song, B. Proteomics Analysis of Xiangcaoliusuobingmi-Treated Capsicum annuum L. Infected with Cucumber Mosaic Virus. Pestic. Biochem. Physiol. 2018, 149, 113–122. [Google Scholar] [CrossRef]
- Zhang, J.; He, F.; Chen, J.; Wang, Y.; Yang, Y.; Hu, D.; Song, B. Purine Nucleoside Derivatives Containing a Sulfa Ethylamine Moiety: Design, Synthesis, Antiviral Activity, and Mechanism. J. Agric. Food Chem. 2021, 69, 5575–5582. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Wang, Q. Research Progress of Anti-Plant Virus Agents Based on Marine Natural Products. Adv. Agrochem. 2023, 2, 31–38. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; Shaaban, M.; Shaaban, K.A.; El-Bondkly, A.M.; Laatsch, H. Essramycin: A First Triazolopyrimidine Antibiotic Isolated from Nature. J. Antibiot. 2008, 61, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, S.; Li, H.; Lu, A.; Wang, Z.; Yao, Y.; Wang, Q. Discovery, Structural Optimization, and Mode of Action of Essramycin Alkaloid and Its Derivatives as Anti-Tobacco Mosaic Virus and Anti-Phytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, L.; Yang, Q.L.; Xu, B.; Fu, X.Z.; Liu, M.; Li, Z.; Zhang, S.M.; Xie, Z.P. Antibacterial and Cytotoxic Bridged and Ring Cleavage Angucyclinones from a Marine Streptomyces sp. Front. Chem. 2020, 8, 586. [Google Scholar] [CrossRef]
- Pakdaman Sardrood, B.; Mohammadi Goltapeh, E. Weeds, Herbicides and Plant Disease Management. In Sustainable Agriculture Reviews 31: Biocontrol; Springer: Cham, Switzerland, 2018; pp. 41–178. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Wang, X.B.; Li, Y.Q.; Ding, J.L.; Lan, M.X.; Gao, X.; Zhao, D.L.; Zhang, C.S.; Wu, G.X. Phytotoxic Azaphilones from the Mangrove-Derived Fungus Penicillium sclerotiorum HY5. Front. Microbiol. 2022, 13, 880874. [Google Scholar] [CrossRef]
- Motti, C.A.; Bourne, D.G.; Burnell, J.N.; Doyle, J.R.; Haines, D.S.; Liptrot, C.H.; Llewellyn, L.E.; Ludke, S.; Muirhead, A.; Tapiolas, D.M. Screening Marine Fungi for Inhibitors of the C4 Plant Enzyme Pyruvate Phosphate Dikinase: Unguinol as a Potential Novel Herbicide Candidate. Appl. Environ. Microbiol. 2007, 73, 1921–1927. [Google Scholar] [CrossRef]
- Loebenstein, G.; Carr, J.P. Natural Resistance Mechanisms of Plants to Viruses; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The Intriguing Chemistry and Biology of Sulfur-Containing Natural Products from Marine Microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Song, C.; Yang, J.; Zhang, M.; Ding, G.; Jia, C.; Qin, J.; Guo, L. Marine Natural Products: The Important Resource of Biological Insecticide. Chem. Biodivers. 2021, 18, e2001020. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.; Nong, X.; Zhou, X.; Luo, Y.; Chen, G. Four New Insecticidal Xanthene Derivatives from the Mangrove-Derived Fungus Penicillium sp. JY246. Mar. Drugs 2019, 17, 649. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, P.; Liu, T.; Wang, X.-F.; Li, Z.-X.; Li, W.; Wang, F.-L. Insecticidal Activities of Chloramphenicol Derivatives Isolated from a Marine Alga-Derived Endophytic Fungus, Acremonium vitellinum, against the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Molecules 2018, 23, 2995. [Google Scholar] [CrossRef]
- Guo, Z.; Gai, C.; Cai, C.; Chen, L.; Liu, S.; Zeng, Y.; Yuan, J.; Mei, W.; Dai, H. Metabolites with Insecticidal Activity from Aspergillus fumigatus JRJ111048 Isolated from Mangrove Plant Acrostichum specioum Endemic to Hainan Island. Mar. Drugs 2017, 15, 381. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Li, C.S.; Shang, Z.; Wang, B.G. Cristatumins A-D, New Indole Alkaloids from the Marine-Derived Endophytic Fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ji, N.; Liu, X.; Li, K.; Zhu, Q.; Xue, Q. Indoloditerpenes from an Algicolous Isolate of Aspergillus oryzae. Bioorg. Med. Chem. Lett. 2010, 20, 5677–5680. [Google Scholar] [CrossRef] [PubMed]
- Dörr, T. Understanding tolerance to cell wall–active antibiotics. Ann. N. Y. Acad. Sci. 2021, 1496, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Pagnussatt, F.A.; Lemos, A.C.; Nicolli, C.P.; Del Ponte, E.M.; Badiale-Furlong, E. Nannochloropsis sp. and Spirulina sp. as a source of antifungal compounds to mitigate contamination by Fusarium graminearum species complex. Curr. Microbiol. 2019, 76, 930–938. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317–322. [Google Scholar] [CrossRef]
- Nair, V.; Schuhmann, I.; Anke, H.; Kelter, G.; Fiebig, H.H.; Helmke, E.; Laatsch, H. Marine bacteria, XLVII–Psychrotolerant bacteria from extreme antarctic habitats as producers of rare bis-and trisindole alkaloids. Planta Medica 2016, 82, 910–918. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- López-Abarrategui, C.; Alba, A.; Silva, O.N.; Reyes-Acosta, O.; Vasconcelos, I.M.; Oliveira, J.T.; Migliolo, L.; Costa, M.P.; Costa, C.R.; Silva, M.R. Functional characterization of a synthetic hydrophilic antifungal peptide derived from the marine snail Cenchritis muricatus. Biochimie 2012, 94, 968–974. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018, 84, e00445-18. [Google Scholar] [CrossRef]
- Zhao, D.L.; Wang, D.; Tian, X.Y.; Cao, F.; Li, Y.Q.; Zhang, C.S. Anti-phytopathogenic and cytotoxic activities of crude extracts and secondary metabolites of marine-derived fungi. Mar. Drugs 2018, 16, 36. [Google Scholar] [CrossRef]
- Zhao, D.; Han, X.; Wang, D.; Liu, M.; Gou, J.; Peng, Y.; Liu, J.; Li, Y.; Cao, F.; Zhang, C. Bioactive 3-decalinoyltetramic acids derivatives from a marine-derived strain of the fungus Fusarium equiseti D39. Front. Microbiol. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Deng, S.; Gu, Z.; Yang, N.; Li, L.; Yue, X.; Que, Y.; Sun, G.; Wang, Z.; Wang, J. Identification and characterization of the peroxin 1 gene MoPEX1 required for infection-related morphogenesis and pathogenicity in Magnaporthe oryzae. Sci. Rep. 2016, 6, 36292. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Chen, X. Screening of marine bioactive antimicrobial compounds for plant pathogens. Mar. Drugs 2021, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Betancur, L.A.; Forero, A.M.; Vinchira-Villarraga, D.M.; Cárdenas, J.D.; Romero-Otero, A.; Chagas, F.O.; Pupo, M.T.; Castellanos, L.; Ramos, F.A. NMR-based metabolic profiling to follow the production of anti-phytopathogenic compounds in the culture of the marine strain Streptomyces sp. PNM-9. Microbiol. Res. 2020, 239, 126507. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Islam, S.; Sheikh, M.A.; Dhingra, S.; Uwambaye, P.; Labricciosa, F.M.; Iskandar, K.; Charan, J.; Abukabda, A.B.; Jahan, D. Quorum sensing: A new prospect for the management of antimicrobial-resistant infectious diseases. Expert Rev. Anti-Infect. Ther. 2021, 19, 571–586. [Google Scholar] [CrossRef]
- Soto-Aceves, M.P.; Cocotl-Yañez, M.; Servín-González, L.; Soberón-Chávez, G. The Rhl quorum-sensing system is at the top of the regulatory hierarchy under phosphate-limiting conditions in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2021, 203, e00475-20. [Google Scholar] [CrossRef]
- De Almeida, C.L.; Falcão, H.D.; Lima, G.R.; Montenegro, C.D.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; De Souza, M.D.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Liu, Y.; Lu, A.; Wang, Z.; Li, Y.; Yang, S.; Wang, Q. Marine-natural-product development: First discovery of nortopsentin alkaloids as novel antiviral, anti-phytopathogenic-fungus, and insecticidal agents. J. Agric. Food Chem. 2018, 66, 4062–4072. [Google Scholar] [CrossRef]
- Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different antifungal activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, L.; Rajashekara, H.; Uppala, L.S.; Ambika, D.S.; Patil, B.; Shankarappa, K.S.; Nath, V.S.; Kavitha, T.R.; Mishra, A.K. Mechanisms of microbial plant protection and control of plant viruses. Plants 2022, 11, 3449. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.S.; Bhargavi, K.; Suman, J.D.; Nanda, C.; Kantal, D.; Vate, N.K.; Shanthanna, P. Exploring Marine Natural Products as Antiviral Agents, Advances and Emerging Opportunities. Uttar Pradesh J. Zool. 2025, 46, 55–65. [Google Scholar] [CrossRef]
- Aina, O.; Bakare, O.O.; Daniel, A.I.; Gokul, A.; Beukes, D.R.; Fadaka, A.O.; Keyster, M.; Klein, A. Seaweed-derived phenolic compounds in growth promotion and stress alleviation in plants. Life 2022, 12, 1548. [Google Scholar] [CrossRef]
- Peng, J.; Shen, X.; El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Bobzin, S.; Huesing, J.; Camp, R. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef]
- Mercurio, P.; Eaglesham, G.; Parks, S.; Kenway, M.; Beltran, V.; Flores, F.; Mueller, J.F.; Negri, A.P. Contribution of transformation products towards the total herbicide toxicity to tropical marine organisms. Sci. Rep. 2018, 8, 4808. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.; Feijão, E.; de Carvalho, R.C.; Matos, A.R.; Fonseca, V.F.; Novais, S.C.; Lemos, M.F. Potential of Asparagopsis armata as a biopesticide for weed control under an invasive seaweed circular-economy framework. Biology 2021, 10, 1321. [Google Scholar] [CrossRef]
- Oršolić, D.; Pehar, V.; Šmuc, T.; Stepanić, V. Comprehensive machine learning based study of the chemical space of herbicides. Sci. Rep. 2021, 11, 11479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).