Marine-Derived Steroids for Cancer Treatment: Search for Potential Selective Glucocorticoid Receptor Agonists/Modulators (SEGRAMs)
Abstract
1. Introduction
2. Marine-Derived Steroids with Anti-Cancer Activity
| No. | Name | Structure | Source | Anti-Cancer Activity | Ref. |
|---|---|---|---|---|---|
| 1 | 5α-cholesta-24-en-3β,20β-diol-23-one | 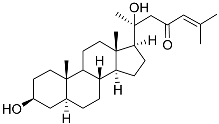 | Crown-of-thorns starfish Acanthaster planci | Cytotoxic activity against luminal A breast cancer cells MCF-7 in MTT assay IC50 49 ± 1.6 μg/mL | [86] |
| 2 | 5α-cholesta-9(11)-en-3β,20β-diol | 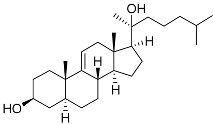 | Crown-of-thorns starfish Acanthaster planci | Cytotoxic activity against luminal A breast cancer cells MCF-7 in MTT assay IC50 57.5 ± 1.5 μg/mL | [86] |
| 3 | Dendrodoristerol | 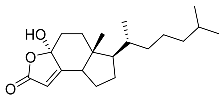 | Sea slug Dendrodoris fumata | Cytotoxic activity against hepatocellular carcinoma cells HepG2, prostate cancer cells LNCaP, breast cancer cells MCF-7, lung adenocarcinoma cells SK-LU-1, epidermal carcinoma cells KB, leukemia cells HL-60 in SRB assay IC50 21.63 ± 2.22, 22.22 ± 1.81, 24.53 ± 2.47, 41.19 ± 3.25, 25.34 ± 3.81, and 21.59 ± 1.38 μM | [87] |
| 4 | (25S)-5α-cholestane-3β,5,6β,15α,16β,26-hexaol | 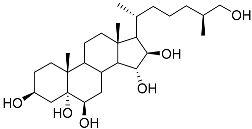 | Mud star Ctenodiscus crispatus | Shows cytotoxic activity against hepatocellular carcinoma cells HepG2 in MTT assay | [88] |
| 5 | (3E)-cholest-4-en-3,6-dione-3-oxime | 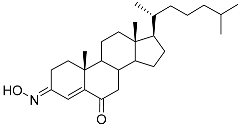 | Sea sponge Cinachyrella australiensis | Cytotoxic activity against hepatocellular carcinoma cells HepG2 in MTT assay IC50 2.91 mg/mL | [89] |
| 6 | Gracilosulfate A | 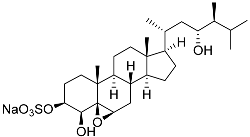 | Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 64.4 ± 14.9 μM | [90] |
| 7 | Gracilosulfate B | 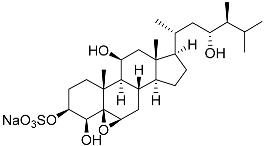 | Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 8 | Gracilosulfate D | 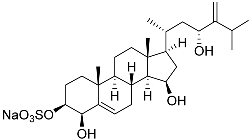 | Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 9 | Gracilosulfate F | 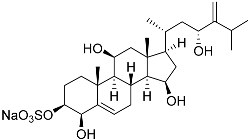 | Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 10 | Gracilosulfate G | 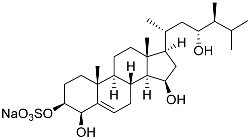 | Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 11 | β-sitosterol-3-O-(3Z)-pentacosenoate |  | Sea sponge Echinoclathria gibbosa | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 64 μM | [91] |
| 12 | 5α-pregna-3β-acetoxy-12β,16β-diol-20-one |  | Sea sponge Echinoclathria gibbosa | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 > 100 μM | [91] |
| 13 | 3α,12α,16α-trihydroxy-24ξ-ethylcholest-25-ene |  | Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 13 ± 1 μM | [92] |
| 14 | 3α,12α,16α-trihydroxy-24R-methylcholest-22E-ene | 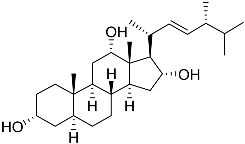 | Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 27 ± 1 μM | [92] |
| 15 | 3α,12α,16α-trihydroxy-24-methylcholest-24(28)-ene | 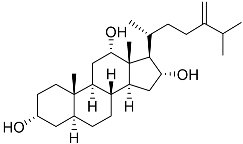 | Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 27 ± 1 μM | [92] |
| 16 | 3α,12α,16α-trihydroxycholestane | 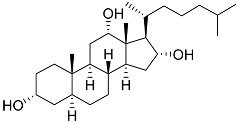 | Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 6.7 ± 0.2 μM | [92] |
| 17 | Archasteroside A |  | [93] | ||
| 18 | Archasteroside B |  | [93] | ||
| 19 | Halityloside A | 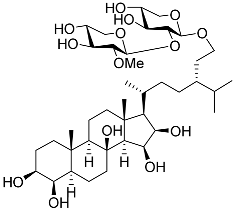 | Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 48.59 ± 2.30 μM | [94] |
| 20 | Halityloside B |  | Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 39.68 ± 2.65 μM | [94] |
| 21 | Culcitoside C5 | 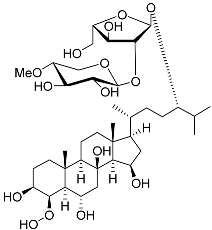 | Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 57.08 ± 1.81 μM | [94] |
| 22 | Halityloside D | 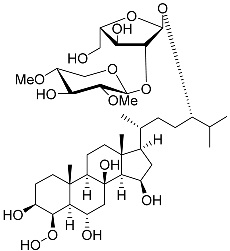 | Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 31.80 ± 1.59 μM | [94] |
| 23 | Spiculiferosides A |  | Starfish Henricia leviuscula spiculifera | Inhibition of colony formation colorectal carcinoma cells HCT 116 at concentration 40 μM was 65% | [95] |
| 24 | Spiculiferosides B | 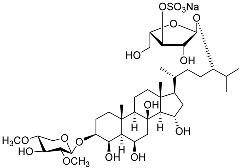 | Starfish Henricia leviuscula spiculifera | Inhibition of colony formation colorectal carcinoma cells HCT 116 at concentration 40 μM was 81% | [95] |
| 25 | Spiculiferosides C | 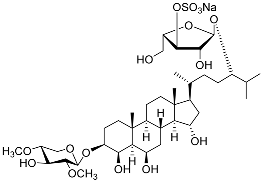 | Starfish Henricia leviuscula spiculifera | Cytotoxic activity against colorectal carcinoma cells HCT 116 in MTS assay IC50 87.6 μM Inhibition of colony formation colorectal carcinoma cells HCT 116 at concentration 40 μM was 87% | [95] |
| 26 | (20R,22E)-24-norcholesta-5,22-diene-3β,21-diol 3,21-disulfate disodium salt | 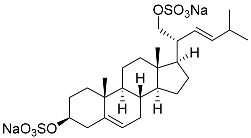 | Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 76% | [111] |
| 27 | (20R,22E)-24-nor-5α-cholest-22-ene-3β,21-diol 3,21-disulfate disodium salt | 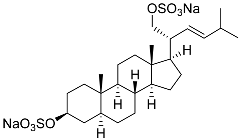 | Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 86% | [111] |
| 28 | (20R)-7-oxo-24-methylcholesta-5,24(28)-diene-3β ,21-diyl disulfate disodium salt | 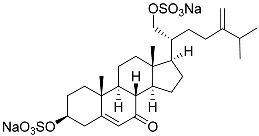 | Starfish Pteraster marsippus | Cytotoxic activity of the mixture of 28 and 29 against human breast carcinoma cells ZR-75-1 in MTS assay IC50 90.4 μM | [96] |
| 29 | (20R)-7-oxo-24-methyl-5α-cholest-24(28)-ene-3 β,21-diyl disulfate disodium salt | 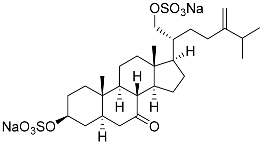 | Starfish Pteraster marsippus | Cytotoxic activity of the mixture of 28 and 29 against human breast carcinoma cells ZR-75-1 in MTS assay IC50 90.4 μM | [96] |
| 30 | (20S,22R)-24-metylcholesta-5,24-diene-3β,22-diol 3,22-disulfate disodium salt | 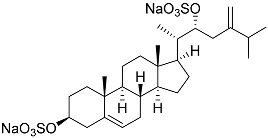 | Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 71% | [111] |
| 31 | (20S,22R)-24-metyl-5α-cholest-24-ene-2β,3α,22-triol 3,22-disulfate disodium salt |  | Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 79% | [111] |
| 32 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 5′Z,11′Z-octadecadienoate | 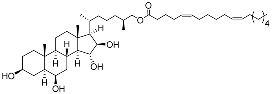 | Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 36% | [97] |
| 33 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 11’Z-octadecenoate | 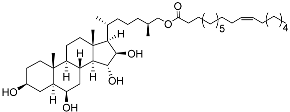 | Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 73% | [97] |
| 34 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 5’Z,11’Z-eicosadienoate | 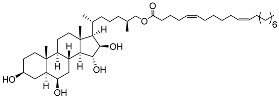 | Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 30% | [97] |
| 35 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 7’Z-eicosenoate | 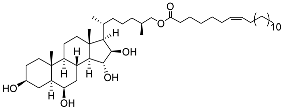 | Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 24% | [97] |
| 36 | (23R)-methoxycholest-5,24-dien-3β-ol | 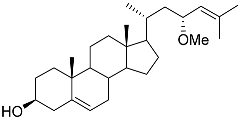 | Colonial bryozoan Cryptosula pallasiana | Cytotoxic activity against hepatocellular carcinoma cells HepG2, gastric carcinoma cells SGC-7901, and leukemia cells HL-60 in MTT assay IC50 12.34 ± 0.12, 18.37 ± 0.17, and 17.64 ± 0.32 μM | [98] |
| 37 | Cerevisterol | 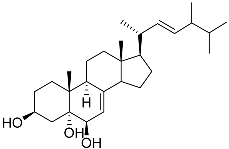 | Marine fungus Penicillium levitum | Cytotoxic activity against hepatocellular carcinoma cells HepG2, lung carcinoma cells A549, and breast cancer cells MCF-7 in MTT assay was not detected | [99] |
| 38 | Ergosterol peroxide | 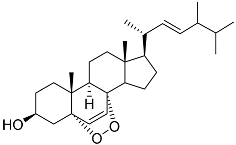 | Marine fungus Penicillium levitum | Cytotoxic activity against hepatocellular carcinoma cells HepG2, lung carcinoma cells A549, and breast cancer cells MCF-7 in MTT assay IC50 16.22, 22.48, and 27.11 μM | [99] |
| 39 | (3β,5α,22E)-ergosta-6,8(14),22-triene-3,5-diol | 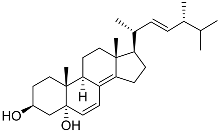 | Marine fungus Penicillium levitum | Cytotoxic activity against hepatocellular carcinoma cells HepG2, lung carcinoma cells A549, and breast cancer cells MCF-7 in MTT assay IC50 2.89, 18.51, and 16.47 μM | [99] |
| 40 | (24E)-stigmasta-24(28)-en-3,6-dione |  | Green algae Tydemania expeditionis | Cytotoxic activity against prostate cancer cells DU-145, prostate cancer cells PC-3, and prostate cancer cells LNCaP in MTT assay IC50 31.27 ± 1.50, 40.59 ± 3.10, and 19.80 ± 3.84 μM | [100] |
| 41 | Fucosterol | 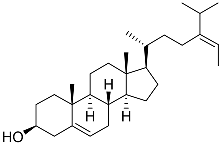 | Green algae Tydemania expeditionis | Cytotoxic activity against prostate cancer cells DU-145, prostate cancer cells PC-3, and prostate cancer cells LNCaP in MTT assay IC50 12.38 ± 2.47, 2.14 ± 0.33, and 1.38 ± 0.07 μM | [100] |
| 42 | Saringosterol | 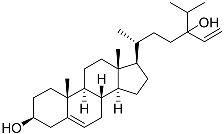 | Green algae Tydemania expeditionis | Cytotoxic activity against prostate cancer cells DU-145, prostate cancer cells PC-3, and prostate cancer cells LNCaP in MTT assay IC50 > 50, > 50, and 41.60 ± 4.26 μM | [100] |
| 43 | Cholest-8-ene-3β,5α,6β,7α-tetraol | 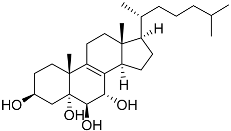 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 40.43 ± 1.45 μM | [101] |
| 44 | Cholest-8(14)-ene-3β,5α,6β,7α-tetraol | 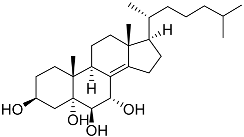 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 5.49 ± 0.22 μM | [101] |
| 45 | Cholest-7-ene-3β,5α,6β-triol | 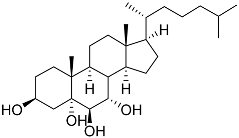 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 74.06 ± 3.46 μM | [101] |
| 46 | Cholest-7-ene-3β,5α,6α,9α-tetraol | 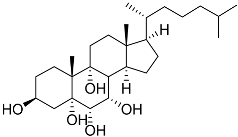 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 27.41 ± 0.50 μM | [101] |
| 47 | Cholest-7-ene-6-one-3β,5α,9α-triol | 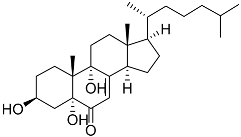 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 24.40 ± 0.46 μM | [101] |
| 48 | Cholest-5-ene-3β,7α-diol | 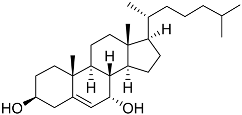 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 29.22 ± 0.17 μM | [101] |
| 49 | Cholest-5-ene-3β,7β-diol |  | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 27.94 ± 0.63 μM | [101] |
| 50 | Cholest-5-ene-7β-methoxy-3β-ol | 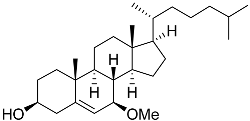 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 9.22 ± 0.67 μM | [101] |
| 51 | Campesterol | 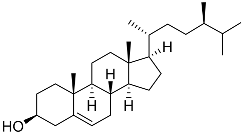 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 22.26 ± 0.59 μM | [101] |
| 52 | Cholest-5-ene-3β-sulfate sodium solt | 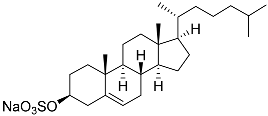 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 68.87 ± 6.08 μM | [101] |
| 53 | Cholest-6-ene-5α,8α-epidioxy-3β-ol | 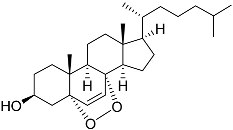 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 6.99 ± 0.28 μM | [101] |
| 54 | Cholest-5-ene-3β-ol | 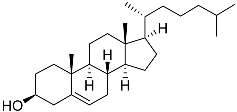 | Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay was not detected | [101] |
| 55 | Klyflaccisteroid A |  | Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29, lung cancer cells A549, and murine leukemia cells P388 in Alamar Blue assay ED50 > 20, 7.7, and >20 μg mL−1 | [102] |
| 56 | Klyflaccisteroid F | 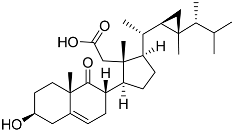 | Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29, lung cancer cells A549, and murine leukemia cells P388 in Alamar Blue assay ED50 > 20, 14.5, and 17.9 μg mL−1 | [102] |
| 57 | Klyflaccisteroid C | 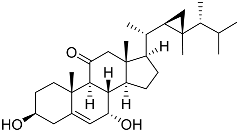 | Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29, lung cancer cells A549, and murine leukemia cells P388 in Alamar Blue assay ED50 8.2, 6.1, and 10.8 μg mL−1 | [102] |
| 58 | Klyflaccisteroid E | 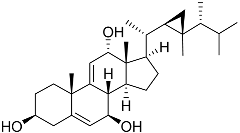 | Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29 and murine leukemia cells P388 in Alamar Blue assay ED50 6.9 and 3.7 μg mL−1 | [102] |
| 59 | Ergosta-24(28)-ene-3β,5α,6β-triol-6-acetate | 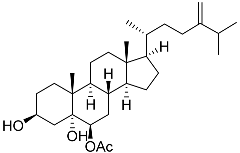 | Soft coral Sinularia conferta | Cytotoxic activity against lung cancer cells A549, cervical adenocarcinoma cells HeLa, and pancreatic epithelioid carcinoma cells PANC-1 in MTT assay IC50 3.64 ± 0.18, 19.34 ± 0.42, and 1.78 ± 0.69 μM | [112] |
| 60 | Dendronestadione | 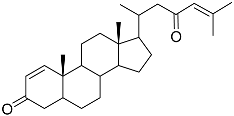 | Soft coral Dendronephthya | Cytotoxic activity against hepatocellular carcinoma cells HepG2, colon cancer cells HT-29, and prostate cancer cells PC-3 in MTT assay IC50 19.1 ± 1.81, 32.4 ± 2.84, and 7.8 ± 0.80 μM | [113] |
| 61 | (22E)-4α,24-dimethyl-5α-cholesta-22,24(28)-dien-3β,8β-diol | 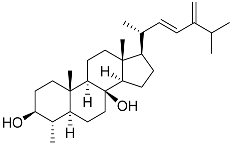 | Soft coral Litophyton mollis | Cytotoxic activity against hepatocellular carcinoma cells HepG2, breast cancer cells MCF-7, and lung carcinoma cells NCI-H1299 in SRB assay IC50 > 50 μM in all cases | [104] |
| 62 | (22E,24R)-7β-acetoxy-24-methylcholesta-5,22-dien-3β,19-diol | 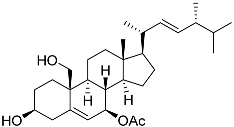 | Soft coral Litophyton mollis | Cytotoxic activity against hepatocellular carcinoma cells HepG2, breast cancer cells MCF-7, and lung carcinoma cells NCI-H1299 in SRB assay IC50 32.5, 8.4, and 15.1 μM | [104] |
3. Potential Glucocorticoid Receptor Modulators from Natural Marine Products
| No. | Name | Structure | Source | Anti-Inflammatory Effects | Reference |
|---|---|---|---|---|---|
| 56 | Klyflaccisteroid F | 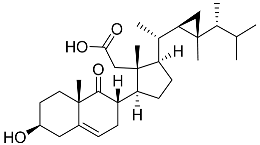 | Soft coral Klyxum flaccidum | Activity in inhibiting the superoxide anion generation 88.26 ± 35.86% at 10 μM and activity in inhibiting elastase release 104.22 ± 6.55% at 10 μM in N-formyl-methionyl-leucyl-phenylalanine/ cytochalasin B (fMLP/CB)-induced neutrophils | [102] |
| 57 | Klyflaccisteroid C | 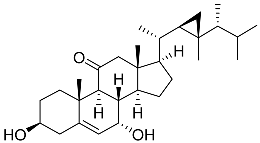 | Soft coral Klyxum flaccidum | Activity in inhibiting the superoxide anion generation 76.24 ± 5.64% at 10 μM and activity in inhibiting elastase release 88.38 ± 1.19% at 10 μM in N-formyl-methionyl-leucyl-phenylalanine/ cytochalasin B (fMLP/CB)-induced neutrophils | [102] |
| 63 | (22E,24S)-9a,15a-dihydroxyergosta-4,6,8(14),22- tetraen-3-one 15-palmitate | 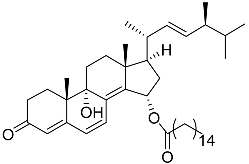 | Marine fungus Penicillium oxalicum HL-44 | Inhibition of expression of pro-inflammatory cytokines TNF-α and INF-β1 on DMXAA-stimulated Raw264.7 cells by 68% and 94% at 20 μM | [137] |
| 64 | (22E, 24R)-ergosta-5,7,22-trien-3β-ol | 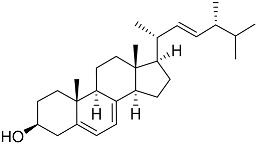 | Mangrove fungus Amorosia sp. | Inhibition of expression of pro-inflammatory cytokines TNF-α, IL-6, and MCP-1 on LPS-activated RAW264.7 M1-type cells by 55%, 50%, and 50% at 10 μM | [138] |
| 65 | Ergosterol | 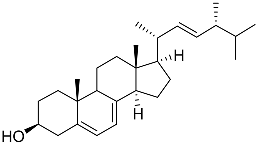 | Marine fungus Samsoniella hepiali W7 | Inhibition of NO production in LPS-activated BV-2-microglia cells by 32.9 ± 1.6% at 1 μM | [139] |
| 66 | Arctiol | 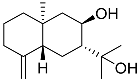 | Marine fungus Eutypella sp. F0219 | Inhibition of NO production in LPS-treated BV-2-microglia cells by 71% at 20 μM | [140] |
| 67 | Persteroid | 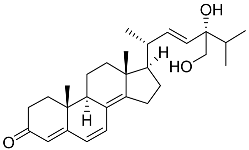 | Marine fungus Penicillium sp. ZYX-Z-143 | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 25.81 ± 0.92 μM | [141] |
| 37 | Cerevisterol | 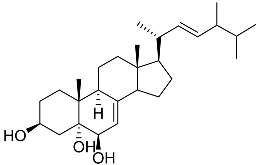 | Marine fungus Penicillium levitum | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 25.45 μg/mL | [99] |
| 38 | Ergosterol peroxide | 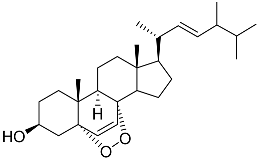 | Marine fungus Penicillium levitum | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 2.85 μg/mL | [99] |
| 39 | (3β,5α,22E)-ergosta-6,8(14),22-triene-3,5-diol | 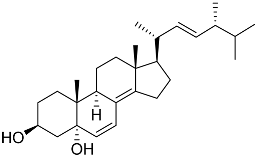 | Marine fungus Penicillium levitum | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 2.79 μg/mL | [99] |
| 68 | Splenocin A | 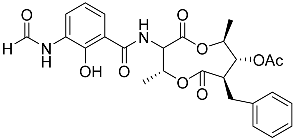 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 on TH2 cells (helper T lymphocytes) IC50 3.1 ± 1.2 nM | [142] |
| 69 | Splenocin B | 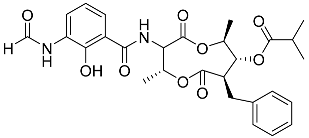 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 1.8 ± 0.2 and 1.6 ± 0.02 nM | [142] |
| 70 | Splenocin C | 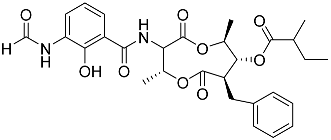 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 6.7 ± 0.2 and 7.3 ± 4.2 nM | [142] |
| 71 | Splenocin D | 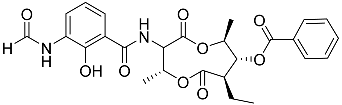 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 47.9 ± 2.9 and 43.7 ± 3.5 nM | [142] |
| 72 | Splenocin E | 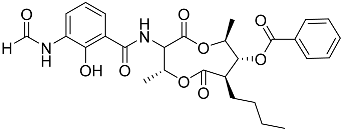 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 16.6 ± 1.8 and 15.9 ± 1.1 nM | [142] |
| 73 | Splenocin F | 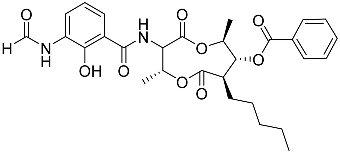 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 9.4 ± 2.8 and 6.8 ± 0.3 nM | [142] |
| 74 | Splenocin G | 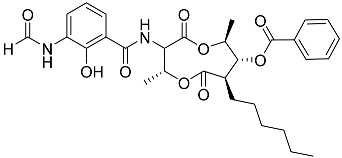 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 5 ± 0.4 and 5.2 ± 0.1 nM | [142] |
| 75 | Splenocin H | 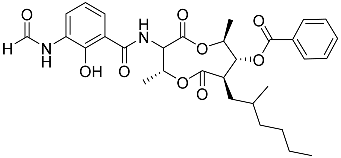 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 4.3 ± 0.5 and 5.1 ± 0.1 nM | [142] |
| 76 | Splenocin I | 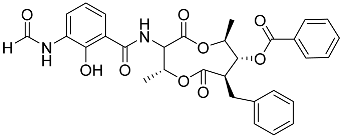 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 15.8 ± 1.0 and 15.2 ± 1.3 nM | [142] |
| 77 | Splenocin J | 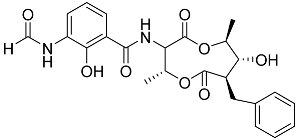 | Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 1022.7 ± 52.3 and 826.3 ± 187.6 nM | [142] |
| 41 | Fucosterol | 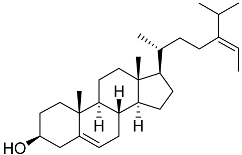 | Green algae Tydemania expeditionis | Hydrophobic interaction with GR via Leu563, Phe623, Leu608, and Met604 (molecular docking analysis) | [143] |
| No. | Compound | Chemical Structure | ΔG, kcal/mol | FF Score, kcal/mol | H-Binding | Hydrophobic Interactions |
|---|---|---|---|---|---|---|
| 78 | 3β,15β-Dihydroxy-(22E, 24R)-ergosta-5,8(14),22-trien-7-one | 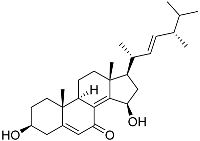 | −7.23 | −1226.71 | - | Val538, ILe539, Lys576, Ala573, Leu544, Trp577 |
| 79 | 24-Methylcholesta-5,24(28)-diene-3β,4α-diol | 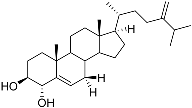 | −7.40 | −1204.67 | H29 Arg611 2.607 | Val543, Trp610, Tyr660 |
| 80 | 24-nor-Cholesta-5,22-diene-3β,7α-diol | 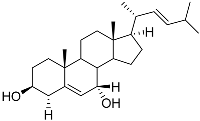 | −6.37 | −1165.36 | - | Met604, Leu566, Leu732, Asn630, Leu563, Tyr735, Phe623, Leu608, Cys736 |
| 81 | Decumbenone C | 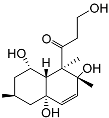 | −8.23 | −1298.75 | H29 Arg611 O 2.666 H24 Asn564 1.682 | Gly567, Trp600, Met604, Met601, Leu732, Met646, Phe623, Leu563, Met560 |
| 82 | Conidiogenone F | 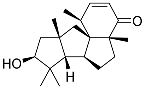 | −8.46 | −1229.36 | - | Gly567, Met604, Met601, Leu732, Trp600, Cys736, Tyr735, Met560, Leu563, Met646, Phe623 |
| −7.91 | −1211.54 | H29 Gln642 2.103 | Met560, Leu563, Leu753, Gly567, Met604, Met646, Leu732, Cys736 | |||
| Dexamethasone | 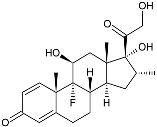 | −10.19 | −1206.31 | H29 Arg611 O 2.146 H27 Gln642 2.351 H26 Thr739 2.115 | Met560, Leu566, Gly567, Trp600, Met601, Met604, Phe623, Met646, Tyr735, Cys736, Thr739, Ile747 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| AChE | Acetyl Cholinesterase |
| AR | Androgen Receptor |
| BACE1 | Beta-Secretase 1 |
| CDK | Cyclin-Dependent Kinase |
| CpdA | Compound A |
| DBD | DNA-Binding Domain |
| ER | Estrogen Receptor |
| ERE | Estrogen Response Element |
| ERK | Extracellular-Regulated Kinase |
| GC | Glucocorticoid |
| GR | Glucocorticoid Receptor |
| GRE | Glucocorticoid Response Element |
| IL | Interleukin |
| INF | Interferon |
| iNOS | Inducible Nitric Oxide Synthase |
| LPS | Lipopolysaccharide |
| LXR-β | Liver X Receptor Beta |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MR | Mineralocorticoid Receptor |
| PR | Progesterone Receptor |
| SEGRAMs | Selective Glucocorticoid Receptor Agonists/Modulators |
| TA | Transactivation |
| TF | Transcription Factor |
| ThR | Thyroid Hormone Receptor |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TR | Transrepression |
| TrkB | Tropomyosin Receptor Kinase B |
| VDR | Vitamin D Receptor |
References
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Sawyers, C.L. The Emergence of Resistance to Targeted Cancer Therapeutics. Pharmacogenomics 2002, 3, 603–623. [Google Scholar] [CrossRef]
- Khamisipour, G.; Jadidi-Niaragh, F.; Jahromi, A.S.; Zandi, K.; Hojjat-Farsangi, M. Mechanisms of Tumor Cell Resistance to the Current Targeted-Therapy Agents. Tumor Biol. 2016, 37, 10021–10039. [Google Scholar] [CrossRef]
- Gentile, C.; Martorana, A.; Lauria, A.; Bonsignore, R. Kinase Inhibitors in Multitargeted Cancer Therapy. Curr. Med. Chem. 2017, 24, 1671–1686. [Google Scholar] [CrossRef]
- Valerio, L.; Matrone, A. Multikinase and Highly Selective Kinase Inhibitors in the Neoadjuvant Treatment of Patients with Thyroid Cancer. Explor. Target. Antitumor Ther. 2025, 6, 1002291. [Google Scholar] [CrossRef]
- Buzatu, I.M.; Tataranu, L.G.; Duta, C.; Stoian, I.; Alexandru, O.; Dricu, A. A Review of FDA-Approved Multi-Target Angiogenesis Drugs for Brain Tumor Therapy. Int. J. Mol. Sci. 2025, 26, 2192. [Google Scholar] [CrossRef]
- Díaz Vico, T.; Martínez-Amores Martínez, B.; Mihic Góngora, L.; Jiménez-Fonseca, P.; Peinado Martín, P.; Grao Torrente, I.; García Muñoz-Nájar, A.; Durán-Poveda, M. Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing. Cancers 2025, 17, 1800. [Google Scholar] [CrossRef]
- Flauto, F.; Damiano, V. The Efficacy and Safety of Multi-Kinase Inhibitors in Adrenocortical Carcinoma: A Systematic Review and Single-Arm Meta-Analysis. Cancers 2025, 17, 2004. [Google Scholar] [CrossRef]
- Guo, C.; Gasparian, A.V.; Zhuang, Z.; Bosykh, D.A.; Komar, A.A.; Gudkov, A.V.; Gurova, K. V 9-Aminoacridine-Based Anticancer Drugs Target the PI3K/AKT/MTOR, NF-ΚB and P53 Pathways. Oncogene 2009, 28, 1151–1161. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sung, B.; Prasad, S.; Webb, L.J.; Aggarwal, B.B. Cancer Drug Discovery by Repurposing: Teaching New Tricks to Old Dogs. Trends Pharmacol. Sci. 2013, 34, 508–517. [Google Scholar] [CrossRef]
- Swamidass, S.J. Mining Small-Molecule Screens to Repurpose Drugs. Brief. Bioinform. 2011, 12, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Agarwal, S.; Readhead, B.; Vinokour, E.; Baida, G.; Bhalla, P.; Kirsanov, K.; Yakubovskaya, M.; Platanias, L.C.; Dudley, J.T.; et al. Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy. J. Investig. Dermatol. 2018, 138, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Blatt, J.; Corey, S.J. Drug Repurposing in Pediatrics and Pediatric Hematology Oncology. Drug Discov. Today 2013, 18, 4–10. [Google Scholar] [CrossRef] [PubMed]
- McCabe, B.; Liberante, F.; Mills, K.I. Repurposing Medicinal Compounds for Blood Cancer Treatment. Ann. Hematol. 2015, 94, 1267–1276. [Google Scholar] [CrossRef]
- Koval, A.; Ahmed, K.; Katanaev, V.L. Inhibition of Wnt Signalling and Breast Tumour Growth by the Multi-Purpose Drug Suramin through Suppression of Heterotrimeric G Proteins and Wnt Endocytosis. Biochem. J. 2016, 473, 371–381. [Google Scholar] [CrossRef]
- Xi, Z.; Jie, W.; Long, Z.; Shasha, S. A Review of Thalidomide and Digestive System Related Diseases. Front. Oncol. 2025, 15, 1543757. [Google Scholar] [CrossRef]
- de Miranda Drummond, P.L.; de Araújo Silva, C.; de Souza, J.E.; Magno, B.A.R.; Battaglia, G.M.; Candido, R.C.F.; Menezes de Pádua, C.A.; Pereira, B.G. Efficacy and Safety of Thalidomide for Oncology-Related Uses Approved in Brazil: An Overview of Systematic Reviews. J. Oncol. Pharm. Pract. 2025; ahead of print. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Zhu, D.; Muthusamy, S.; Deshpande, M.; Kiruthiga, N.; Theivendren, P.; Rajalakshmi, K.; Wu, S.; Zhu, C. Exploring Alkaloids and Flavonoids from Natural Sources: Emerging Natural Agents for Inhibiting Cervical Cancer Progression through Apoptosis Induction, Anti-Inflammatory Effects, and Oxidative Stress Reduction. Pathol. Res. Pr. 2025, 272, 156092. [Google Scholar] [CrossRef]
- Al Khalily, I.; Megantara, S.; Aulifa, D. Targeting Molecular Pathways in Breast Cancer Using Plant-Derived Bioactive Compounds: A Comprehensive Review. J. Exp. Pharmacol. 2025, 17, 375–401. [Google Scholar] [CrossRef]
- Dodonova, S.A.; Zhidkova, E.M.; Kryukov, A.A.; Valiev, T.T.; Kirsanov, K.I.; Kulikov, E.P.; Budunova, I.V.; Yakubovskaya, M.G.; Lesovaya, E.A. Synephrine and Its Derivative Compound A: Common and Specific Biological Effects. Int. J. Mol. Sci. 2023, 24, 17537. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yao, S.; Chen, L.; Dong, Q.; Luo, R.; Zheng, K.; Liu, J.; Liu, Y.; Chen, Y.; et al. Inflammatory Bowel Disease Induces Colorectal Cancer: Risk Factors, Triggering Mechanisms, and Treatment with Phyto-Derivatives. Phytother. Res. 2025, 39, 3386–3418. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Bechelany, M.; Karav, S. Polyphenols in Foods and Their Use in the Food Industry: Enhancing the Quality and Nutritional Value of Functional Foods. Int. J. Mol. Sci. 2025, 26, 5803. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.S.; Chingizova, E.A.; Pislyagin, E.A.; Yurchenko, E.A.; Klimovich, A.A.; Zelepuga, E.A.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S. Mechanisms of Action of Sea Cucumber Triterpene Glycosides Cucumarioside A0-1 and Djakonovioside A Against Human Triple-Negative Breast Cancer. Mar. Drugs 2024, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Borkunov, G.V.; Leshchenko, E.V.; Berdyshev, D.V.; Popov, R.S.; Chingizova, E.A.; Shlyk, N.P.; Gerasimenko, A.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Chausova, V.E.; et al. New Piperazine Derivatives Helvamides B–C from the Marine-Derived Fungus Penicillium Velutinum ZK-14 Uncovered by OSMAC (One Strain Many Compounds) Strategy. Nat. Prod. Bioprospect 2024, 14, 32. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Girich, E.V.; Yurchenko, E.A. Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies. Mar. Drugs 2021, 19, 88. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Yurchenko, A.N.; Van Minh, C.; Aminin, D.L. Achievements in the Study of Marine Low-Molecular Weight Biologically Active Metabolites from the Vietnamese Territorial Waters as a Result of Expeditions Aboard the Research Vessel ‘Akademik Oparin’ (2004–2017). Chem. Biodivers. 2019, 16, e1800654. [Google Scholar] [CrossRef]
- Hajdaś, G.; Koenig, H.; Pospieszny, T. Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids. Int. J. Mol. Sci. 2025, 26, 3203. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular Cholesterol Delivery, Intracellular Processing and Utilization for Biosynthesis of Steroid Hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, R. Revisiting the Role of Steroidal Therapeutics in the 21st Century: An Update on FDA Approved Steroidal Drugs (2000–2024). RSC Med. Chem. 2025, 16, 2902–2918. [Google Scholar] [CrossRef]
- Samuel, S.; Nguyen, T.; Choi, H.A. Pharmacologic Characteristics of Corticosteroids. J. Neurocrit. Care 2017, 10, 53–59. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Fuller, P.J.; Yang, J.; Young, M.J.; Cole, T.J. Mechanisms of Ligand-Mediated Modulation of Mineralocorticoid Receptor Signaling. Mol. Cell. Endocrinol. 2025, 600, 112504. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.J.; Appleton, M.; Kipp, Z.A.; Loria, A.S.; Min, B.; Hinds, T.D. Glucocorticoids, Their Uses, Sexual Dimorphisms, and Diseases: New Concepts, Mechanisms, and Discoveries. Physiol. Rev. 2024, 104, 473–532. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Lange, C.A.; Levin, E.R. Membrane-Initiated Estrogen, Androgen, and Progesterone Receptor Signaling in Health and Disease. Endocr. Rev. 2022, 43, 720–742. [Google Scholar] [CrossRef] [PubMed]
- Agnoletto, A.; Brisken, C. Hormone Signaling in Breast Development and Cancer. Adv. Exp. Med. Biol. 2025, 1464, 279–307. [Google Scholar]
- Quistini, A.; Chierigo, F.; Fallara, G.; Depalma, M.; Tozzi, M.; Maggi, M.; Jannello, L.M.I.; Pellegrino, F.; Mantica, G.; Terracciano, D.; et al. Androgen Receptor Signalling in Prostate Cancer: Mechanisms of Resistance to Endocrine Therapies. Res. Rep. Urol. 2025, 17, 211–223. [Google Scholar] [CrossRef]
- Chakrabarti, D.; Albertsen, P.; Adkins, A.; Kishan, A.; Murthy, V.; Parker, C.; Pathmanathan, A.; Reid, A.; Sartor, O.; Van As, N.; et al. The Contemporary Management of Prostate Cancer. CA Cancer J. Clin. 2025. Early View. [Google Scholar] [CrossRef]
- Mansour, R.; Abunasser, M.; Sharaf, B.; Abdel-Razeq, H. Update in the Clinical Utilization of Chemoprevention for Breast Cancer: A Narrative Review. Front. Oncol. 2025, 15, 1435253. [Google Scholar] [CrossRef]
- Zulkipli, N.N.; Zakaria, R.; Wan Taib, W.R. Bibliometric Analysis of The Global Research Trends on The Application of Tamoxifen in The Treatment of Breast Cancer Over The Past 50 Years. Malays. J. Med. Sci. 2025, 32, 35–55. [Google Scholar] [CrossRef]
- Hiltunen, J.; Helminen, L.; Paakinaho, V. Glucocorticoid Receptor Action in Prostate Cancer: The Role of Transcription Factor Crosstalk. Front. Endocrinol. 2024, 15, 1437179. [Google Scholar] [CrossRef] [PubMed]
- Posani, S.H.; Gillis, N.E.; Lange, C.A. Glucocorticoid Receptors Orchestrate a Convergence of Host and Cellular Stress Signals in Triple Negative Breast Cancer. J. Steroid Biochem. Mol. Biol. 2024, 243, 106575. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.B.; Conzen, S.D. Glucocorticoid Receptor-Mediated Oncogenic Activity Is Dependent on Breast Cancer Subtype. J. Steroid Biochem. Mol. Biol. 2024, 243, 106518. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, E.M.; Lylova, E.S.; Grigoreva, D.D.; Kirsanov, K.I.; Osipova, A.V.; Kulikov, E.P.; Mertsalov, S.A.; Belitsky, G.A.; Budunova, I.; Yakubovskaya, M.G.; et al. Nutritional Sensor REDD1 in Cancer and Inflammation: Friend or Foe? Int. J. Mol. Sci. 2022, 23, 9686. [Google Scholar] [CrossRef]
- Faggiano, A.; Mazzilli, R.; Natalicchio, A.; Adinolfi, V.; Argentiero, A.; Danesi, R.; D’Oronzo, S.; Fogli, S.; Gallo, M.; Giuffrida, D.; et al. Corticosteroids in Oncology: Use, Overuse, Indications, Contraindications. An Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) Multidisciplinary Consensus Position Paper. Crit. Rev. Oncol. Hematol. 2022, 180, 103826. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Yemelyanov, A.Y.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. Antitumor Effect of Non-Steroid Glucocorticoid Receptor Ligand CpdA on Leukemia Cell Lines CEM and K562. Biochemistry 2011, 76, 1242–1252. [Google Scholar] [CrossRef]
- Lesovaya, E.; Yemelyanov, A.; Kirsanov, K.; Popa, A.; Belitsky, G.; Yakubovskaya, M.; Gordon, L.I.; Rosen, S.T.; Budunova, I. Combination of a Selective Activator of the Glucocorticoid Receptor Compound A with a Proteasome Inhibitor as a Novel Strategy for Chemotherapy of Hematologic Malignancies. Cell Cycle 2013, 12, 133–144. [Google Scholar] [CrossRef]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A—A Selective Activator of the Glucocorticoid Receptor with Anti-Inflammatory and Anti-Cancer Activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Chudakova, D.; Baida, G.; Zhidkova, E.M.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. The Long Winding Road to the Safer Glucocorticoid Receptor (GR) Targeting Therapies. Oncotarget 2022, 13, 408–424. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Tilova, L.R.; Fetisov, T.I.; Kirsanov, K.I.; Kulikov, E.P.; Enikeev, A.D.; Budunova, I.V.; Badun, G.A.; Chernysheva, M.G.; Shirinian, V.Z.; et al. Synthesis and Anti-Cancer Activity of the Novel Selective Glucocorticoid Receptor Agonists of the Phenylethanolamine Series. Int. J. Mol. Sci. 2024, 25, 8904. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Oleynik, E.S.; Mikhina, E.A.; Stepanycheva, D.V.; Grigoreva, D.D.; Grebenkina, L.E.; Gordeev, K.V.; Savina, E.D.; Matveev, A.V.; Yakubovskaya, M.G.; et al. Synthesis and Anti-Cancer Activity In Vitro of Synephrine Derivatives. Biomolecules 2024, 15, 2. [Google Scholar] [CrossRef]
- Clarisse, D.; Van Moortel, L.; Van Leene, C.; Gevaert, K.; De Bosscher, K. Glucocorticoid Receptor Signaling: Intricacies and Therapeutic Opportunities. Trends Biochem. Sci. 2024, 49, 431–444. [Google Scholar] [CrossRef]
- Van Moortel, L.; Gevaert, K.; De Bosscher, K. Improved Glucocorticoid Receptor Ligands: Fantastic Beasts, but How to Find Them? Front. Endocrinol. 2020, 11, 559673. [Google Scholar] [CrossRef]
- Hsu, S.-J.; He, M.; Salomé-Abarca, L.F.; Choi, Y.H.; Wang, M. Uncovering Anti-Inflammatory Activity of Ginsenoside Rg1 in a Wound-Inured Zebrafish Model by GC-MS-Based Chemical Profiling. Planta Med. 2025, 91, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Hennen, S.; Gautrois, M.; Khalil, F.; Lockhart, A. Novel Selective Glucocorticoid Receptor Modulator GRM-01 Demonstrates Dissociation of Anti-Inflammatory Effects from Adverse Effects on Glucose and Bone Metabolism. Front. Pharmacol. 2025, 16, 1542351. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, E.M.; Lylova, E.S.; Savinkova, A.V.; Mertsalov, S.A.; Kirsanov, K.I.; Belitsky, G.A.; Yakubovskaya, M.G.; Lesovaya, E.A. A Brief Overview of the Paradoxical Role of Glucocorticoids in Breast Cancer. Breast Cancer 2020, 14, 1178223420974667. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Halima, M.; Xie, Y.; Schaaf, M.J.M.; Meijer, A.H.; Wang, M. Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells 2020, 9, 1107. [Google Scholar] [CrossRef]
- Karra, A.G.; Tziortziou, M.; Kylindri, P.; Georgatza, D.; Gorgogietas, V.A.; Makiou, A.; Krokida, A.; Tsialtas, I.; Kalousi, F.D.; Papadopoulos, G.E.; et al. Boswellic Acids and Their Derivatives as Potent Regulators of Glucocorticoid Receptor Actions. Arch. Biochem. Biophys. 2020, 695, 108656. [Google Scholar] [CrossRef]
- Morsy, M.A.; Patel, S.S.; El-Sheikh, A.A.K.; Savjani, J.K.; Nair, A.B.; Shah, J.N.; Venugopala, K.N. Computational and Biological Comparisons of Plant Steroids as Modulators of Inflammation through Interacting with Glucocorticoid Receptor. Mediat. Inflamm. 2019, 2019, 3041438. [Google Scholar] [CrossRef]
- Brown, M.N.; Fuhr, R.; Beier, J.; Su, H.-L.; Chen, Y.; Forsman, H.; Hamrén, U.W.; Jackson, H.; Aggarwal, A. Efficacy and Safety of AZD7594, an Inhaled Non-Steroidal Selective Glucocorticoid Receptor Modulator, in Patients with Asthma: A Phase 2a Randomized, Double Blind, Placebo-Controlled Crossover Trial. Respir. Res. 2019, 20, 37. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, T.; Zhang, F.; Lei, C.; Zhu, Y.; Luo, G.; Xiao, D. Bioequivalence Study of Fluticasone Propionate Nebuliser Suspensions in Healthy Chinese Subjects. Front. Pharmacol. 2025, 15, 1452596. [Google Scholar] [CrossRef]
- Hunt, H.; Donaldson, K.; Strem, M.; Zann, V.; Leung, P.; Sweet, S.; Connor, A.; Combs, D.; Belanoff, J. Assessment of Safety, Tolerability, Pharmacokinetics, and Pharmacological Effect of Orally Administered CORT125134: An Adaptive, Double-Blind, Randomized, Placebo-Controlled Phase 1 Clinical Study. Clin. Pharmacol. Drug Dev. 2018, 7, 408–421. [Google Scholar] [CrossRef]
- Kuna, P.; Aurivillius, M.; Jorup, C.; Prothon, S.; Taib, Z.; Edsbäcker, S. Efficacy and Tolerability of an Inhaled Selective Glucocorticoid Receptor Modulator—AZD5423—In Chronic Obstructive Pulmonary Disease Patients: Phase II Study Results. Basic. Clin. Pharmacol. Toxicol. 2017, 121, 279–289. [Google Scholar] [CrossRef]
- Werkström, V.; Prothon, S.; Ekholm, E.; Jorup, C.; Edsbäcker, S. Safety, Pharmacokinetics and Pharmacodynamics of the Selective Glucocorticoid Receptor Modulator AZD5423 after Inhalation in Healthy Volunteers. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Conrado, D.J.; Krishnaswami, S.; Shoji, S.; Kolluri, S.; Hey-Hadavi, J.; McCabe, D.; Rojo, R.; Tammara, B.K. Predicting the Probability of Successful Efficacy of a Dissociated Agonist of the Glucocorticoid Receptor from Dose–Response Analysis. J. Pharmacokinet. Pharmacodyn. 2016, 43, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Bareille, P.; Hardes, K.; Donald, A.C. Efficacy and Safety of Once-Daily GW870086 a Novel Selective Glucocorticoid in Mild-Moderate Asthmatics: A Randomised, Two-Way Crossover, Controlled Clinical Trial. J. Asthma 2013, 50, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Harcken, C.; Scholl, P.; Nabozny, G.; Thomson, D.; Bianchi, D. Clinical Profile of the Functionally Selective Glucocorticoid Receptor Agonist BI 653048 in Healthy Male Subjects. Expert Opin. Investig. Drugs 2019, 28, 489–496. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Chingizova, E.A.; Aminin, D.L.; Yurchenko, A.N. Marine Fungi: In Search of New Antibacterial Drugs. Mol. Biol. 2025, 59, 43–59. [Google Scholar] [CrossRef]
- Borkunov, G.V.; Kirichuk, N.N.; Chausova, V.E.; Popov, R.S.; Zhuravleva, O.I.; Chingizova, E.A.; Yurchenko, E.A.; Isaeva, M.P.; Yurchenko, A.N. Differences in Metabolite Profiles and Bioactivities of Intra-Strain Variants of Marine Fungus Penicillium Antarcticum KMM 4668. Metabolites 2025, 15, 77. [Google Scholar] [CrossRef]
- Chingizova, E.A.; Yurchenko, E.A.; Chingizov, A.R.; Klimovich, A.A.; Pislyagin, E.A.; Menchinskaya, E.S.; Kuzmich, A.S.; Trinh, P.T.H.; Ngoc, N.T.D.; Van, T.T.T.; et al. The Effects of Marine Fungal Asterripeptides A–C on In Vitro and In Vivo Staphylococcus Aureus Skin Infection. Pharmaceuticals 2024, 17, 1345. [Google Scholar] [CrossRef]
- Belousova, E.B.; Zhuravleva, O.I.; Yurchenko, E.A.; Oleynikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Chausova, V.E.; Khudyakova, Y.V.; Menshov, A.S.; Popov, R.S.; et al. New Anti-Hypoxic Metabolites from Co-Culture of Marine-Derived Fungi Aspergillus Carneus KMM 4638 and Amphichorda sp. KMM 4639. Biomolecules 2023, 13, 741. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Oleinikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Pelageev, D.N.; Rasin, A.B.; Menshov, A.S.; Popov, R.S.; Kim, N.Y.; Chingizova, E.A.; et al. New Antibacterial Chloro-Containing Polyketides from the Alga-Derived Fungus Asteromyces Cruciatus KMM 4696. J. Fungi 2022, 8, 454. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Chingizova, E.A.; Antonov, A.S.; Shlyk, N.P.; Borkunov, G.V.; Berdyshev, D.V.; Chausova, V.E.; Kirichuk, N.N.; Khudyakova, Y.V.; Chingizov, A.R.; et al. New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium Yezoense KMM 4679. Mar. Drugs 2024, 22, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Belousova, E.B.; Oleinikova, G.K.; Antonov, A.S.; Khudyakova, Y.V.; Rasin, A.B.; Popov, R.S.; Menchinskaya, E.S.; Trinh, P.T.H.; Yurchenko, A.N.; et al. Cytotoxic Drimane-Type Sesquiterpenes from Co-Culture of the Marine-Derived Fungi Aspergillus Carneus KMM 4638 and Beauveria Felina (=Isaria Felina) KMM 4639. Mar. Drugs 2022, 20, 584. [Google Scholar] [CrossRef] [PubMed]
- Krautforst, K.; Kulbacka, J.; Fornasier, M.; Mocci, R.; Porcheddu, A.; Pusceddu, A.; Moccia, D.; Murgia, S.; Bazylińska, U. Caulerpin Delivery via Pluronic-Free Cubosomes: Unlocking the Therapeutic Potential of a Pigment from an Invasive Marine Algae. Mol. Pharm. 2025, 22, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Raksat, A.; Atanu, M.S.H.; McDermid, K.J.; Wall, M.M.; Chang, B.L.; Wongwiwatthananukit, S.; Chang, L.C. Bioactive Constituents from the Edible Seaweed Halymenia Hawaiiana (Rhodophyta). Pharm. Biol. 2025, 63, 447–459. [Google Scholar] [CrossRef]
- Asoudeh-Fard, A.; Soltanmohammadi, F.; Kashkouie Jahromi, M.; Javanmardi, F.; Roosta, A.; Zareian Jahromi, M.; Parsaei, A. Potential Anticancer Properties of Tetraselmis Suecica Extract against Oral and Colorectal Cancer Cells. Med. Oncol. 2025, 42, 282. [Google Scholar] [CrossRef]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef]
- Hussain, A.; Sohail, A.; Akash, M.S.H.; Iqbal, S.; Rehman, K.; Imran, M.; Khan, S.; Ayub, M.A.; Wang, D.; Ahmed, D.; et al. Marine Life as a Source of Anti-Prostate Cancer Agents: An Updated Overview (2003–2023). Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 7971–8074. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Y.; Li, Q.; Su, M.; Guo, Y.; Jin, X. Recent Advances in Natural Products Derived from Marine Echinoderms and Endophytic Microbes: Chemical Insights and Therapeutic Potential. Mar. Drugs 2025, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Mar. Drugs 2024, 22, 433. [Google Scholar] [CrossRef]
- Islam, F.; Mitra, S.; Emran, T.B.; Khan, Z.; Nath, N.; Das, R.; Sharma, R.; Al Awadh, A.A.; Park, M.N.; Kim, B. Natural Small Molecules in Gastrointestinal Tract and Associated Cancers: Molecular Insights and Targeted Therapies. Molecules 2022, 27, 5686. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Abd El Hafez, M.S.M.; Aziz Okbah, M.A.E.; Ibrahim, H.A.H.; Hussein, A.A.E.R.; El Moneim, N.A.A.; Ata, A. First Report of Steroid Derivatives Isolated from Starfish Acanthaster Planci with Anti-Bacterial, Anti-Cancer and Anti-Diabetic Activities. Nat. Prod. Res. 2022, 36, 5545–5552. [Google Scholar] [CrossRef]
- Huong, P.T.M.; Phong, N.V.; Thao, N.P.; Binh, P.T.; Thao, D.T.; Van Thanh, N.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Minh, C. Van Dendrodoristerol, a Cytotoxic C20 Steroid from the Vietnamese Nudibranch Mollusk Dendrodoris Fumata. J. Asian Nat. Prod. Res. 2020, 22, 193–200. [Google Scholar] [CrossRef]
- Quang, T.H.; Lee, D.; Han, S.J.; Kim, I.C.; Yim, J.H.; Kim, Y.; Oh, H. ChemInform Abstract: Steroids from the Cold Water Starfish Ctenodiscus Crispatus with Cytotoxic and Apoptotic Effects on Human Hepatocellular Carcinoma and Glioblastoma Cells. ChemInform 2015, 46. [Google Scholar] [CrossRef]
- Xiao, D.-J.; Peng, X.-D.; Deng, S.-Z.; Ma, W.-J.; Wu, H.-M. Structure Elucidation of (3E)-Cholest-4-En-3,6-Dione-3-Oxime in Marine Sponge Cinachyrella Australiensis from the South China Sea. Chin. J. Org. Chem. 2005, 25, 1606–1609. [Google Scholar]
- Shubina, L.K.; Makarieva, T.N.; Denisenko, V.A.; Popov, R.S.; Dyshlovoy, S.A.; Grebnev, B.B.; Dmitrenok, P.S.; von Amsberg, G.; Stonik, V.A. Gracilosulfates A–G, Monosulfated Polyoxygenated Steroids from the Marine Sponge Haliclona Gracilis. Mar. Drugs 2020, 18, 454. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Abd-Elrazek, A.E.E.; Hassanean, H.A.; Youssef, D.T.A.; van Soest, R. New Compounds from the Red Sea Marine Sponge Echinoclathria Gibbosa. Phytochem. Lett. 2014, 9, 51–58. [Google Scholar] [CrossRef]
- Holland, I.P.; McCluskey, A.; Sakoff, J.A.; Gilbert, J.; Chau, N.; Robinson, P.J.; Motti, C.A.; Wright, A.D.; van Altena, I.A. Steroids from an Australian Sponge Psammoclema sp. J. Nat. Prod. 2009, 72, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Ivanchina, N.V.; Huong, T.T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Dyshlovoy, S.A.; Long, P.Q.; Stonik, V.A. Two New Asterosaponins, Archasterosides A and B, from the Vietnamese Starfish Archaster Typicus and Their Anticancer Properties. Bioorg. Med. Chem. Lett. 2010, 20, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Ngoan, B.T.; Hanh, T.T.H.; Vien, L.T.; Diep, C.N.; Thao, N.P.; Thao, D.T.; Van Thanh, N.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; et al. Asterosaponins and Glycosylated Polyhydroxysteroids from the Starfish Culcita Novaeguineae and Their Cytotoxic Activities. J. Asian Nat. Prod. Res. 2015, 17, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Tolkanov, D.K.; Malyarenko, T.V.; Malyarenko, O.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V.; Dmitrenok, P.S. Sulfated Polyhydroxysteroid Glycosides from the Sea of Okhotsk Starfish Henricia Leviuscula Spiculifera and Potential Mechanisms for Their Observed Anti-Cancer Activity against Several Types of Human Cancer Cells. Mar. Drugs 2024, 22, 294. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Malyarenko, O.S.; Ermakova, S.P.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V. Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster Marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures. Mar. Drugs 2022, 20, 164. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Kicha, A.A.; Malyarenko, O.S.; Zakharenko, V.M.; Kotlyarov, I.P.; Kalinovsky, A.I.; Popov, R.S.; Svetashev, V.I.; Ivanchina, N.V. New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster Patagonicus and Their Anticancer Activity. Mar. Drugs 2020, 18, 260. [Google Scholar] [CrossRef]
- Tian, X.-R.; Gao, Y.-Q.; Tian, X.-L.; Li, J.; Tang, H.-F.; Li, Y.-S.; Lin, H.-W.; Ma, Z.-Q. New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula Pallasiana. Mar. Drugs 2017, 15, 120. [Google Scholar] [CrossRef]
- Hoang, C.K.; Le, C.H.; Nguyen, D.T.; Tran, H.T.N.; Luu, C.V.; Le, H.M.; Tran, H.T.H. Steroid Components of Marine-Derived Fungal Strain Penicillium Levitum N33.2 and Their Biological Activities. Mycobiology 2023, 51, 246–255. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Tian, H.-Y.; Li, J.; Jin, L.; Luo, C.; Ye, W.-C.; Jiang, R.-W. Steroids with Inhibitory Activity against the Prostate Cancer Cells and Chemical Diversity of Marine Alga Tydemania Expeditionis. Fitoterapia 2012, 83, 973–978. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Kim, E.J.; Kang, J.I.; Kang, H.K.; Cuong, N.X.; Nam, N.H.; Van Kiem, P.; Van Minh, C.; Kim, Y.H. Steroidal Constituents from the Edible Sea Urchin Diadema Savignyi Michelin Induce Apoptosis in Human Cancer Cells. J. Med. Food 2015, 18, 45–53. [Google Scholar] [CrossRef]
- Tsai, C.-R.; Huang, C.-Y.; Chen, B.-W.; Tsai, Y.-Y.; Shih, S.-P.; Hwang, T.-L.; Dai, C.-F.; Wang, S.-Y.; Sheu, J.-H. New Bioactive Steroids from the Soft Coral Klyxum Flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Alarif, W.M.; Asfour, H.Z.; Ayyad, S.-E.N.; Khedr, A.; Badria, F.A.; Al-lihaibi, S.S. Cytotoxic Effects of Three New Metabolites from Red Sea Marine Sponge, Petrosia sp. Env. Toxicol. Pharmacol. 2014, 37, 928–935. [Google Scholar] [CrossRef]
- Eissa, A.H.; Abdel-Tawab, A.M.; Hamed, E.S.A.E.; El-Ablack, F.Z.N.; Ayyad, S.-E. Cytotoxic Evaluation of New Polyhydroxylated Steroids from the Red Sea Soft Coral Litophyton Mollis (Macfadyen, 1936). Nat. Prod. Res. 2024, 38, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Y.; Wang, H.-N.; Sun, L.-X.; Sun, J.; Jin, S.-H.; Dai, F.-X.; Sai, C.-M.; Zhang, Z. Bioactive Steroids from Marine-Derived Fungi: A Review (2015–2023). J. Asian Nat. Prod. Res. 2025, 27, 1236–1262. [Google Scholar] [CrossRef]
- Khaledi, M.; Moradipoodeh, B.; Moradi, R.; Baghbadorani, M.A.; Mahdavinia, M. Antiproliferative and Proapoptotic Activities of Sea Cucumber H. Leucospilota Extract on Breast Carcinoma Cell Line (SK-BR-3). Mol. Biol. Rep. 2022, 49, 1191–1200. [Google Scholar] [CrossRef]
- Heidary Jamebozorgi, F.; Yousefzadi, M.; Firuzi, O.; Nazemi, M.; Zare, S.; Chandran, J.N.; Schneider, B.; Baldwin, I.T.; Jassbi, A.R. Cytotoxic Furanosesquiterpenoids and Steroids from Ircinia Mutans Sponges. Pharm. Biol. 2021, 59, 573–581. [Google Scholar] [CrossRef]
- Hadisaputri, Y.E.; Andika, R.; Sopyan, I.; Zuhrotun, A.; Maharani, R.; Rachmat, R.; Abdulah, R. Caspase Cascade Activation During Apoptotic Cell Death of Human Lung Carcinoma Cells A549 Induced by Marine Sponge Callyspongia Aerizusa. Drug Des. Dev. Ther. 2021, 15, 1357–1368. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Rodríguez-Pérez, C.; Herranz-López, M.; Martín-García, B.; Gómez-Caravaca, A.-M.; Arráez-Román, D.; Segura-Carretero, A.; Barrajón-Catalán, E.; Micol, V. Marine Invertebrate Extracts Induce Colon Cancer Cell Death via ROS-Mediated DNA Oxidative Damage and Mitochondrial Impairment. Biomolecules 2019, 9, 771. [Google Scholar] [CrossRef]
- Thi Duy Ngoc, N.; Yurchenko, E.A.; Thi Hoai Trinh, P.; Menchinskaya, E.S.; Thi Dieu, T.V.; Savagina, A.D.; Minin, A.; Thinh, P.D.; Khanh, H.H.N.; Thi Thanh Van, T.; et al. Secondary Metabolites of Vietnamese Marine Fungus Penicillium Chermesinum 2104NT-1.3 and Their Cardioprotective Activity. Reg. Stud. Mar. Sci. 2025, 81, 104003. [Google Scholar] [CrossRef]
- Kicha, A.A.; Malyarenko, T.V.; Kuzmich, A.S.; Malyarenko, O.S.; Kalinovsky, A.I.; Popov, R.S.; Tolkanov, D.K.; Ivanchina, N.V. Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster Marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells. Mar. Drugs 2024, 22, 43. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Van Thanh, N.; Chi, N.T.P.; Dang, N.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Van Kiem, P.; Minh, C. Van Cytotoxic Steroids from the Vietnamese Soft Coral Sinularia Conferta. Chem. Pharm. Bull. 2017, 65, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ghandourah, M.A. Cytotoxic Ketosteroids from the Red Sea Soft Coral Dendronephthya sp. Open Chem 2023, 21. [Google Scholar] [CrossRef]
- Adcock, I.M.; Mumby, S. Glucocorticoids. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–196. [Google Scholar]
- Tan, C.K.; Wahli, W. A Trilogy of Glucocorticoid Receptor Actions. Proc. Natl. Acad. Sci. USA 2016, 113, 1115–1117. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Specificity and Sensitivity of Glucocorticoid Signaling in Health and Disease. Best. Pr. Res. Clin. Endocrinol. Metab. 2015, 29, 545–556. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Pofi, R.; Caratti, G.; Ray, D.W.; Tomlinson, J.W. Treating the Side Effects of Exogenous Glucocorticoids; Can We Separate the Good From the Bad? Endocr. Rev. 2023, 44, 975–1011. [Google Scholar] [CrossRef]
- De Bosscher, K.; Beck, I.M.; Ratman, D.; Berghe, W.V.; Libert, C. Activation of the Glucocorticoid Receptor in Acute Inflammation: The SEDIGRAM Concept. Trends Pharmacol. Sci. 2016, 37, 4–16. [Google Scholar] [CrossRef]
- Sundahl, N.; Bridelance, J.; Libert, C.; De Bosscher, K.; Beck, I.M. Selective Glucocorticoid Receptor Modulation: New Directions with Non-Steroidal Scaffolds. Pharmacol. Ther. 2015, 152, 28–41. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for Modulation of the Nuclear Receptor Superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Hudson, W.H.; de Vera, I.M.S.; Nwachukwu, J.C.; Weikum, E.R.; Herbst, A.G.; Yang, Q.; Bain, D.L.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Cryptic Glucocorticoid Receptor-Binding Sites Pervade Genomic NF-ΚB Response Elements. Nat. Commun. 2018, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Hudson, W.H.; Kossmann, B.R.; de Vera, I.M.S.; Chuo, S.-W.; Weikum, E.R.; Eick, G.N.; Thornton, J.W.; Ivanov, I.N.; Kojetin, D.J.; Ortlund, E.A. Distal Substitutions Drive Divergent DNA Specificity among Paralogous Transcription Factors through Subdivision of Conformational Space. Proc. Natl. Acad. Sci. USA 2016, 113, 326–331. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Wuescher, L.; Marino, J.S.; Hill, J.W.; Eng, C.; Hinds, T.D. Glucocorticoid Receptor β Stimulates Akt1 Growth Pathway by Attenuation of PTEN. J. Biol. Chem. 2014, 289, 17885–17894. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Ben Hamida, O.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dexamethasone, a Synthetic Glucocorticoid, Induces the Activity of Androgen Receptor in Human Dermal Papilla Cells. Skin Pharmacol. Physiol. 2022, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Burnstein, K.L.; Maiorino, C.A.; Dai, J.L.; Cameron, D.J. Androgen and Glucocorticoid Regulation of Androgen Receptor CDNA Expression. Mol. Cell. Endocrinol. 1995, 115, 177–186. [Google Scholar] [CrossRef]
- Pecci, A.; Ogara, M.F.; Sanz, R.T.; Vicent, G.P. Choosing the Right Partner in Hormone-Dependent Gene Regulation: Glucocorticoid and Progesterone Receptors Crosstalk in Breast Cancer Cells. Front. Endocrinol. 2022, 13, 1037177. [Google Scholar] [CrossRef]
- Wan, Y.; Nordeen, S.K. Overlapping but Distinct Gene Regulation Profiles by Glucocorticoids and Progestins in Human Breast Cancer Cells. Mol. Endocrinol. 2002, 16, 1204–1214. [Google Scholar] [CrossRef]
- Hundertmark, S.; Buhler, H.; Rudolf, M.; Weitzel, H.; Ragosch, V. Inhibition of 11 Beta-Hydroxysteroid Dehydrogenase Activity Enhances the Antiproliferative Effect of Glucocorticosteroids on MCF-7 and ZR-75-1 Breast Cancer Cells. J. Endocrinol. 1997, 155, 171–180. [Google Scholar] [CrossRef]
- Meyer, M.-E.; Gronemeyer, H.; Turcotte, B.; Bocquel, M.-T.; Tasset, D.; Chambon, P. Steroid Hormone Receptors Compete for Factors That Mediate Their Enhancer Function. Cell 1989, 57, 433–442. [Google Scholar] [CrossRef]
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the Glucocorticoid Receptor Is Associated with Poor Prognosis in Estrogen Receptor-Negative Breast Cancer. Cancer Res. 2011, 71, 6360–6370. [Google Scholar] [CrossRef]
- West, D.C.; Pan, D.; Tonsing-Carter, E.Y.; Hernandez, K.M.; Pierce, C.F.; Styke, S.C.; Bowie, K.R.; Garcia, T.I.; Kocherginsky, M.; Conzen, S.D. GR and ER Coactivation Alters the Expression of Differentiation Genes and Associates with Improved ER+ Breast Cancer Outcome. Mol. Cancer Res. 2016, 14, 707–719. [Google Scholar] [CrossRef]
- Karmakar, S.; Jin, Y.; Nagaich, A.K. Interaction of Glucocorticoid Receptor (GR) with Estrogen Receptor (ER) α and Activator Protein 1 (AP1) in Dexamethasone-Mediated Interference of ERα Activity. J. Biol. Chem. 2013, 288, 24020–24034. [Google Scholar] [CrossRef]
- Miranda, T.B.; Voss, T.C.; Sung, M.-H.; Baek, S.; John, S.; Hawkins, M.; Grøntved, L.; Schiltz, R.L.; Hager, G.L. Reprogramming the Chromatin Landscape: Interplay of the Estrogen and Glucocorticoid Receptors at the Genomic Level. Cancer Res. 2013, 73, 5130–5139. [Google Scholar] [CrossRef]
- Voss, T.C.; Schiltz, R.L.; Sung, M.-H.; Yen, P.M.; Stamatoyannopoulos, J.A.; Biddie, S.C.; Johnson, T.A.; Miranda, T.B.; John, S.; Hager, G.L. Dynamic Exchange at Regulatory Elements during Chromatin Remodeling Underlies Assisted Loading Mechanism. Cell 2011, 146, 544–554. [Google Scholar] [CrossRef]
- Volden, P.A.; Conzen, S.D. The Influence of Glucocorticoid Signaling on Tumor Progression. Brain Behav. Immun. 2013, 30, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Chen, Y.-H.; Bian, H.-H.; Zhang, J.-P.; Su, L.; Han, H.; Zhang, W. Anti-Inflammatory Ergosteroid Derivatives from the Coral-Associated Fungi Penicillium Oxalicum HL-44. Molecules 2023, 28, 7784. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, C.; Ye, Y.; Xu, Z.; Zhao, Q.; Luo, X.; Liu, Y.; Guo, P. Anti-Inflammatory Compounds from the Mangrove Endophytic Fungus Amorosia sp. SCSIO 41026. Front. Microbiol. 2022, 13, 976399. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-B.; Wu, T.-Z.; Yang, L.-H.; He, X.-W.; Liu, W.-Y.; Zhang, K.; Xie, C.-L.; Xie, M.-M.; Zhang, Y.; Yang, X.-W.; et al. Hepialiamides A–C: Aminated Fusaric Acid Derivatives and Related Metabolites with Anti-Inflammatory Activity from the Deep-Sea-Derived Fungus Samsoniella Hepiali W7. Mar. Drugs 2023, 21, 596. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Su, R.; Chen, M.-T.; Li, J.-Y.; Chen, H.-Y.; Yang, L.; Liu, F.-F.; Liu, J.; Xu, C.-J.; Li, W.-S.; et al. Ent-Eudesmane Sesquiterpenoids with Anti-Neuroinflammatory Activity from the Marine-Derived Fungus Eutypella sp. F0219. Phytochemistry 2024, 223, 114121. [Google Scholar] [CrossRef]
- Dai, L.-T.; Yang, L.; Wang, Z.-P.; Guo, J.-C.; Ma, Q.-Y.; Xie, Q.-Y.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Persteroid, a New Steroid from the Marine-Derived Fungus Penicillium sp. ZYX-Z-143. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Strangman, W.K.; Kwon, H.C.; Broide, D.; Jensen, P.R.; Fenical, W. Potent Inhibitors of Pro-Inflammatory Cytokine Production Produced by a Marine-Derived Bacterium. J. Med. Chem. 2009, 52, 2317–2327. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Moon, I.S. Deciphering Molecular Mechanism of the Neuropharmacological Action of Fucosterol through Integrated System Pharmacology and In Silico Analysis. Mar. Drugs 2019, 17, 639. [Google Scholar] [CrossRef]
- Hagiwara, H.; Wakita, K.; Inada, Y.; Hirose, S. Fucosterol Decreases Angiotensin Converting Enzyme Levels with Reduction of Glucocorticoid Receptors in Endothelial Cells. Biochem. Biophys. Res. Commun. 1986, 139, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, E.A.; Khmel, O.O.; Nesterenko, L.E.; Aminin, D.L. The Kelch/Nrf2 Antioxidant System as a Target for Some Marine Fungal Metabolites. Oxygen 2023, 3, 374–385. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Popov, R.S.; Yurchenko, E.A.; Stonik, V.A. Oxysterols from a Marine Sponge Inflatella sp. and Their Action in 6-Hydroxydopamine-Induced Cell Model of Parkinson’s Disease. Mar. Drugs 2018, 16, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Vishchuk, O.S.; Denisenko, V.A.; Slinkina, N.N.; Smetanina, O.F. Decumbenone C, a New Cytotoxic Decaline Derivative from the Marine Fungus Aspergillus Sulphureus KMM 4640. Arch. Pharm. Res. 2012, 35, 1757–1762. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Zhuravleva, O.I.; Khmel, O.O.; Oleynikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Chausova, V.E.; Kalinovsky, A.I.; Berdyshev, D.V.; Kim, N.Y.; et al. New Cyclopiane Diterpenes and Polyketide Derivatives from Marine Sediment-Derived Fungus Penicillium Antarcticum KMM 4670 and Their Biological Activities. Mar. Drugs 2023, 21, 584. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Denisenko, V.A.; Ermakova, S.P.; Slinkina, N.N.; Dmitrenok, P.S.; Kim, N.Y. Secondary Metabolites from a Marine-Derived Fungus Aspergillus Carneus Blochwitz. Phytochemistry 2012, 80, 123–131. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Leshchenko, E.V.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Pislyagin, E.A.; Kim, N.Y.; Berdyshev, D.V.; et al. Piltunines A–F from the Marine-Derived Fungus Penicillium Piltunense KMM 4668. Mar. Drugs 2019, 17, 647. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Kalinovsky, A.I.; Pushilin, M.A.; Glazunov, V.P.; Khudyakova, Y.V.; Kirichuk, N.N.; Ermakova, S.P.; Dyshlovoy, S.A.; Yurchenko, E.A.; et al. Oxirapentyns F–K from the Marine-Sediment-Derived Fungus Isaria Felina KMM 4639. J. Nat. Prod. 2014, 77, 1321–1328. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Chingizova, E.A.; Oleinikova, G.K.; Starnovskaya, S.S.; Antonov, A.S.; Kirichuk, N.N.; Menshov, A.S.; Popov, R.S.; Kim, N.Y.; Berdyshev, D.V.; et al. Anthraquinone Derivatives and Other Aromatic Compounds from Marine Fungus Asteromyces Cruciatus KMM 4696 and Their Effects against Staphylococcus Aureus. Mar. Drugs 2023, 21, 431. [Google Scholar] [CrossRef]
- Dalinova, A.; Smirnov, S.; Dubovik, V.; Senderskiy, I.; Stepanycheva, E.; Kovalenko, N.; Berestetskiy, A. Production and Toxicological Characterization of Secondary Metabolites of Pyrenophora Tritici-Repentis. J. Plant Dis. Prot. 2025, 132, 149. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Sobolevskaya, M.P.; Antonov, A.S.; Denisenko, V.A.; Popov, R.S.; Khudyakova, Y.V.; Kirichuk, N.N.; Kuz’mich, A.S.; Pislyagin, E.A.; et al. New Thomimarine E from Marine Isolate of the Fungus Penicillium Thomii. Chem. Nat. Compd. 2017, 53, 290–294. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Yurchenko, E.A.; Afiyatullov, S.S. Metabolites of the Marine Fungus Penicillium Citrinum Associated with a Brown Alga Padina sp. Chem. Nat. Compd. 2016, 52, 111–112. [Google Scholar] [CrossRef]
- Girich, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Trinh, P.T.H.; Ngoc, N.T.D.; Pivkin, M.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; et al. Neuroprotective Metabolites from Vietnamese Marine Derived Fungi of Aspergillus and Penicillium Genera. Mar. Drugs 2020, 18, 608. [Google Scholar] [CrossRef]
- Yurchenko, A.; Trinh, P.; Girich (Ivanets), E.; Smetanina, O.; Rasin, A.; Popov, R.; Dyshlovoy, S.; von Amsberg, G.; Menchinskaya, E.; Thanh Van, T.; et al. Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus Flocculosus. Mar. Drugs 2019, 17, 579. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Kalinovsky, A.I.; Pushilin, M.A.; Slinkina, N.N.; Yurchenko, E.A.; Afiyatullov, S.S. 4-Methoxy-3-Methylgoniothalamin from Marine-Derived Fungi of the Genus Penicillium. Russ. Chem. Bull. 2011, 60, 760–763. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Phan, T.T.H.; Ngo, N.T.D.; Zhuravleva, O.I.; Rasin, A.B.; Dyshlovoy, S.A.; Menchinskaya, E.S.; Pislyagin, E.A.; et al. Auroglaucin-Related Neuroprotective Compounds from Vietnamese Marine Sediment-Derived Fungus Aspergillus Niveoglaucus. Nat. Prod. Res. 2020, 34, 2589–2594. [Google Scholar] [CrossRef]
- Antonov, A.S.; Leshchenko, E.V.; Zhuravleva, O.I.; Dyshlovoy, S.A.; von Amsberg, G.; Popov, R.S.; Denisenko, V.A.; Kirichuk, N.N.; Afiyatullov, S.S. Naphto-Γ-Pyrones from the Marine-Derived Fungus Aspergillus Foetidus. Nat. Prod. Res. 2021, 35, 131–134. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Sobolevskaya, M.P.; Gerasimenko, A.V.; Khudyakova, Y.V.; Kirichuk, N.N.; Mikhailov, V.V. New 3-[2′(R)-Hydroxybutyl]-7-Hydroxyphthalide from Marine Isolate of the Fungus Penicillium Claviforme. Chem. Nat. Compd. 2015, 51, 111–115. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; von Amsberg, G.; Afiyatullov, S.S. Metabolites of the Marine Fungus Aspergillus Candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem. Nat. Compd. 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Berdyshev, D.V.; Yurchenko, E.A.; Antonov, A.S.; Borkunov, G.V.; Kirichuk, N.N.; Chausova, V.E.; Kalinovskiy, A.I.; Popov, R.S.; Khudyakova, Y.V.; et al. Bioactive Polyketides from the Natural Complex of the Sea Urchin-Associated Fungi Penicillium Sajarovii KMM 4718 and Aspergillus Protuberus KMM 4747. Int. J. Mol. Sci. 2023, 24, 16568. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Min′ko, E.M.; Ivanets, E.V.; Afiyatullov, S.S. New Diorcinol J Produced by Co-Cultivation of Marine Fungi Aspergillus Sulphureus and Isaria Felina. Chem. Nat. Compd. 2016, 52, 227–230. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkova, E.M.; Savina, E.D.; Yurchenko, E.A.; Lesovaya, E.A. Marine-Derived Steroids for Cancer Treatment: Search for Potential Selective Glucocorticoid Receptor Agonists/Modulators (SEGRAMs). Mar. Drugs 2025, 23, 399. https://doi.org/10.3390/md23100399
Zhidkova EM, Savina ED, Yurchenko EA, Lesovaya EA. Marine-Derived Steroids for Cancer Treatment: Search for Potential Selective Glucocorticoid Receptor Agonists/Modulators (SEGRAMs). Marine Drugs. 2025; 23(10):399. https://doi.org/10.3390/md23100399
Chicago/Turabian StyleZhidkova, Ekaterina M., Ekaterina D. Savina, Ekaterina A. Yurchenko, and Ekaterina A. Lesovaya. 2025. "Marine-Derived Steroids for Cancer Treatment: Search for Potential Selective Glucocorticoid Receptor Agonists/Modulators (SEGRAMs)" Marine Drugs 23, no. 10: 399. https://doi.org/10.3390/md23100399
APA StyleZhidkova, E. M., Savina, E. D., Yurchenko, E. A., & Lesovaya, E. A. (2025). Marine-Derived Steroids for Cancer Treatment: Search for Potential Selective Glucocorticoid Receptor Agonists/Modulators (SEGRAMs). Marine Drugs, 23(10), 399. https://doi.org/10.3390/md23100399








