Vaterite/Fucoidan Hybrid Microparticles: Fabrication, Loading of Lactoferrin, Structural Characteristics and Functional Properties
Abstract
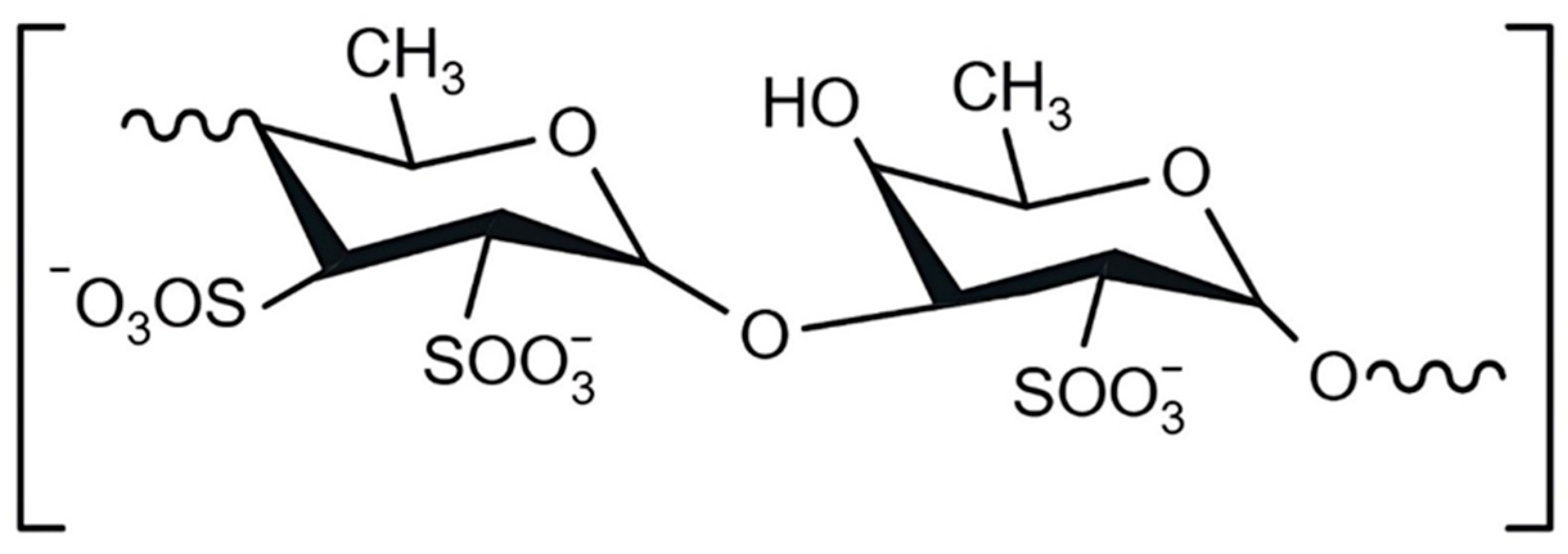
1. Introduction
2. Results and Discussion
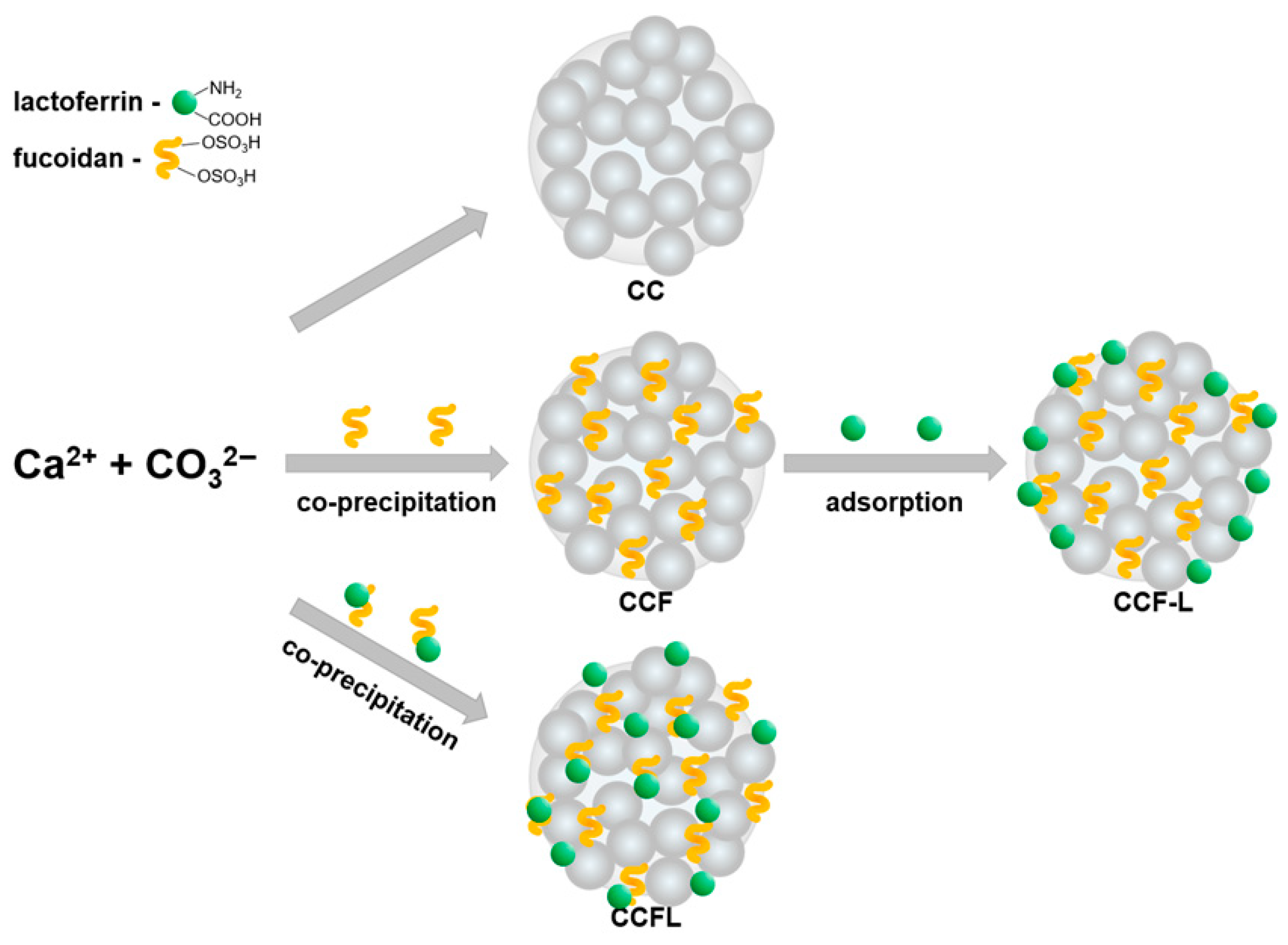
2.1. Obtaining Hybrid Microparticles and Incorporating Fucoidan
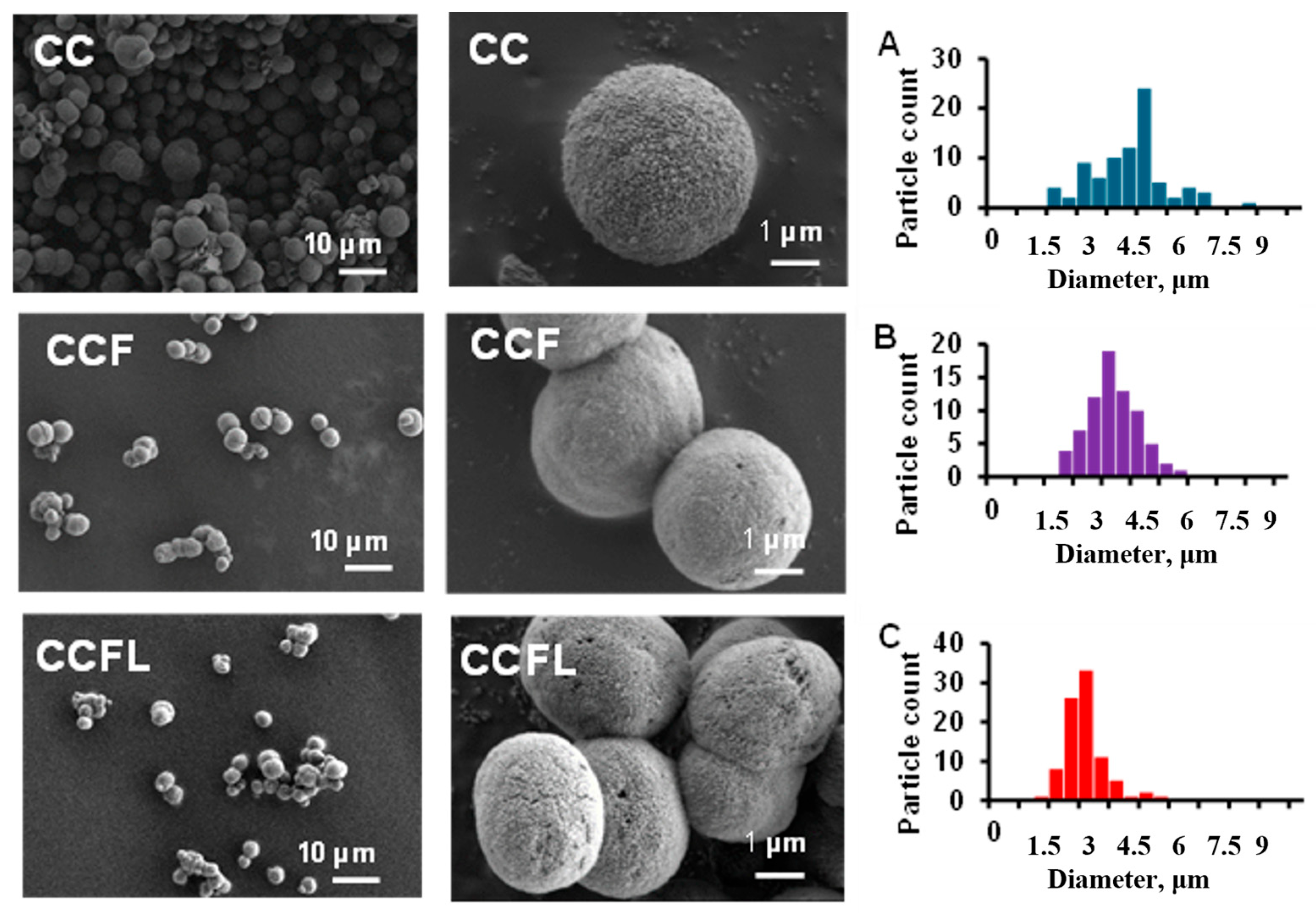
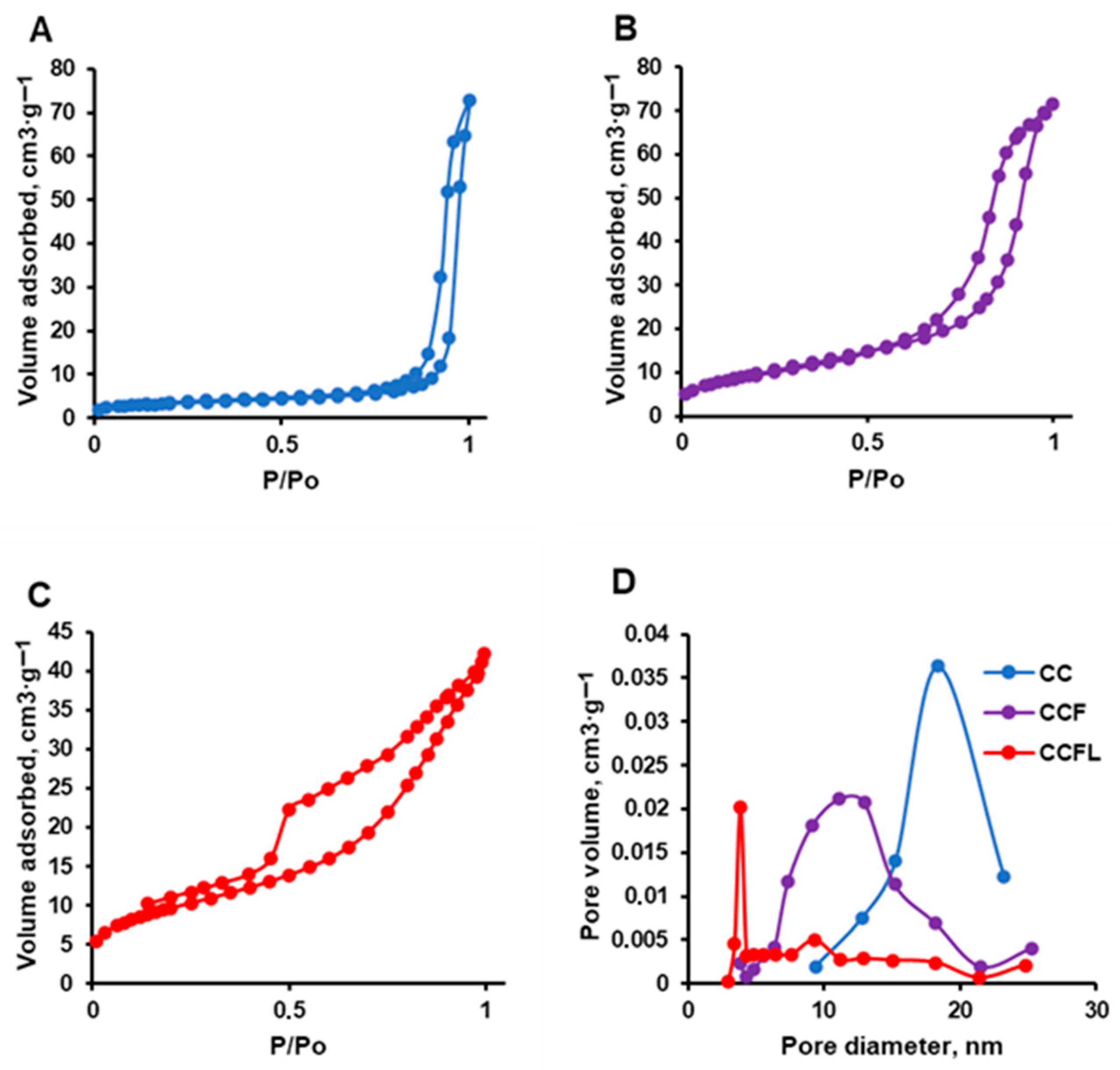
2.2. Physicochemical Properties of Microparticles
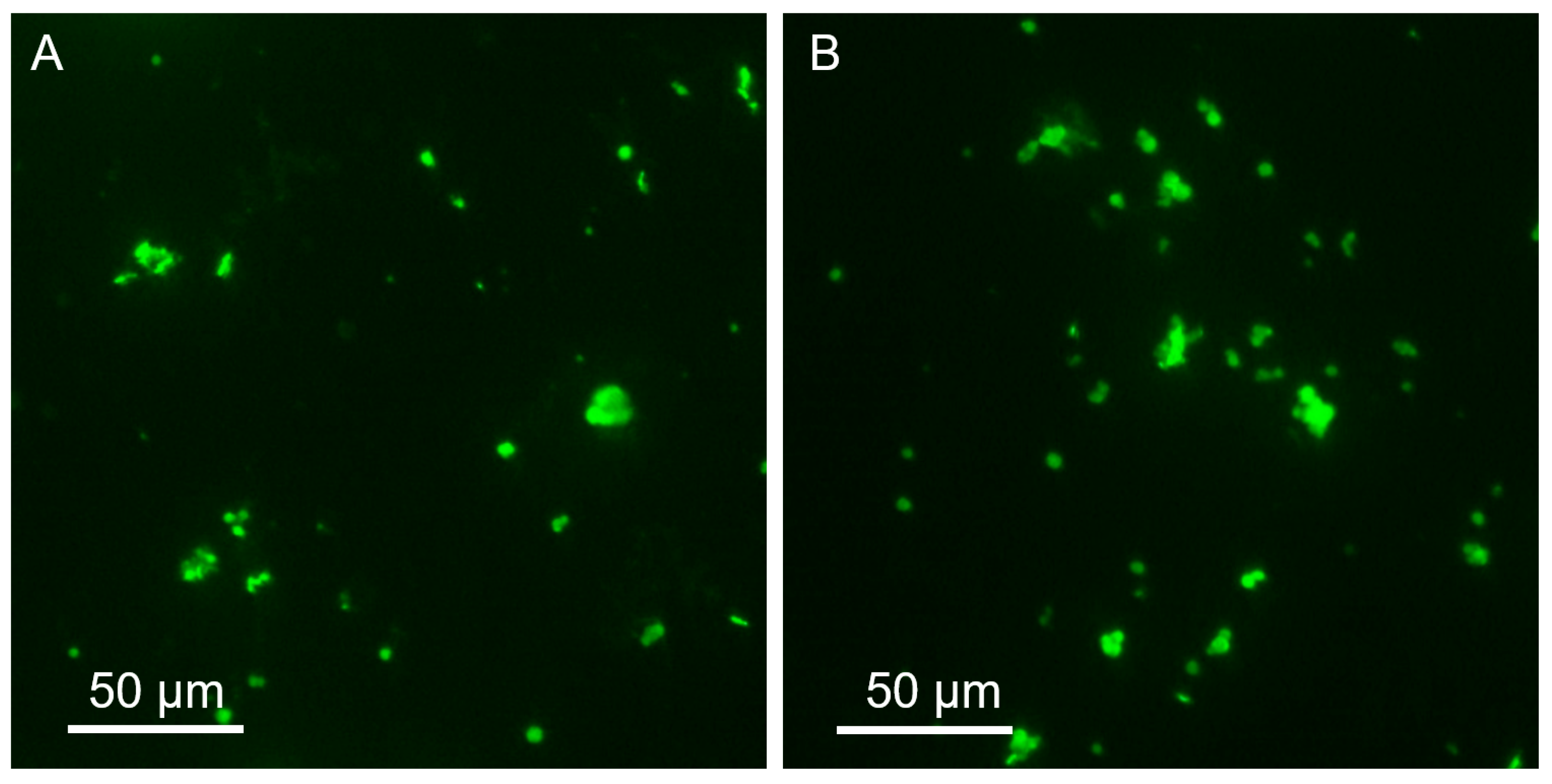
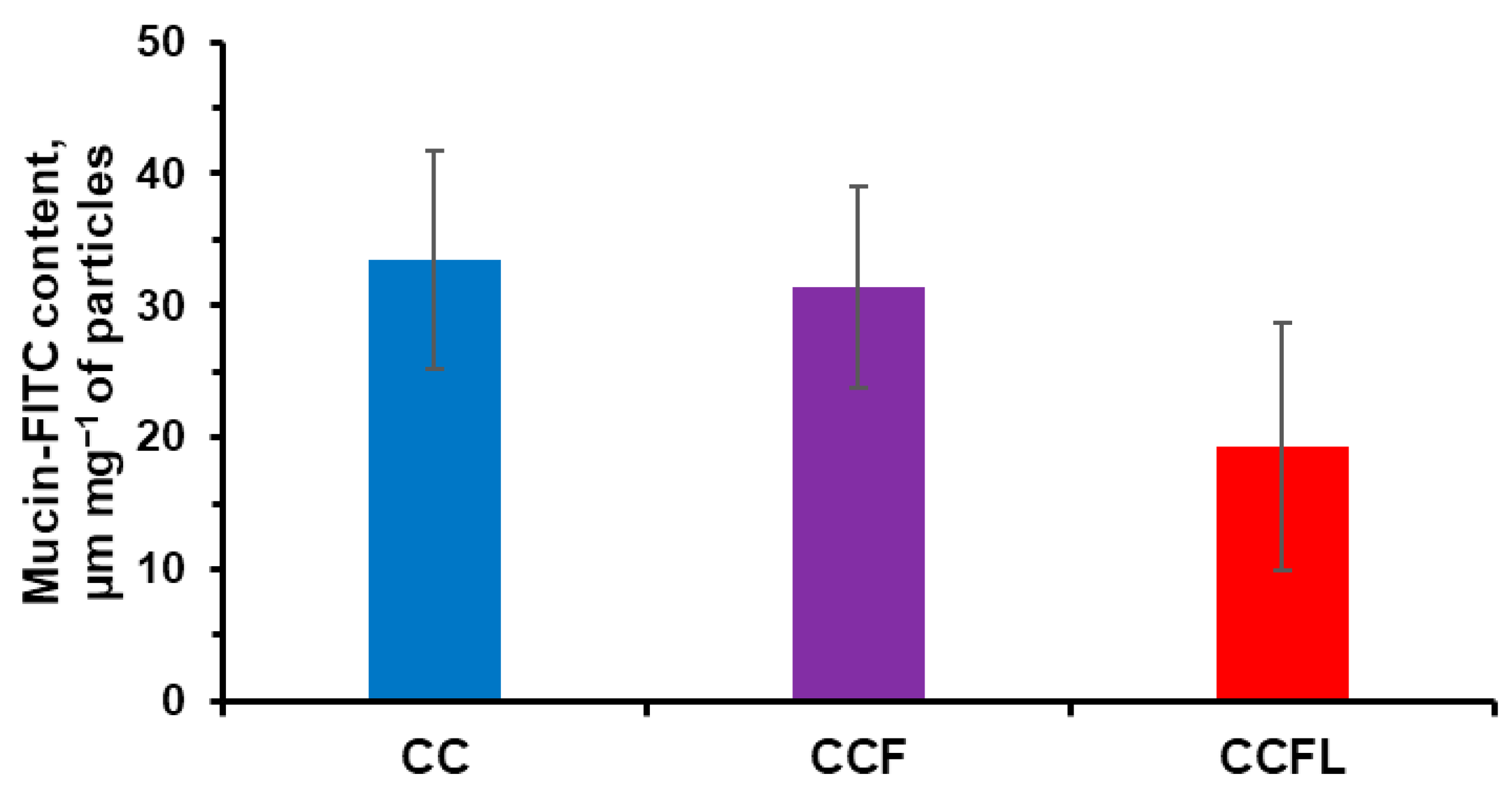
2.3. Mucosal Adhesive Properties of Microparticles
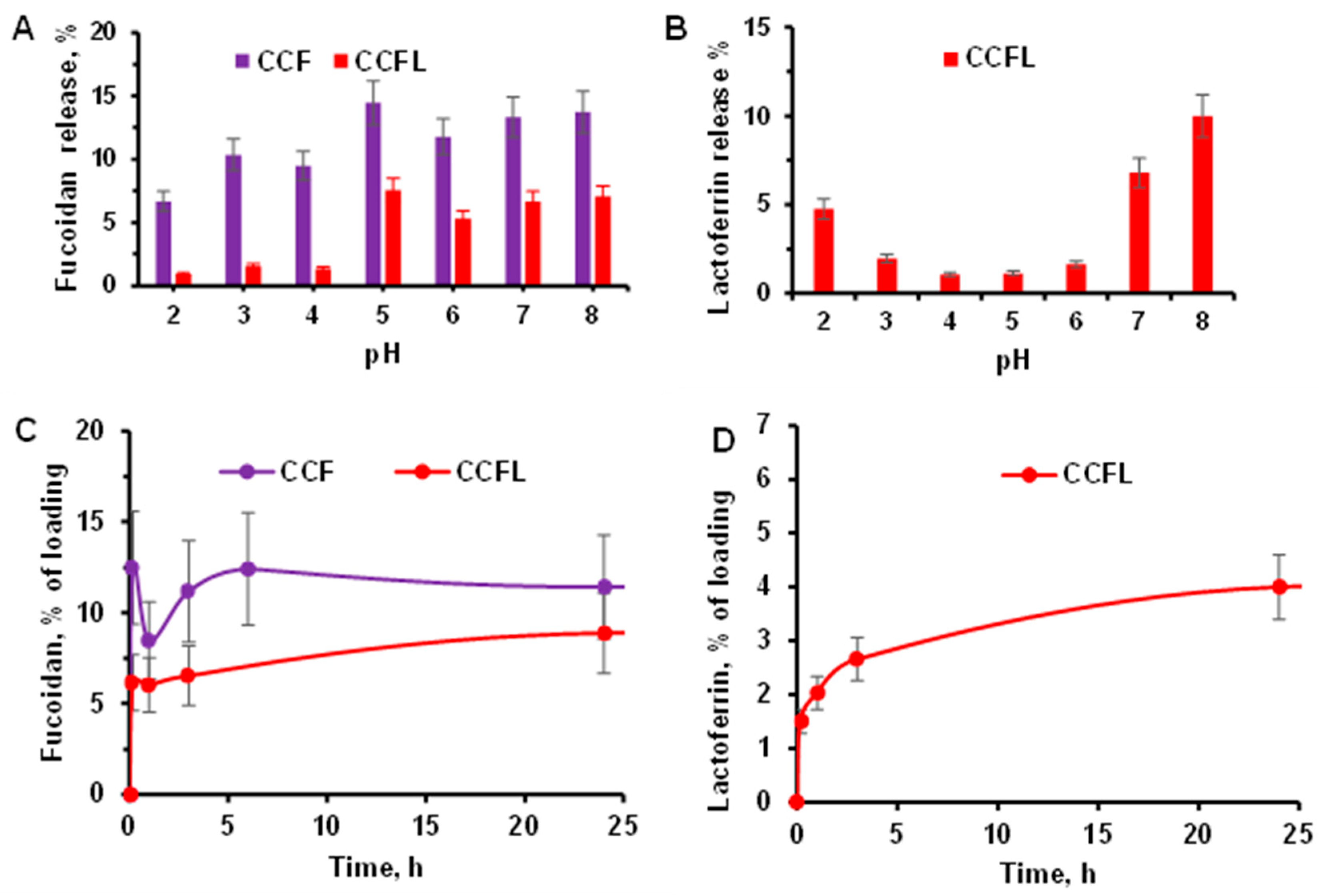
2.4. Stability of Hybrid Microparticles and Release of Components
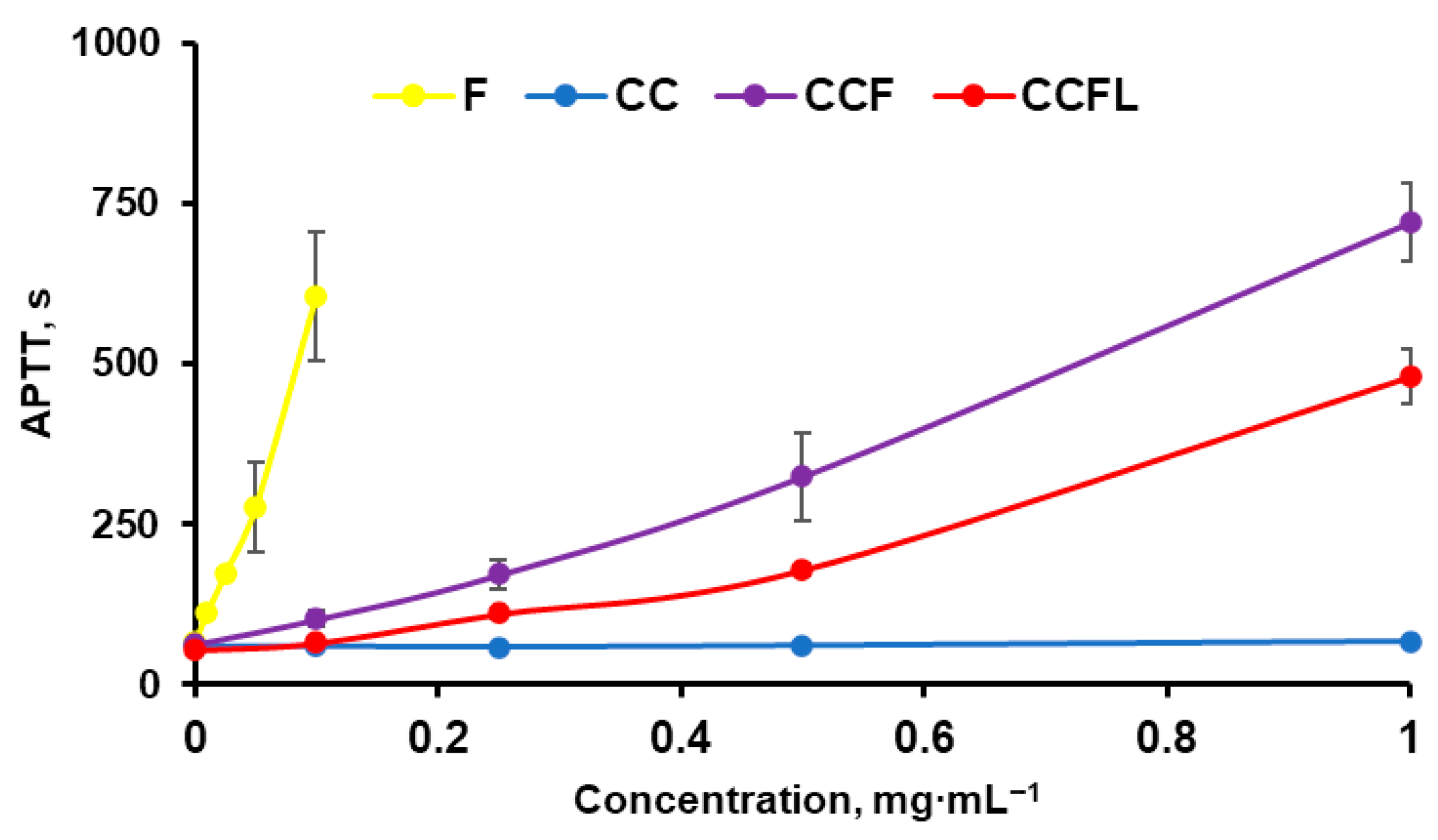
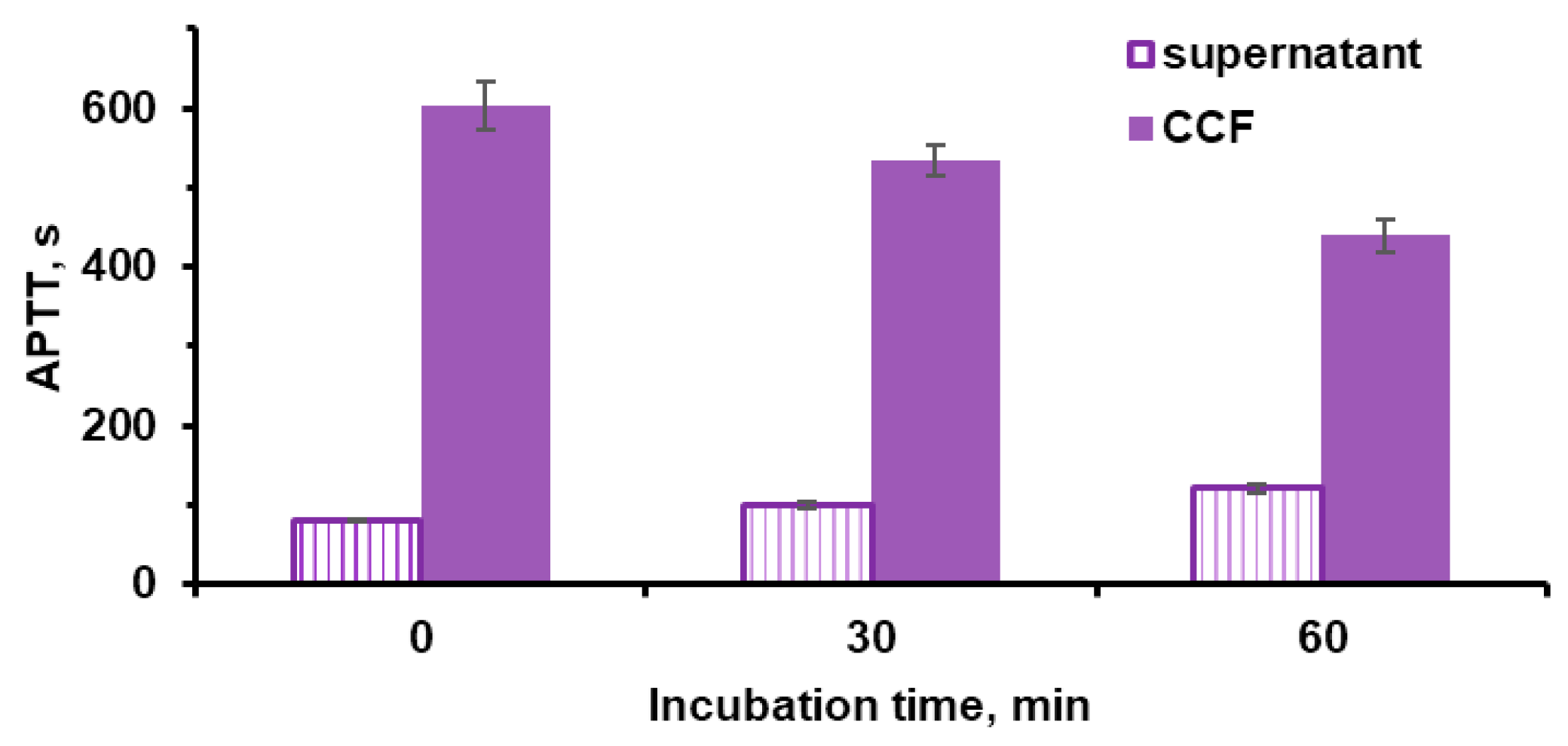
2.5. Anticoagulant Properties of Microparticles
2.6. Effects of Microparticles and Their Components on Bacteria Bacillus subtilis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Vaterite Microparticles
3.3. Characterization of the Microcrystals
3.3.1. Scanning Electron Microscopy
3.3.2. Low-Temperature Nitrogen Adsorption–Desorption
3.3.3. Electrophoretic Light Scattering
3.3.4. X-Ray Diffraction Analysis
3.3.5. Thermogravimetric Analysis
3.3.6. Coagulometry
3.3.7. FITC-Mucin Adsorption on Microparticles
3.3.8. Fluorescence Microscopy
3.4. Component Release Studies
3.5. Evaluation of Biopolymer and Particle Effects on Bacillus subtilis Culture
3.5.1. Bacterial Cultivation
3.5.2. Determination of Nucleic Acid Concentration
3.6. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APTT | Activated partial thromboplastin time |
| BET | Brunauer–Emmett–Teller |
| CC | Microparticles of vaterite (calcium carbonate) |
| CCF | Microparticles of vaterite with fucoidan |
| CCFL | Vaterite microparticles with co-precipitated FL complex |
| CCF-L | Microparticles CCF with adsorbed lactoferrin |
| DLS | Dynamic light scattering |
| Dparticle | Microparticle diameter |
| Dpores | Microparticle pore size |
| EDS | Energy-dispersive X-ray spectroscopy |
| F | Fucoidan |
| FL | Fucoidan and lactoferrin complex |
| FSC | Forward signal fluorescence channels |
| FITC | Fluorescein isothiocyanate |
| L | Lactoferrin |
| Mucin-FITC (MF) | Fluorescein isothiocyanate-labeled mucin |
| S | Microparticle area |
| SEM | Scanning electron microscopy |
| SSC | Side scatter signals fluorescence channels |
| TGA | Thermogravimetric analysis |
| V | Microparticle pore volume |
| XRD | X-ray diffraction analysis |
References
- Haggag, Y.A.; Abd Elrahman, A.A.; Ulber, R.; Zayed, A. Fucoidan in Pharmaceutical Formulations: A Comprehensive Review for Smart Drug Delivery Systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Gasilova, E.R.; Skorik, Y.A. Nano-Sized Fucoidan Interpolyelectrolyte Complexes: Recent Advances in Design and Prospects for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 2615. [Google Scholar] [CrossRef]
- Zahariev, N.; Katsarov, P.; Lukova, P.; Pilicheva, B. Novel Fucoidan Pharmaceutical Formulations and Their Potential Application in Oncology-A (Review). Polymers 2023, 15, 3242. [Google Scholar] [CrossRef]
- Zahariev, N.; Pilicheva, B. A Novel Method for the Preparation of Casein-Fucoidan Composite Nanostructures. Polymers 2024, 16, 1818. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.M.; Reis, C.P.; Pacheco, R. Marine-Derived Compounds Combined with Nanoparticles: A Focus on the Biomedical and Pharmaceutical Sector. Mar. Drugs 2025, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Lee, G.; Hyun, J.; Ryu, B. Systematic Characteristics of Fucoidan: Intriguing Features for New Pharmacological Interventions. Int. J. Mol. Sci. 2024, 25, 11771. [Google Scholar] [CrossRef]
- Chadwick, M.; Carvalho, L.G.; Vanegas, C.; Dimartino, S. A Comparative Review of Alternative Fucoidan Extraction Techniques from Seaweed. Mar. Drugs 2025, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides and Their Importance for Human Health. In Blue Biotechnology: Production and Use of Marine Molecules, 2nd ed.; Barre, L.A., Bates, S.S., Eds.; Wiley-VCH Publisher: Weinheim, Germany; Hoboken, NJ, USA, 2018; pp. 485–529. [Google Scholar]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qin, Y.; Jiang, B.; Chen, J.; Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf. B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Wei, Z.; Xu, Y.; Xue, C. Clove essential oil Pickering emulsions stabilized with lactoferrin/fucoidan complexes: Stability and rheological properties. Polymers 2023, 15, 1820. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, R.; Xu, X.; Chang, Y.; Xue, C. The characterization of fucoidan sodium caseinate electrostatic complexes with application for pH-triggered release: Microstructure and digestive behavior. Food Res. Int. 2025, 207, 116076. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, X.; Zheng, L.; Chang, Q.; Xi, C. Interactions and Phase Behaviors Between Whey Protein Isolate and Fucoidan: Effects of Protein/Polysaccharide Mixing Ratio and pH. 2024. Available online: https://ssrn.com/abstract=4710502 (accessed on 30 January 2024).
- Duan, W.; Chen, L.; Liu, F.; Li, X.; Wu, Y.; Cheng, L.; Liu, J.; Ai, C.; Huang, Q.; Zhou, Y. The properties and formation mechanism of ovalbumin-fucoidan complex. Int. J. Biol. Macromol. 2023, 241, 124644. [Google Scholar] [CrossRef]
- Burova, T.V.; Grinberg, N.V.; Dubovik, A.S.; Plashchina, I.G.; Usov, A.I.; Grinberg, V.Y. β-Lactoglobulin–fucoidan nanocomplexes: Energetics of formation, stability, and oligomeric structure of the bound protein. Food Hydrocoll. 2022, 129, 107666. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Duan, L.; Xie, X.; Zeng, X.; Huang, Y.; Li, Y.; Zhi, Z.; Pang, J.; Wu, C. Fucoidan-based delivery systems: From fabrication strategies to applications. Food Res. Int. 2025, 218, 116752. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Mahmood, S.; Bilal, M.; Manzoor, M.F.; Qamar, S.A.; Qi, X. Fucoidan-based nanomaterial and its multifunctional role for pharmaceutical and biomedical applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 354–380. [Google Scholar] [CrossRef]
- Obiedallah, M.M.; Melekhin, V.V.; Menzorova, Y.A.; Bulya, E.T.; Minin, A.S.; Mironov, M.A. Fucoidan coated liposomes loaded with novel antituberculosis agent: Preparation, evaluation, and cytotoxicity study. Pharm. Dev. Technol. 2024, 29, 311–321. [Google Scholar] [CrossRef]
- Elbi, S.; Nimal, T.R.; Rajan, V.K.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B Biointerfaces 2017, 160, 40–47. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, R.Y. Preparation and characterization of antioxidant nanoparticles composed of chitosan and fucoidan for antibiotics delivery. Mar. Drugs 2014, 12, 4379–4398. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nguyen, V.P.; Manivasagan, P.; Jung, M.J.; Kim, S.W.; Oh, J.; Kang, H.W. Doxorubicin-fucoidan-gold nanoparticles composite for dual-chemo-photothermal treatment on eye tumors. Oncotarget 2017, 8, 113719–113733. [Google Scholar] [CrossRef]
- Cunha, L.; Rosa da Costa, A.M.; Lourenço, J.P.; Buttini, F.; Grenha, A. Spray-dried fucoidan microparticles for pulmonary delivery of antitubelcular drugs. J. Microencapsul. 2018, 35, 392–405. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan–fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef]
- Sezer, A.D.; Akbuga, J. Fucosphere—New microsphere carriers for peptide and protein delivery: Preparation and in vitro characterization. J. Microencapsul. 2006, 23, 513–522. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, D.Y.; Shin, W.S. Fucoidan improves the structural integrity and the molecular stability of-lactoglobulin. Food Sci. Biotechnol. 2018, 27, 1247–1255. [Google Scholar] [CrossRef]
- Sezer, A.D.; Akbuga, J. Comparison on in vitro characterization of fucospheres and chitosan microspheres encapsulated plasmid DNA (pGM-CSF): Formulation design and release characteristics. AAPS PharmSciTech 2009, 10, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Ali, A.; Ahmed, M.; Zia, M.; Haq, I.; Kim, S.J. In vitro antileishmanial, antibacterial, antifungal and anticancer activity of fucoidan from Undaria pinnatifida. Int. J. Biosci. 2017, 11, 219–227. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoglu, F.; Ogurtan, Z.; Bas, A.L.; Akbuga, J. The use of fucosphere in the treatment of dermal burns in rabbits. Eur. J. Pharm. Biopharm. 2008, 69, 189–198. [Google Scholar] [CrossRef]
- Volodkin, D. CaCO3 templated micro-beads and -capsules for bioapplications. Adv. Colloid. Interface Sci. 2014, 207, 306–324. [Google Scholar] [CrossRef]
- Trushina, D.B.; Borodina, T.N.; Belyakov, S.; Antipina, M.N. Calcium carbonate vaterite particles for drug delivery: Advances and challenges. Mater Today Adv. 2022, 14, 100214. [Google Scholar] [CrossRef]
- Vikulina, A.; Voronin, D.; Fakhrullin, R.F.; Vinokurov, V.A.; Volodkin, D. Naturally derived nano- and micro- drug delivery vehicles: Halloysite, vaterite and nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef]
- Vikulina, A.; Webster, J.; Voronin, D.; Ivanov, E.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Mesoporous additive-free vaterite CaCO3 crystals of untypical sizes: From submicron to Giant. Mater. Des. 2021, 197, 109220. [Google Scholar] [CrossRef]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef]
- Paravastu, V.K.K.; Yarraguntla, S.R.; Suvvari, A. Role of nanocomposites in drug delivery. GSC Biol. Pharm. Sci. 2018, 8, 94–103. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Fakhrullin, R. Mesoporous inorganic nanoscale particles for drug adsorption and controlled release. Ther. Deliv. 2018, 9, 287–301. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Huang, X.; Wu, G. Biomimetic synthesis of calcium carbonate with different morphologies and polymorphs in the presence of bovine serum albumin and soluble starch. Mater. Sci. Eng. C Mater Biol. Appl. 2017, 79, 457–464. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Maltseva, L.N.; Filatova, L.Y.; Mosievich, D.V.; Mishin, P.I.; Bogomiakova, M.E.; Lebedeva, O.S.; Murina, M.A.; Klinov, D.V.; Obraztsova, E.A.; et al. Influence of natural polysaccharides on the morphology and properties of hybrid vaterite microcrystals. Heliyon 2024, 10, e33801. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Le-Deygen, I.M.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Hybrid CaCO3-mucin crystals: Effective approach for loading and controlled release of cationic drugs. Mat. Des. 2019, 182, 108020. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Sholina, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Vikulina, A.S.; Volodkin, D. Self-assembled mucin-containing microcarriers via hard templating on CaCO3 crystals. Micromachines 2018, 9, 307. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Wu, Z.; Wan, Y.; Wei, Q.; Hu, Q.; Guo, Y. Additive application for modulating physicochemical properties and antitumor effects of CaCO3 nanodelivery systems. J. Drug Del. Sci. Technol. 2024, 100, 106080. [Google Scholar] [CrossRef]
- Wang, P.; Tong, F.; Luo, J.; Li, Z.; Wei, J.; Liu, Y. Fucoidan-mediated anisotropic calcium carbonate nanorods of pH-responsive drug release for antitumor therapy. Front. Bioeng. Biotechnol. 2022, 10, 845821. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Liu, X.; Zheng, Y.; Han, K.; Liu, H.; Wu, C.; Li, J.; Fan, S.; Peng, W.; et al. The effect of lactoferrin in aging: Role and potential. Food. Funct. 2022, 13, 501–513. [Google Scholar] [CrossRef]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2022, 32, e2261. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Wei, Y.S.; Feng, K.; Li, S.F.; Hu, T.G.; Linhardt, R.J.; Zong, M.H.; Wu, H. Oral fate and stabilization technologies of lactoferrin: A systematic review. Crit. Rev. Food. Sci. Nutr. 2022, 62, 6341–6358. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, W.; Wei, L.; Ma, J. Lactoferrin in cancer: Focus on mechanisms and translational medicine. Biochim. Biophys. Acta Rev. Cancer. 2025, 1880, 189330. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Gajda-Morszewski, P.; Śpiewak-Wojtyła, K.; Oszajca, M.; Brindell, M. Strategies for Oral Delivery of Metal-Saturated Lactoferrin. Curr. Protein Pept. Sci. 2019, 20, 1046–1051. [Google Scholar] [CrossRef]
- Kilic, E.; Novoselova, M.V.; Lim, S.H.; Pyataev, N.A.; Pinyaev, S.I.; Kulikov, O.A.; Sindeeva, O.A.; Mayorova, O.A.; Murney, R.; Antipina, M.N.; et al. Formulation for oral delivery of lactoferrin based on bovine serum albumin and tannic acid multilayer microcapsules. Sci. Rep. 2017, 7, 44159. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Lim, S.H.; Lau, H.H.; Antipina, M.; Khin, Y.W.; Chia, C.Y.; Harris, P.; Weeks, M.; Berry, C.; Hurford, D.; et al. Surface-reacted calcium carbonate microparticles as templates for lactoferrin encapsulation. J. Coll. Int. Sci. 2021, 594, 362–371. [Google Scholar] [CrossRef]
- Cao, L.; Li, J.; Parakhonskiy, B.; Skirtach, A.G. Intestinal-specific oral delivery of lactoferrin with alginate-based composite and hybrid CaCO3-hydrogel beads. Food Chem. 2024, 451, 39205. [Google Scholar] [CrossRef]
- Mosievich, D.V.; Balabushevich, N.G.; Mishin, P.I.; Le-Deygen, I.M.; Filatova, L.Y.; Panasenko, O.M.; Murina, M.A.; Vakhrusheva, T.V.; Barinov, N.A.; Pobeguts, O.V.; et al. Functional activity features of lactoferrin-fucoidan complexes in in vitro model systems. Biomeditsinskaya Khimiya, 2025; 71, in press. [Google Scholar]
- Balabushevich, N.G.; Kovalenko, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Mucin adsorption on vaterite CaCO3 microcrystals for the prediction of mucoadhesive properties. J. Colloid Interface Sci. 2019, 545, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, L.; Sun, Z.; Ji, Y.; Wang, D.; Mei, L.; Shen, P.; Li, Z.; Tang, S.; Zhang, H.; et al. Fucoidan as a marine-origin prebiotic modulates the growth and antibacterial ability of Lactobacillus rhamnosus. Int. J. Biol. Macromol. 2021, 180, 599–607. [Google Scholar] [CrossRef]
- Ongena, R.; Dierick, M.; Vanrompay, D.; Cox, E. Lactoferrin impairs pathogen virulence through its proteolytic activity. Front. Vet. Sci. 2024, 11, 1428156. [Google Scholar] [CrossRef] [PubMed]
- Grammatikova, N.E.; Rezvan, S.P.; Nemtsova, E.R.; Bezborodova, O.A.; Tutykhina, I.L.; Naroditsky, B.S.; Yakubovsky, R.I. In vitro study of antimicrobial activity of lactoferrins from various sources. Antibiot. Khimioter. 2010, 55, 4–9. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Pontes, J.F.; Guerreiro, F.; Afonso, I.T.; Lollo, G.; Torres, M.D.; Domínguez, H.; Costa, A.M.R.D.; Grenha, A. Fucoidan from Fucus vesiculosus: Evaluation of the Impact of the Sulphate Content on Nanoparticle Production and Cell Toxicity. Mar. Drugs 2023, 21, 115. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Borzenkova, N.V.; Izumrudov, V.A.; Larionova, N.I.; Bezborodova, O.A.; Nemtsova, E.R.; Yakubovskaya, R.I. Polyelectrolyte complexes of lactoferrin and pH sensitive microparticles on their basis. Appl. Biochem. Microbiol. 2014, 50, 232–240. [Google Scholar] [CrossRef]
- Chikhirzhina, E.V.; Starkova, T.J.; Polyanichko, A.M. Interaction between Chromosomal Protein HMGB1 and DNA Studied by DNA-Melting Analysis. J. Spectrosc. 2014, 8, 1–5. [Google Scholar] [CrossRef]
- Repici, A.; Hasan, A.; Capra, A.P.; Scuderi, S.A.; Paterniti, I.; Campolo, M.; Ardizzone, A.; Esposito, E. Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers? Mar. Drugs 2024, 22, 336. [Google Scholar] [CrossRef]


| Sample | Dparticle, µm | Content, % | S, m2g−1 | V, cm3g−1 | Dpores, nm | ζ-Potential, mV | |||
|---|---|---|---|---|---|---|---|---|---|
| Fucoidan | Sulfur | Lactoferrin | |||||||
| Determination Method | |||||||||
| TGA | Dubois | EDS | Lowry | ||||||
| CC | 4.8 ± 1.2 | 0 | 12 ± 1 | 0.04 | 19.6 | −7 ± 2 | |||
| CCF | 4.4 ± 0.9 | 4.7 ± 0.2 | 10.3 ± 4.5 | 1 | 36 ± 4 | 0.08 | 10.0 | −11 ± 1 | |
| CCFL | 3.2 ± 0.5 | 10.1 ± 1.3 | 0.7 | 2.7 ± 0.3 | 35 ± 4 | 0.05 | 5.4 | −12 ± 1 | |
| Fucoidan | 100 | −56 ± 1 | |||||||
| Lactoferrin | 100 | 10 ± 1 | |||||||
| Complex FL | −48 ± 1 | ||||||||
| Particles | Volume, mL | ||||
|---|---|---|---|---|---|
| 0.1 M Tris-HCl | 10 mg mL−1 Fucoidan | 50 mg mL−1 Lactoferrin | 1 M CaCl2 | 1 M Na2CO3 | |
| CC | 9.0 | - | - | 3.0 | 3.0 |
| CCF | 1.5 | 7.5 | - | 3.0 | 3.0 |
| CCFL | - | 2.5 | 1.5 | 3.0 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosievich, D.V.; Balabushevich, N.G.; Mishin, P.I.; Filatova, L.Y.; Murina, M.A.; Pobeguts, O.V.; Galyamina, M.A.; Obraztsova, E.A.; Grigorieva, D.V.; Gorudko, I.V.; et al. Vaterite/Fucoidan Hybrid Microparticles: Fabrication, Loading of Lactoferrin, Structural Characteristics and Functional Properties. Mar. Drugs 2025, 23, 428. https://doi.org/10.3390/md23110428
Mosievich DV, Balabushevich NG, Mishin PI, Filatova LY, Murina MA, Pobeguts OV, Galyamina MA, Obraztsova EA, Grigorieva DV, Gorudko IV, et al. Vaterite/Fucoidan Hybrid Microparticles: Fabrication, Loading of Lactoferrin, Structural Characteristics and Functional Properties. Marine Drugs. 2025; 23(11):428. https://doi.org/10.3390/md23110428
Chicago/Turabian StyleMosievich, Daniil V., Nadezhda G. Balabushevich, Pavel I. Mishin, Lyubov Y. Filatova, Marina A. Murina, Olga V. Pobeguts, Maria A. Galyamina, Ekaterina A. Obraztsova, Daria V. Grigorieva, Irina V. Gorudko, and et al. 2025. "Vaterite/Fucoidan Hybrid Microparticles: Fabrication, Loading of Lactoferrin, Structural Characteristics and Functional Properties" Marine Drugs 23, no. 11: 428. https://doi.org/10.3390/md23110428
APA StyleMosievich, D. V., Balabushevich, N. G., Mishin, P. I., Filatova, L. Y., Murina, M. A., Pobeguts, O. V., Galyamina, M. A., Obraztsova, E. A., Grigorieva, D. V., Gorudko, I. V., Sokolov, A. V., Shmeleva, E. V., Panasenko, O. M., & Mikhalchik, E. V. (2025). Vaterite/Fucoidan Hybrid Microparticles: Fabrication, Loading of Lactoferrin, Structural Characteristics and Functional Properties. Marine Drugs, 23(11), 428. https://doi.org/10.3390/md23110428








