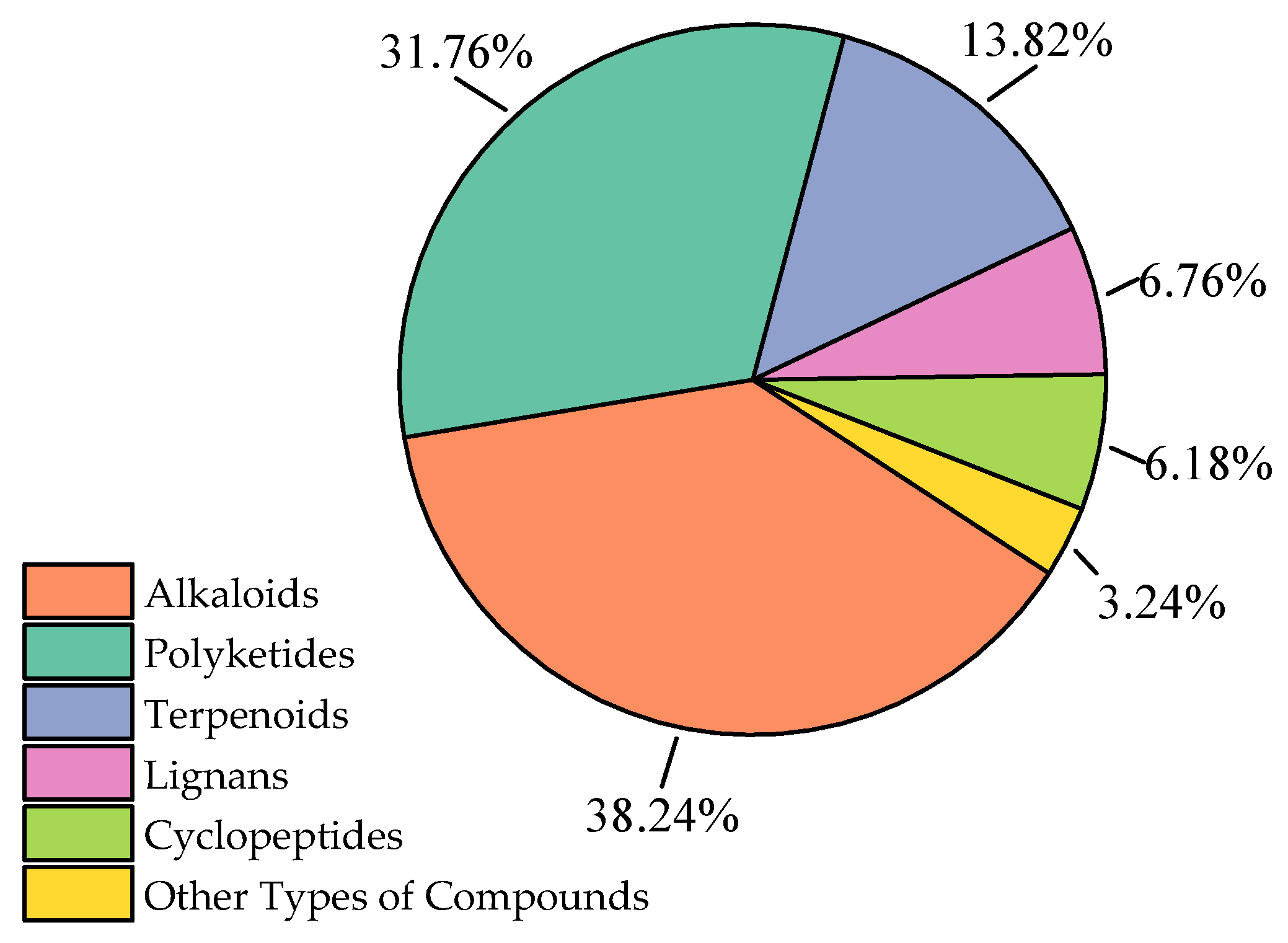
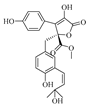
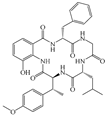
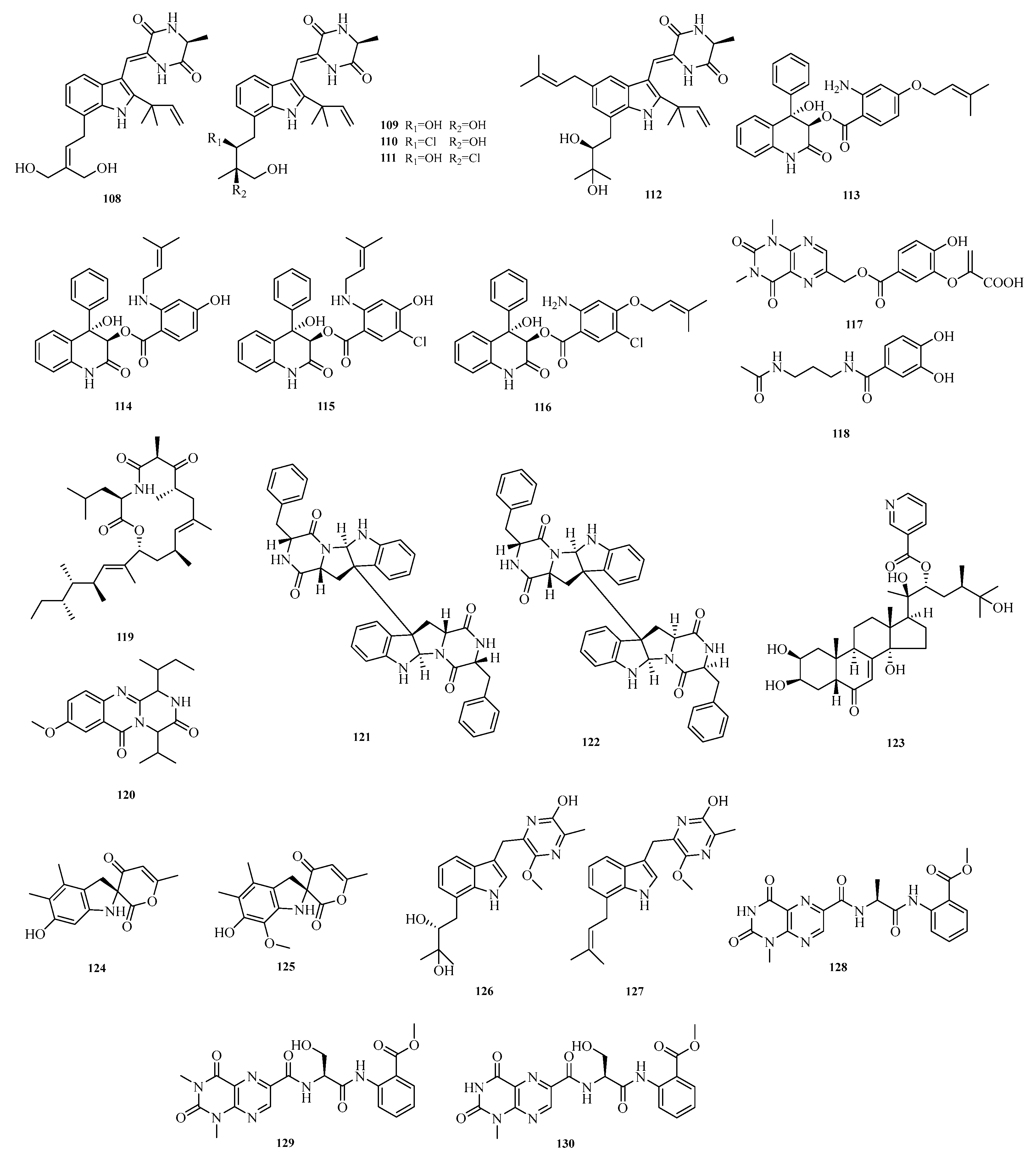2.1. Alkaloids
Alkaloids, nitrogen-containing organic compounds widely distributed in nature, have been increasingly identified from marine-derived
Aspergillus, in addition to plants, animals, and other microorganisms. Their structures typically feature nitrogen-containing cyclic cores—a characteristic that contributes to remarkable structural diversity, particularly among those isolated from marine
Aspergillus [
8]. Owing to their unique architecures shaped by the extreme marine environment, marine
Aspergillus-derived alkaloids exhibit prominent and diverse biological activities with significant pharmaceutical and agricultural potential [
9,
10,
11]. Such distinctive properties make marine Aspergillus-derived alkaloids a focal point in natural product chemistry and drug development.
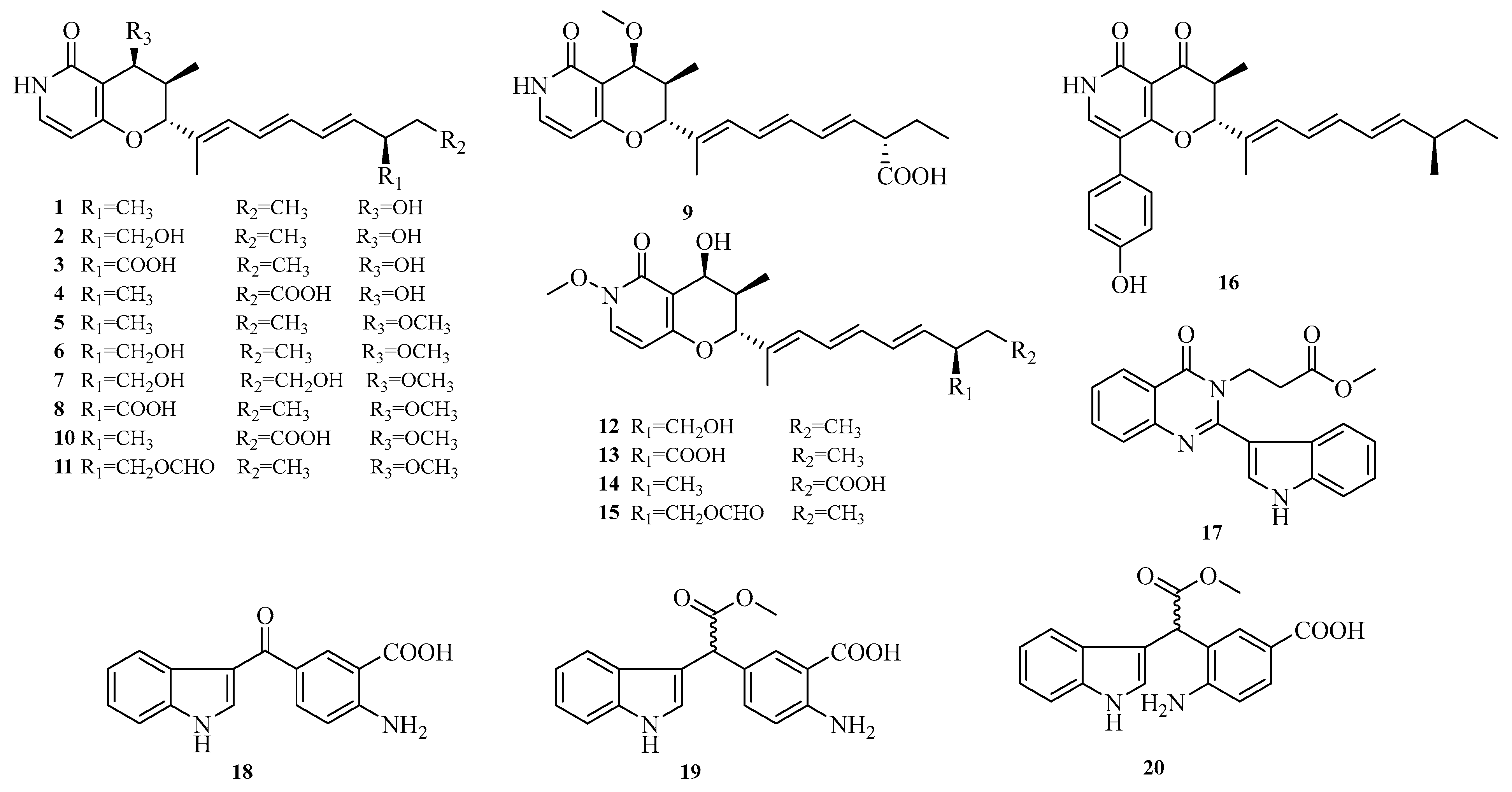
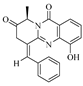
Wei-Chen Chen et al. isolated 16 undescribed pyranopyridone alkaloids, aculeapyridones A–P (
1–
16), from the co-culture extract of the mangrove-derived fungus
Aspergillus aculeatinus WHUF0198 and the mangrove-associated
Penicillium sp. DM27 through bioactivity-guided fractionation. Among them, compounds
12–
15 with unique N-methoxy groups were identified as activated products of fungal co-culture. The hepatoprotective activity of these compounds against acetaminophen-induced acute liver injury was evaluated in vitro. Results showed that compounds
1–
7,
9,
10, and
12–
15 significantly increased cell viability and reduced alanine aminotransferase (ALT) levels in acetaminophen-treated mouse hepatocytes at 5.0 μM or 10.0 μM [
12]. Additionally, Lai-Hui Dai et al. reported four new alkaloids (
17–
20) isolated from the culture of marine-derived
Aspergillus fumigatus AF1 [
9]. The structures of compounds
1–
20 are shown in
Figure 2.
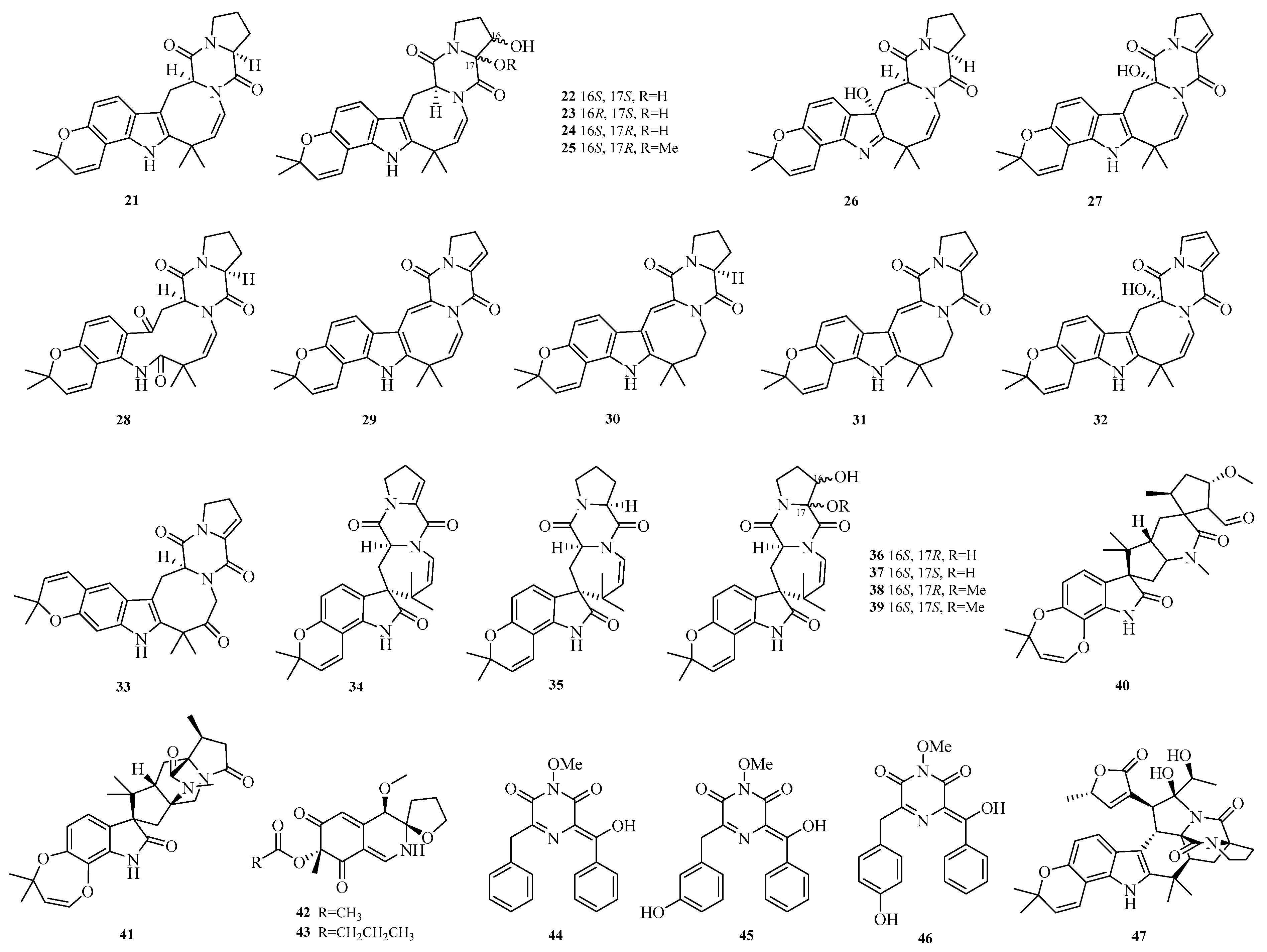
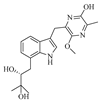
Jingshuai Wu et al. investigated the deep-sea sediment-derived fungus
Aspergillus puulaauensis F77 and successfully isolated
19 undescribed austamide-type diketopiperazines, named versicoines A–S (
21–
39). Compound
34 effectively reduced NO production and the expression of iNOS and COX-2 proteins in LPS-induced BV2 cells, suppressed LPS-triggered NF-κB signaling pathway and subsequent NLRP3 inflammasome activation. Compounds
22,
23,
36, and
37 exhibited mild cytotoxicity with cell viability rates of 70.0–75.0% [
13].
Philomina Panin Edjah et al. isolated two new paralectins (PHQ), aculeaquamides B and C (
40–
41), from the co-culture of mangrove-derived
Aspergillus aculeatinus WHUF0198 and mangrove-associated
Penicillium sp. DM27 [
14]. Yao-Yao Zheng et al. studied the sea hare-derived fungus
Aspergillus terreus RA2905 and identified two alkaloids, azasperones E and F (
42–
43) [
15]. Sarani Kankanamge et al. cultured the Australian marine sediment-derived fungus
Aspergillus noonimiae CMB-M0339 and obtained rare 2,6-diketopiperazine alkaloids noonazines A–C (
44–
46) [
16]. Zheng-Biao Zou et al. isolated a rare stephacidin-asperochratide hybrid, stephaochratidin A (
47), from deep-sea
Aspergillus ochraceus. Activity assays showed that stephaochratidin A (
47) significantly inhibited ferroptosis with an EC
50 value of 15.4 μM, acting by downregulating heme oxygenase 1 (HMOX-1) expression and suppressing lipid peroxidation [
17]. The structures of compounds
21–
47 are shown in
Figure 3.
Zhibo Hu et al. isolated four diketomorpholine alkaloids (
48–
51) and one indole diketopiperazine alkaloid (
52) from the seagrass-derived
Aspergillus alabamensis SYSU-6778. Compounds
48 and
49 exhibited potent inhibitory activity against the fish pathogen
Edwardsiella ictalurid, with minimum inhibitory concentrations (MICs) of 10.0 μM [
18]. Yura Ha et al. obtained phthalimidinic acid A (
53) and phthalimidinic acid B (
54) from the marine sediment-derived
Aspergillus sp. ZZ1861. Both compounds showed antifungal activity against
Candida albicans, with MIC values of 1.6 and 3.1 μM, respectively [
19].
Geng-Si Zhang et al. isolated the new natural products secofumitremorgins C (
55) and D (
56) from the salt pan-derived
Aspergillus fumigatus GXIMD00544. Activity assays showed that compound
55 exhibited antifungal spore germination activity against
Fusarium sacchari-related plant pathogenic fungi, with a 53.0% inhibition rate at 100.0 μM. Additionally, compound
55 demonstrated antifouling potential against
Balanus amphitrite larval settlement, achieving a 96% inhibition rate at 100.0 μM [
20]. Harol Ricardo Arias Cardona et al. reported an undescribed isoprenylated indole derivative, hydroxyhomamide (
57), from the marine sponge-associated fungus
Aspergillus fischeri MMERU 23 [
21]. Bingying Tang et al. isolated a new ascandinine T (
58) from the Antarctic sponge-derived fungus
Aspergillus candidus HDN15-152 [
22]. The structures of compounds
48–
58 are shown in
Figure 4.
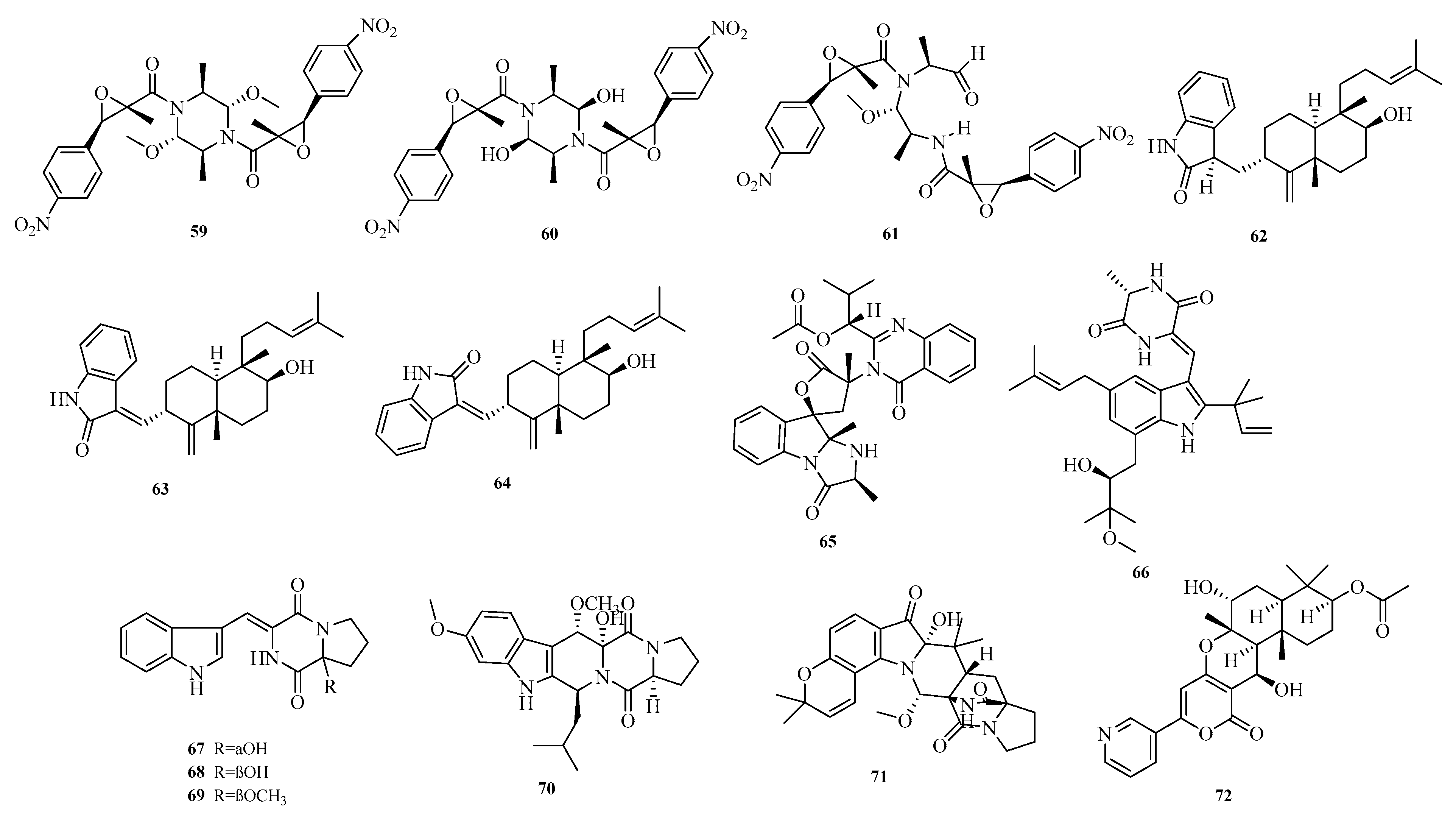
Xiaomei Huang et al. isolated three dimeric nitrobenzyl trans-epoxyamides (
59–
61) from the culture of deep-sea-derived
Aspergillus terreus MCCC M28183. Activity assays showed that compound
59 exhibited moderate inhibitory activity against human gastric cancer cell line MKN28 with an IC
50 value below 10.0 μM [
23]. Hao-Yu Yu et al. reported three new oxyindole diterpenoid alkaloids, emeniveol B–D (
62–
64), from the marine sediment-derived
Aspergillus sp. MCCC 3A00392 [
24]. Elisa Doro-Goldsmith et al. isolated a novel tryptophan derivative, 12
S-deoxynorquinoline (
65), from the marine ascidian-derived fungus
Aspergillus clavatus AS-107 [
25].
Dina H El-Kashef et al. isolated a new isoprenylated indole diketopiperazine alkaloid, rubrumline P (
66), from the fermented culture of
Aspergillus chevalieri, a marine sediment-derived fungus collected at a depth of 15 m near the Lighthouse of Dahab, Red Sea, Egypt. Compound rubrumline P (
66) was confirmed to exhibit cytotoxic activity against PANC-1 cancer cells with an IC
50 value of 25.8 μM. Although the underlying mechanism remains elusive, cell cycle analysis showed a slight increase in the sub-G1 peak following treatment with compound
66 [
26]. Cangzhu Sun et al. emphasized the importance of fungi as a source of novel bioactive natural products and isolated asperindopiperazines A–C (
67–
69) from Mariana Trench-associated
Aspergillus sp. SY2601 [
27]. Yi-Hao Che et al. isolated a novel diketopiperazine derivative, 8
R-methoxy-9
R-hydroxy-fumigaclavine C (
70), from
Aspergillus fumigatus CYH-5 collected from a seahorse cold seep [
28].
Yu Chen et al. isolated and identified a new derivative, aspertaichamide A (
71), from the endophytic fungus
Aspergillus taichungensis 299 derived from the marine red alga
Gelidium amansii. In vitro cytotoxicity assays showed that new compound
71 reduced AGS cell viability in a concentration-dependent manner, with an IC
50 value of 1.7 μM. Further studies indicated that
71 might induce programmed cell death in AGS cells via an apoptotic pathway [
29]. Zhu Chen et al. obtained a new alkaloid, pyripyropene U (
72), from the marine sponge-derived
Aspergillus sp. SCSIO41420 [
30]. The structures of compounds
59–
72 are shown in
Figure 5.
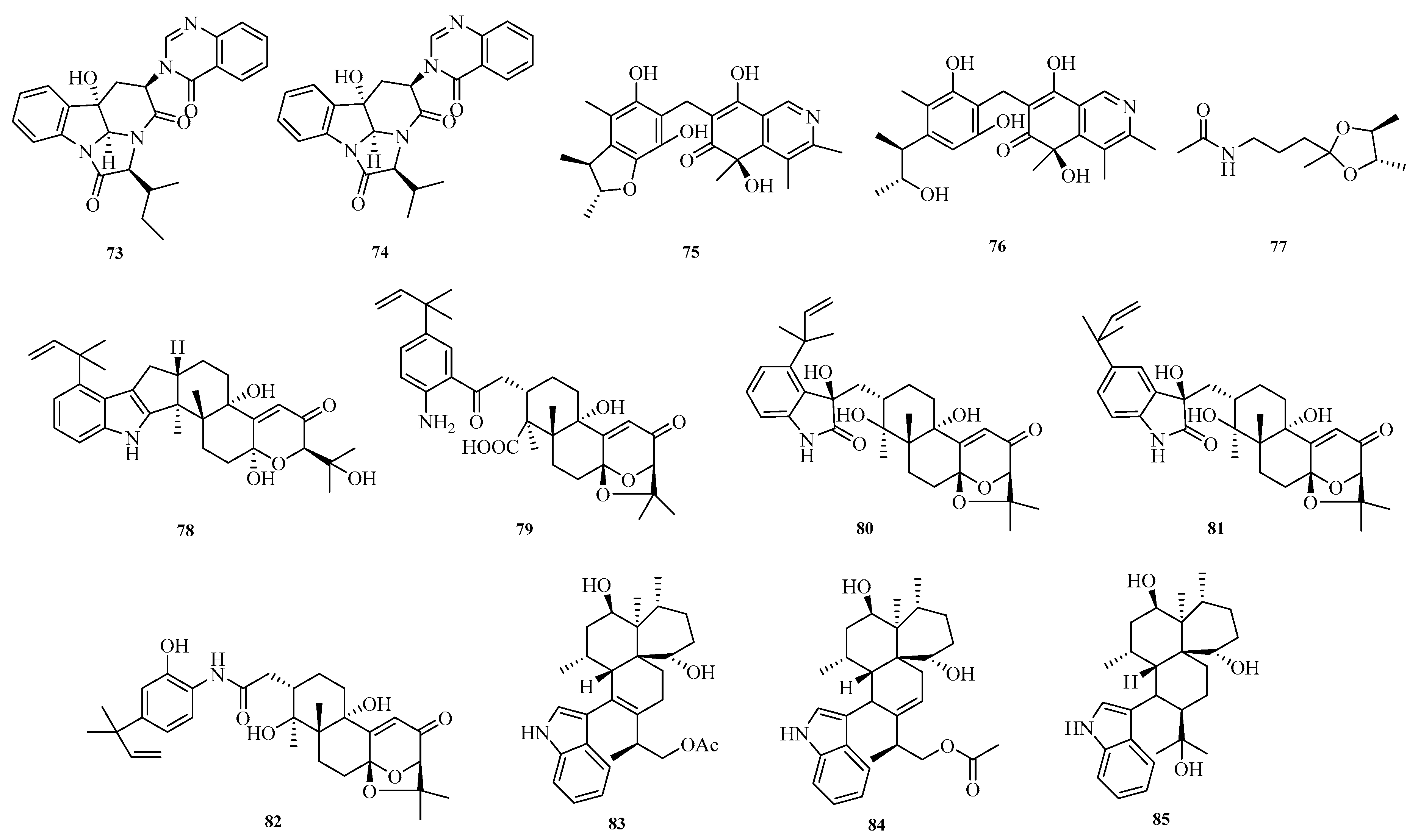
Mangaladoss Fredimoses et al. isolated two new alkaloids, chaetominines A (
73) and B (
74), from the marine sponge-derived fungus
Aspergillus versicolor SCSIO XWS04 F52. Activity assays showed that compounds
73 and
74 exhibited cytotoxic activity against leukemia K562 and colon cancer SW1116 cells, with half-maximal inhibitory concentration (IC
50) values ranging from 7.5 to 12.5 μM [
31]. Shui-Hua Lin et al. conducted a systematic chemical study on the deep-sea-derived fungus
Aspergillus versicolor 170217, isolating three new alkaloids: citriquinolinone A–B (
75–
76) and
N-(3-((2′
S,3′
S)-2′,3′,5′-trimethyl-1,3-dioxolan-2-yl)propyl)acetamide (
77) [
32].
Fei Zhang et al. isolated and identified the fungus
Aspergillus sp. ZF-104 from marine soft corals collected in Haikou Bay, China. Eight undescribed indole-diterpenoid alkaloids, penerpenes O–V (
78–
85), were isolated and characterized from this strain. The inhibitory activity of these compounds against protein tyrosine phosphatase 1B (PTP1B) was evaluated. In the PTP1B inhibition assay, compounds
78,
79, and
84 showed activities comparable to that of the positive control [
33]. The structures of compounds
73–
85 are shown in
Figure 6.
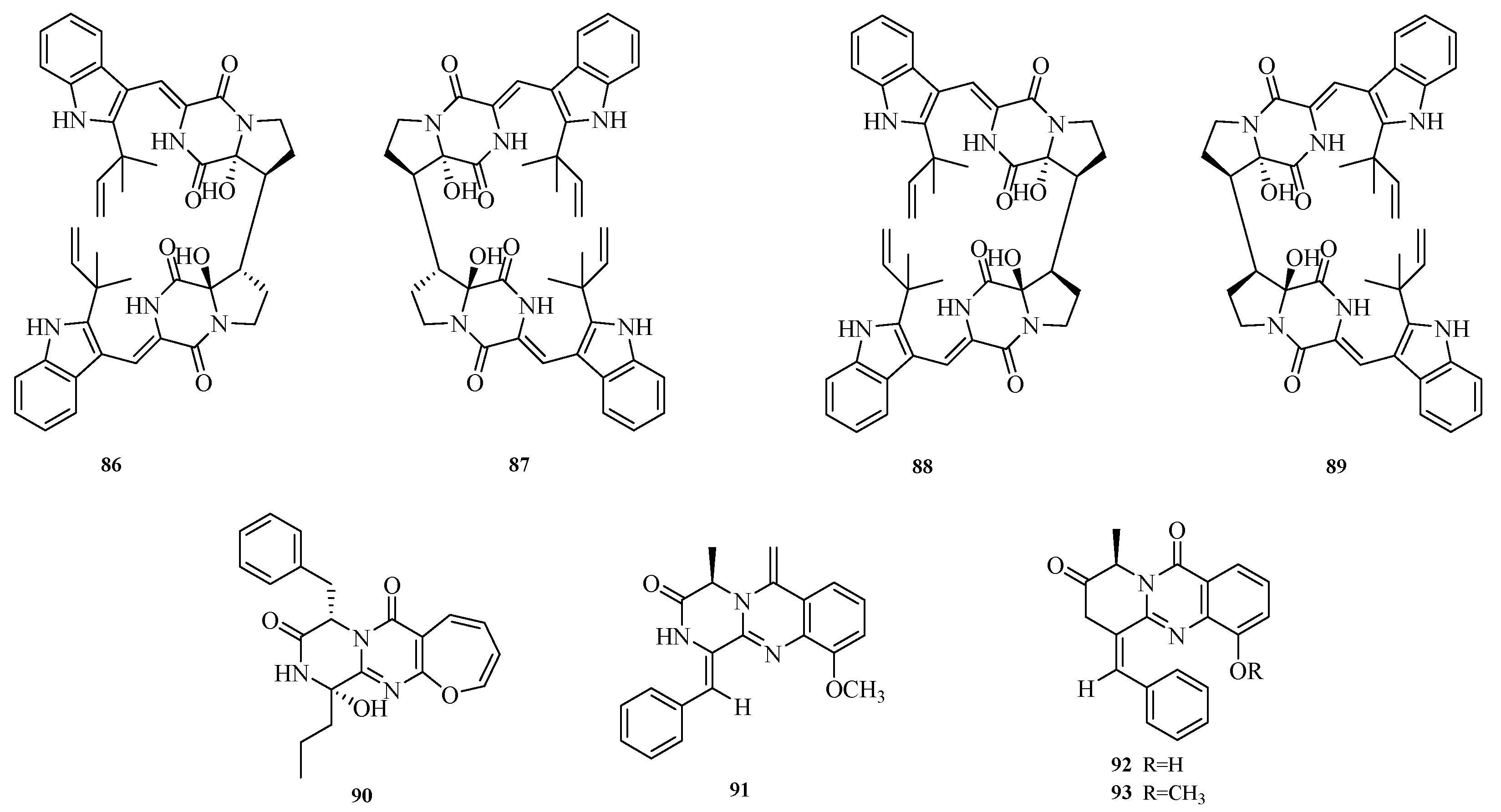
Ying-Jie Zhao et al. obtained two pairs of new dimeric diketopiperazine alkaloids, (±)-dibrevianamides Q1 and Q2 ((±)-1 and (±)-2) (
86–
89), from marine-derived
Aspergillus sp. Activity assays showed that compounds
86 and
89 exhibited resistance to H1N1 virus with half-maximal inhibitory concentration (IC
50) values of 12.6 and 19.5 μM, respectively. Compound
86 also demonstrated significant activity against
Mycobacterium tuberculosis with a minimum inhibitory concentration (MIC) of 10.2 μM [
34]. Qi Hong et al. conducted a secondary metabolite study on the filamentous fungus
Aspergillus puniceus FAHY0085 isolated from a South China Sea coral sample, isolating four undescribed alkaloids, including an oxyepin-containing diketopiperazine-type alkaloid (
90) and three 4-quinazolone alkaloids (
91–
93). The transcriptional activation of liver X receptor α (LXRα) by the isolated compounds was evaluated. Results showed that the new diketopiperazine puniceloid F (
92) exhibited significant transcriptional activation activity against LXRα with half-maximal effective concentration (EC
50) values ranging from 2.0 to 15.0 μM [
35]. The structures of compounds
86–
93 are shown in
Figure 7.
Komal Anjum et al. isolated a novel alkaloid with a pyridoindole hydroxymethylpiperazine dione structure, aspergill alkaloid A (
94), from the deep-sea-derived fungus
Aspergillus sp. HDN20-1401. Antibacterial assays showed that aspergill alkaloid A (
94) exhibited inhibitory activity against Bacillus cereus with a minimum inhibitory concentration (MIC) of 12.5 μM [
36]. Yu-Liang Dong et al. isolated multiple compounds from
Aspergillus versicolor AS-212, an endophyte derived from the deep-sea coral
Hemicorallium cf.
imperiale collected from the Magellan Seamounts in the Western Pacific. The isolation yielded four new oxazine-based pyrimidine alkaloids, versicoxepines A–D (
95–
98), and two quinolinone analogs: 3-hydroxy-6-methoxy-4-phenylquinolin-2(1
H)-one (
99) and 3-methoxy-6-hydroxy-4-phenylquinolin-2(1
H)-one (
100). Activity tests showed that compound
99 exhibited antibacterial activity against aquatic pathogens
Vibrio harveyi and
V. alginolyticus with an MIC of 8.0 μM [
37].
Yu-Liang Dong et al. isolated and identified two new quinazoline diketopiperazine alkaloids, versicomide E (
101) and cottoquinazoline H (
102), from the endophytic fungus
Aspergillus versicolor AS-212 associated with deep-sea corals. In antibacterial assays, compound
102 exhibited inhibitory effects against
Vibrio harveyi and
Vibrio parahaemolyticus with an MIC of 9.0 μM [
38].
Jun-Qiu Mao et al. studied the marine-derived fungus
Aspergillus sclerotiorum ST0501 from the South China Sea, from which three new alkaloids, sclerotioloids A–C (
103–
105), were obtained. Sclerotioloid B (
104) showed inhibition of LPS-induced NO production, with an inhibition rate 28.9% higher than that of dexamethasone (25.9%) [
39]. Jin-Shan Hu et al. isolated two new indolediketopiperazine alkaloids (IDAs), namely (+)-19-epi-sclerotiamide (
106) and (−)-19-epi-sclerotiamide (
107), from the epiphytic fungus
Aspergillus versicolor CGF9-1-2 associated with soft corals [
40]. The structures of compounds
94–
107 are shown in
Figure 8.
Li-Hong Yan et al. isolated and characterized five new antibacterial indolediketopiperazine alkaloids from a deep-sea cold seep-derived
Aspergillus chevalieri, namely 24,25-dihydroxyvariecolorin G (
108), 25-hydroxyrubrumazine B (
109), 22-chloro-25-hydroxyrubrumazine B (
110), 25-hydroxyvariecolorin F (
111), and 27-epi-aspechinulin D (
112). Activity assays showed that compounds
108–
112 exhibited inhibitory activity against various pathogens with minimum inhibitory concentration (MIC) values ranging from 4.0 to 32.0 μM [
41]. Zhibo Hu et al. isolated and identified six new benzoic acid-containing alkaloids from seagrass-derived
Aspergillus candidus, namely asperalins A–D (
113–
116), asperaluhalazine A (
117), and
N-(3-acetamidopropyl)-3,4-dihydroxybenzamide (
118). Compounds
115 and
116 showed strong activity against
Staphylococcus aureus,
Streptococcus iniae, and
Streptococcus parauberis, with MIC values of 10.1, 5.0 and 10.1 μM, respectively [
42].
Florent Magot et al. isolated 77 microbial strains from the seafloor at a depth of 2454 m in the Fram Strait, Arctic Ocean. Using the one-strain-many-compounds (OSMAC) cultivation method, they isolated a new polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid macrolide, heteroamidin A (
119), and a new quinazoline, (−)-isocarbonolide A (
120) [
43]. Xinyang Li et al. isolated two new dimeric diketopiperazine stereoisomers (
121–
122) from the culture broth of an
Aspergillus strain derived from the intestine of Lip Tarico [
44]. Zhong-Hui Huang et al. isolated one previously undescribed compound, punicesterones A (
123), from the deep-sea-derived fungal strain
Aspergillus puniceus SCSIO z021 [
45].
Yao-Yao Zheng et al. conducted a chemical study on the marine sediment-derived fungus
Aspergillus terreus PPS1, successfully isolating and identifying seven previously undescribed alkaloids, namely asperspiroids A and B (
124–
125), astepyrazinol C (
126); two luhalazine peptides, scytalols C and D (
127–
128); and scytalols E and F (
129–
130). Activity evaluation results showed that astepyrazinol C (
126) exhibited significant inhibitory activity against lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW264.7 macrophages, with an inhibition rate of 37.4% at 20.0 μM [
46]. The structures of compounds
108–
130 are shown in
Figure 9.
The sources and biological activities of compounds
1–
130 are summarized in
Table 1.
2.2. Polyketides
Polyketide compounds are a family of natural products synthesized by polyketide synthases (PKSs). Their carbon skeletons are formed through modular extension of acetyl/malonyl units [
47]. Depending on the type of PKS (Type I, II, or III), these compounds can form linear, cyclic, or highly modified complex structures, including macrolides (e.g., erythromycin), aromatic polyketides (e.g., tetracycline), and polyethers (e.g., amphotericin B). Their structural diversity arises from combinations of post-modification reactions such as ketone reduction, cyclization, and methylation. These compounds exhibit broad-spectrum biological activities.
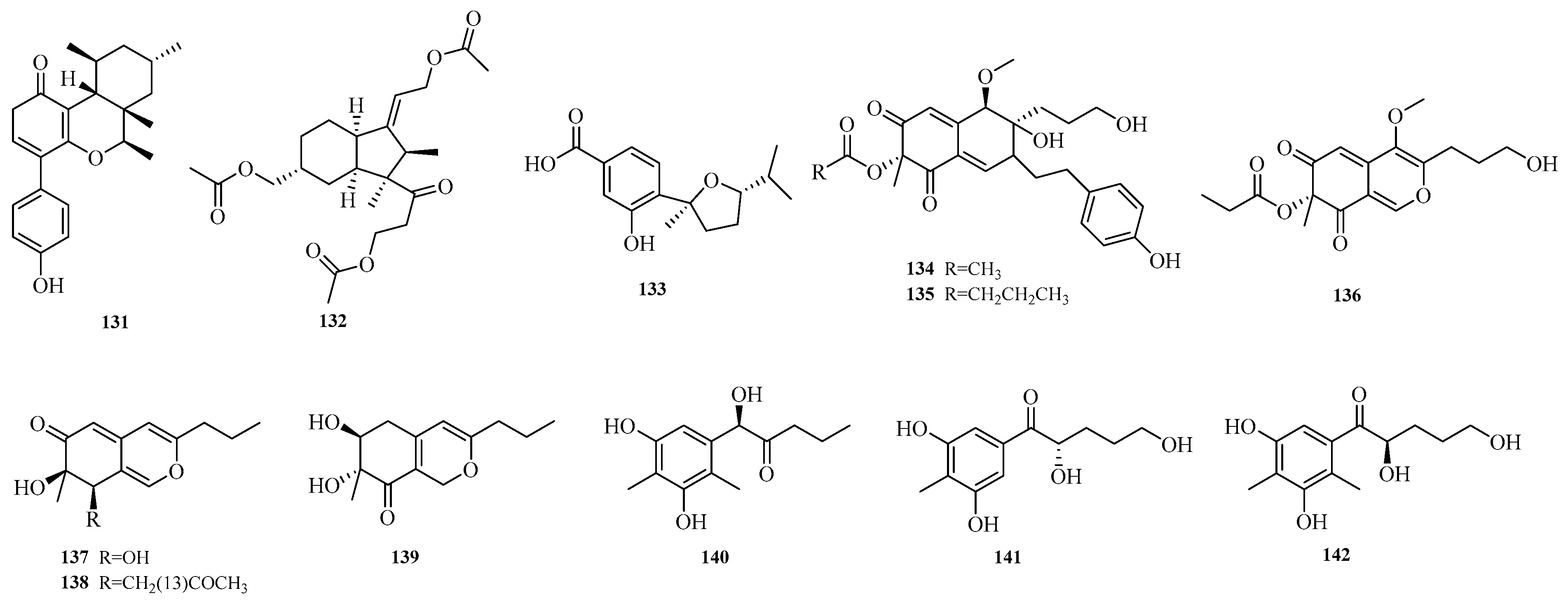
Wei-Chen Chen et al. isolated one undescribed polyketide, aculeapyridones Q (
131), from the co-culture extract of mangrove-derived fungus
Aspergillus aculeatinus WHUF0198 and mangrove-associated fungal
Penicillium sp. DM27 via bioactivity-guided fractionation [
12]. Yue Jiang et al. isolated asperhydrindane A (
132) from the mangrove-derived fungus
Aspergillus terreus GXIMD 03,158 [
48]. Xu-Meng Ren et al. isolated (7
R,10
R)-11-dehydroxy-iso-10-hydroxysydowic acid (
133) from the deep-sea-derived fungus
Aspergillus sydowii DFFSCS007 [
49]. Yao-Yao Zheng et al. conducted a study on the sea hare-derived fungus
Aspergillus terreus RA2905, identifying nine new polyketide compounds, namely azasperones C–D, G–J (
134–
139) and preazasperones A–C (
140–
142) [
15]. The structures of compounds
131–
142 are shown in
Figure 10.
Yu-Pei He et al. conducted a study on the marine fungus
Aspergillus versicolor CGF9-1-2, aiming to discover undescribed compounds. They successfully isolated four new polyketides, including decumbenone E (
143), decumbenone F (
144), 2′-epi-8-
O-methylnidurufin (
145), and (−)-phomoindene A (
146). In vitro screening for TDP1 inhibitory activity of all isolated compounds showed that compound
145 exhibited weak inhibitory activity against TDP1 with an IC
50 value of 33.00±5.10 μM [
50]. Ailiman Abulaizi et al. isolated a marine-derived fungal strain
Aspergillus sp. ITBBc1 from corals collected in the South China Sea, Hainan Province. In-depth chemical investigation of the fermented extract of this strain yielded four new secondary metabolites (
147–
150), named megastigmanones A-C and prenylterphenyllin H [
51]. Sarani Kankanamge et al. performed culture analysis on
Aspergillus noonimiae CMB-M0339, a fungus derived from Australian marine sediments, obtaining a new aza-nonaketide, noonaphilone A (
151) [
16].
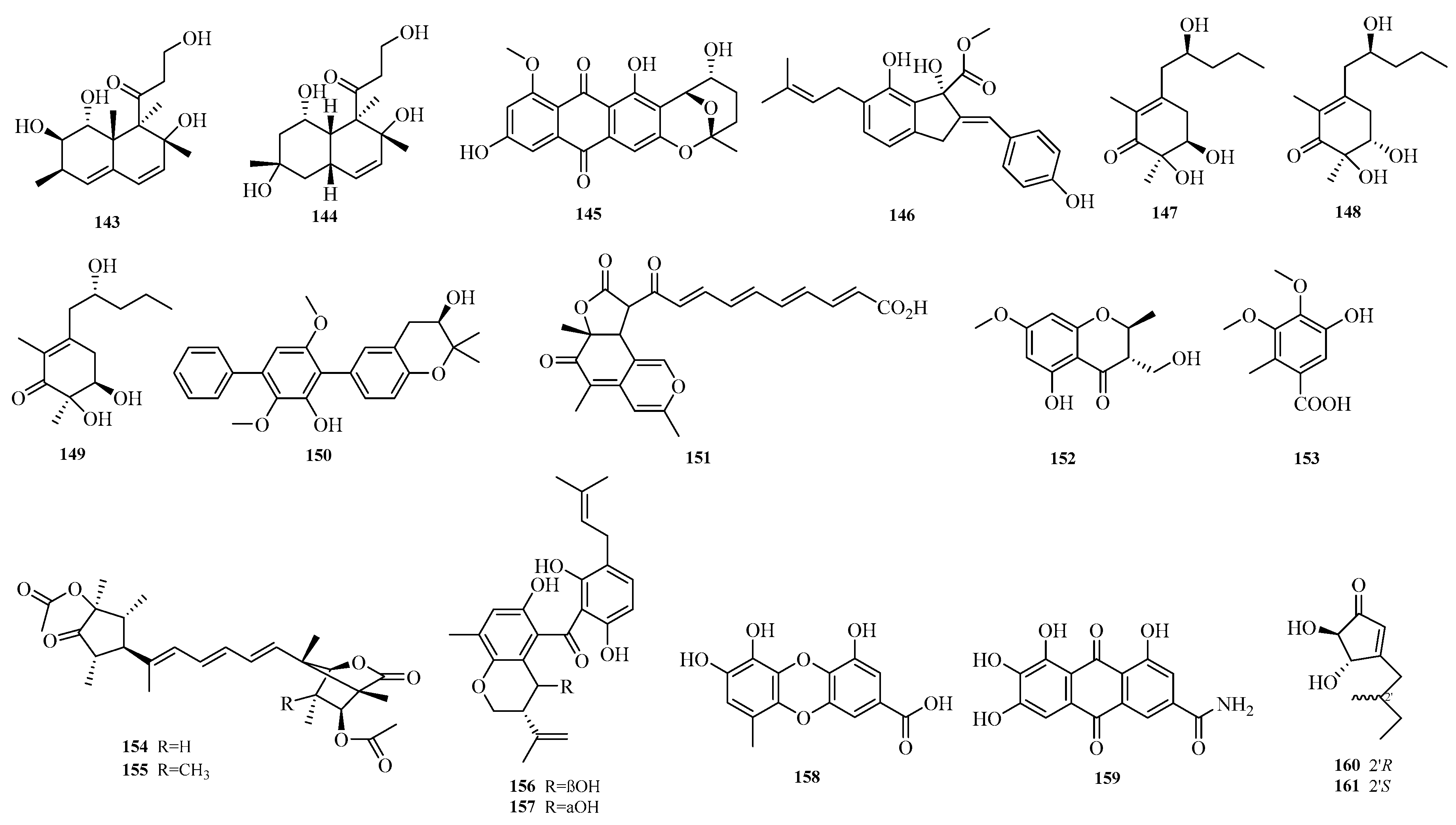
Zhibo Hu et al. isolated one chromone (
152) and one benzoic acid derivative (
153) from the seagrass-derived
Aspergillus alabamensis SYSU-6778 [
18]. Yura Ha et al. obtained emericelactones F and G (
154–
155), 20
R,25
S-preshamixanthone (
156), 20
R,25
R-preshamixanthone (
157), aspergilol G (
158), and 2-hydroxyemodic amide (
159) from the marine sediment-derived
Aspergillus sp. ZZ1861. Aspergilol G (
158) and 2-hydroxyemodic amide (
159) exhibited antifungal activity against Candida albicans with minimum inhibitory concentration (MIC) values of 1.6 and 3.1 μM, respectively [
19]. Guang-Yu Zhang et al. identified terreins A and B (
160–
161) from the coral-derived fungus
Aspergillus terreus [
52]. The structures of compounds
143–
161 are shown in
Figure 11.
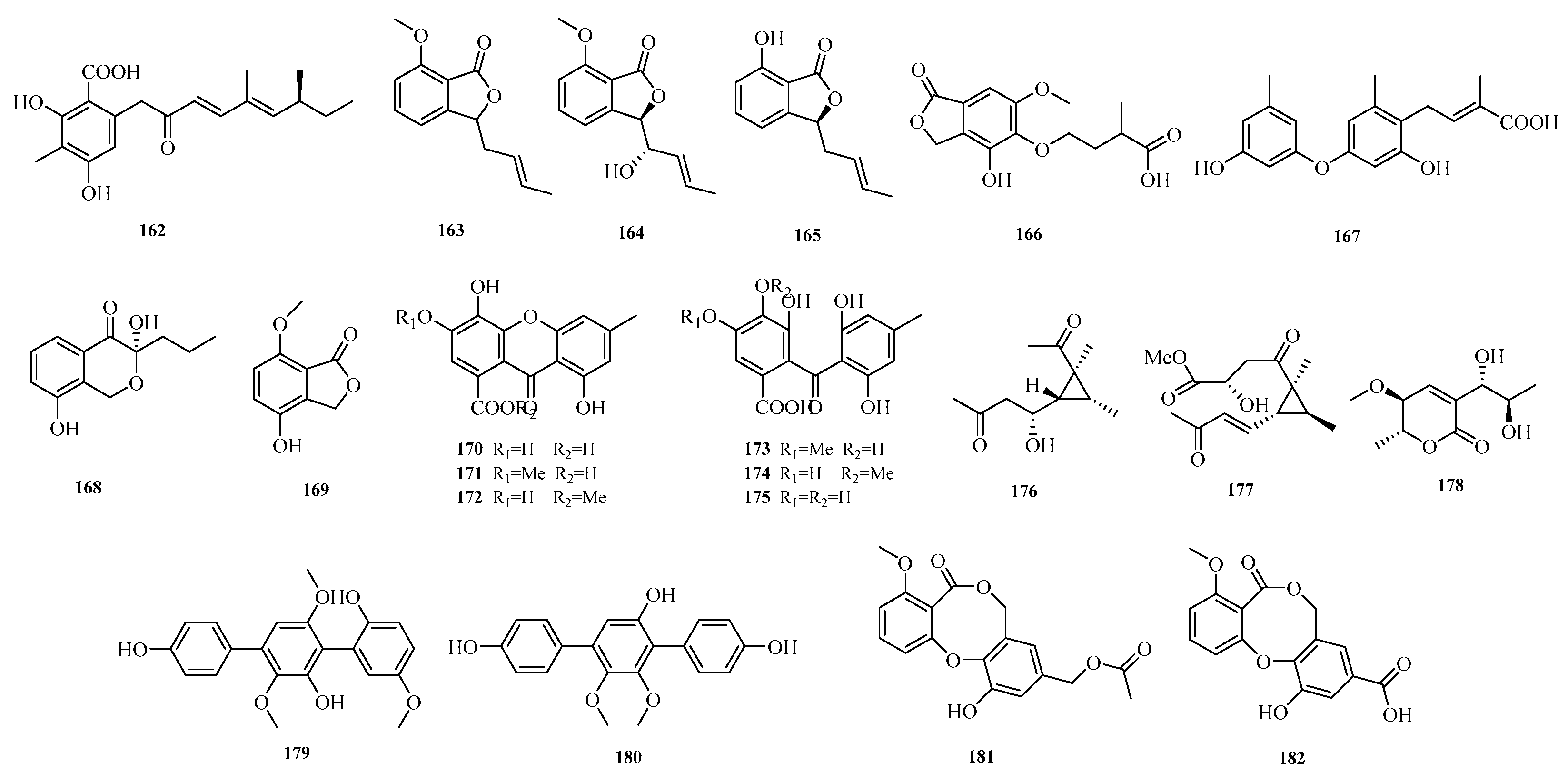
Yi-Hao Che et al. isolated seven new phenol derivatives, namely subversins A–E (
162–
166), subversic acid A (
167), and epi-wortmannine G (
168), as well as one new natural product 4-hydroxy-7-methoxyphthalide (
169), from the fungus
Aspergillus subversicolor CYH-17 collected from a seahorse cold seep [
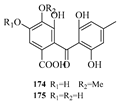
53]. Chun-Ju Lu et al. isolated six benzophenone derivatives, carneusones A–F (
170–
175), from the marine sponge-derived fungal strain
Aspergillus carneus GXIMD00543. Using lipopolysaccharide (LPS)-induced RAW 264.7 cells, they evaluated the effect of these compounds on nitric oxide (NO) secretion. The results showed that compounds
174 and
175 exhibited moderate anti-inflammatory activity with half-maximal effective concentration (EC
50) values of 34.6 ± 0.9 and 20.2 ± 1.8 μM, respectively [
54]. Hao-Yu Yu et al. isolated two new polyketides, hamavellone C and (+)-Stagonospone A (
176-
177), from the marine sediment-derived
Aspergillus sp. MCCC 3A00392. Highlighting the importance of fungi as a source of novel bioactive natural products [
24]. Cangzhu Sun et al. isolated 5-methoxy-8,9-dihydroxy-8,9-deoxyaspyrone (
178) from the Mariana Trench-related
Aspergillus sp. SY2601 [
27].
Yanbo Zeng et al. obtained two undescribed compounds, asperterphenylcins A–B (
179–
180), and another two undescribed compounds, asperdiphenylcins A–B (
181–
182), from the marine-derived fungus
Aspergillus candidus HM5-4 isolated from a South China Sea sponge. Activity assays showed that compound
179 exhibited strong inhibitory activity against
Neoscytalidium dimidiatum, with an inhibition zone diameter of 31.7 ± 2.6 mm at a concentration of 10.0 μg/disk; compound
180 displayed potent inhibitory activity against
α-glucosidase, with an IC
50 value of 1.3 ± 0.2 μM [
55]. The structures of compounds
162–
182 are shown in
Figure 12.
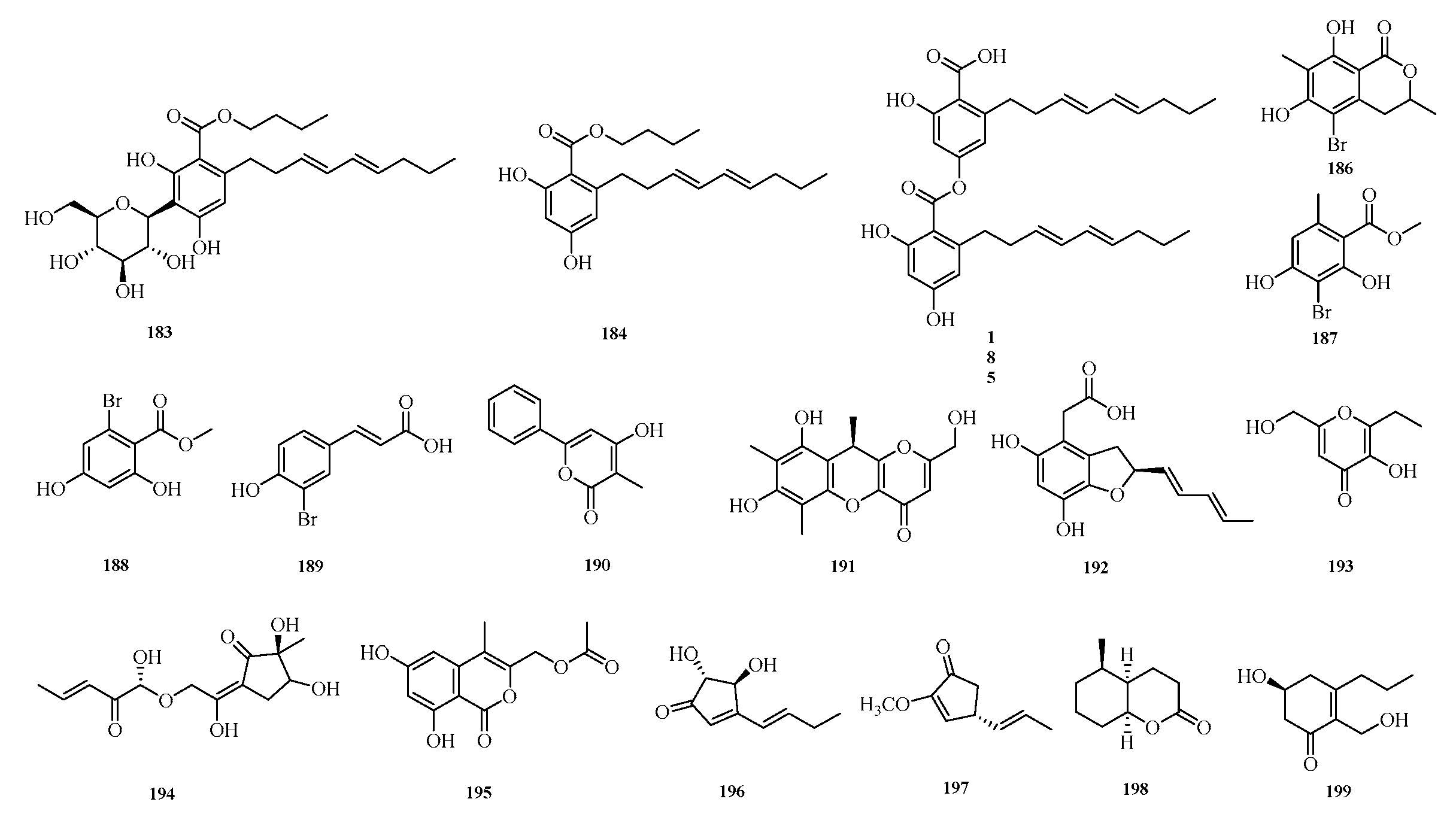
Jingjing Xue et al. successfully isolated three phenolic compounds, namely carnemycin H–I (
183–
184) and stromemycin B (
185), from secondary metabolites of a marine-derived
Aspergillus strain. Antibacterial activity evaluation of the isolated compounds against
Ralstonia solanacearum (bacterial wilt pathogen) showed that compound
185 exhibited excellent inhibitory activity with a minimum inhibitory concentration (MIC) of 3.0 μM, which was comparable to that of streptomycin sulfate. Furthermore, compound
185 significantly altered the morphology of
R. solanacearum and inhibited the activity of succinate dehydrogenase (SDH), thereby interfering with the growth of R. solanacearum [
7].
Chao Li et al. conducted a study on the starfish-derived fungus
Aspergillus sp. WXF1904, isolating one new brominated isocoumarin, namely 5-bromo-6,8-dihydroxy-3,7-dimethylisocoumarin (
186), as well as four new natural products: methyl 3-bromo-2,4-dihydroxy-6-methylbenzoate (
187), methyl 2-bromo-4,6-dihydroxybenzoate (
188), (
E)-3-(3-bromo-4-hydroxyphenyl)acrylic acid (
189), and 4-hydroxy-3-methyl-6-phenyl-2
H-pyran-2-one (
190). Evaluation of the acetylcholinesterase and pancreatic lipase inhibitory activities of these compounds showed that the new compound
186 exhibited weak inhibitory activity against acetylcholinesterase, while compounds
187 and
190 displayed weak inhibitory activity against pancreatic lipase [
56].
Ying Chen et al. conducted a study on the coral-derived fungus
Aspergillus austwickii SCSIO41227 from the Beibu Gulf, obtaining three previously uncharacterized compounds, asperpentenones C–E (
191–
193). Bioassay results showed that compound
191 exhibited significant NA inhibitory activity with a half-maximal inhibitory concentration (IC
50) of 31.3 μM, while compound
192 displayed weak inhibitory activity against PL [
57]. Mangaladoss Fredimoses et al. isolated a new oxytetracycline derivative (
194) from the marine sponge-derived fungus
Aspergillus versicolor SCSIO XWS04 F52 [
31]. Shui-Hua Lin et al. performed a systematic chemical investigation on the deep-sea-derived fungus
Aspergillus versicolor 170217, isolating (6,8-dihydroxy-4-methyl-1-oxo-1
H-isochromen-3-yl)methyl (
195) [
32].
Youmin Ying et al. isolated the fungus
Aspergillus terreus F6-3 from the body surface of Johnius belangerii collected from the coastal waters of Hainan Province, China. From this fungal strain, they identified two previously undescribed compounds, asperterreinones A–B (
196–
197), and one new compound, (±)-asperterreinin A (
198) [
58]. Zhibo Hu et al. isolated a new cyclohexanone derivative, insuetone A (
199), from the seagrass-derived fungus
Aspergillus insuetus SYSU6925. Activity assays showed that compound
199 exhibited weak to moderate antifungal activity against four phytopathogenic fungi, with minimum inhibitory concentration (MIC) values in the range of 50.0 μM [
59]. The structures of compounds
183–
199 are shown in
Figure 13.
Weibo Zhao et al. isolated three new phenolic compounds, namely epicocconigrones C–D (
200–
201) and flavimycin C (
202), from the fermentation culture of a deep-sea sediment-derived fungus
Aspergillus insulicola. Evaluation of their
α-glucosidase inhibitory activity showed that compound
200 exhibited strong inhibitory effect on
α-glucosidase with a half-maximal inhibitory concentration (IC
50) of 17.0 μM, which was significantly higher than that of the positive control acarbose (IC
50 = 823.0 μM). This indicates that compound
200 has the potential to be a promising lead compound for new hypoglycemic drugs [
60]. Jun Wu et al. isolated five new dimeric tetrahydroanthraquinones, aculeaxanthones A–E (
203–
207), from the fungus
Aspergillus aculeatinus WHUF0198. Compound
205 showed cytotoxicity against the Bel-7402 cell line (IC
50 = 2.0 μM) [
61].
Yuanli Li et al. successfully obtained seven new phenolic bisabolane sesquiterpenoids (
208–
214) from the deep-sea-derived fungus
Aspergillus versicolor YPH93. Evaluation of the effects of all compounds on ferroptosis showed that compound
214 exerted an inhibitory effect on erastin/RSL3-induced ferroptosis, with a half-maximal effective concentration (EC
50) in the range of 2.0 to 4.0 μM [
62]. The structures of compounds
200–
214 are shown in
Figure 14.
Xin Qi et al. conducted a study on the sponge-derived fungus
Aspergillus sp. SCSIO41315, from which they isolated 21 new terphenyl derivatives, namely asperterphenyls A-N and the enantiomers of asperterphenyls B–H (
215–
235). Activity assays revealed that asperterphenyl A (
215) exhibited neuraminidase inhibitory activity with a half-maximal inhibitory concentration (IC
50) of 1.8 ± 0.5 μM, and it could effectively inhibit infections by various H1N1 virus strains with IC
50 values ranging from 0.7 ± 0.3 to 1.5 ± 0.6 μM. Its mechanism of action involves reducing virus plaque formation in a dose-dependent manner, indicating that asperterphenyl A (
215) holds potential as a promising antiviral compound in the pharmaceutical field [
63].
Baiq Nila Sari Ningsih et al. isolated a new nonapeptide enantiomer, ent-epiheveadride (
236), from the marine-derived fungus
Aspergillus chevalieri PSU-AMF79. Activity assays showed that compound
236 exhibited antifungal activity against
Cryptococcus neoformans ATCC90113 (flucytosine-resistant) and
Candida albicans NCPF3153, with minimum inhibitory concentration (MIC) values of 128.0 μM and 200.0 μM, respectively [
64]. Ze’en Xiao et al. isolated a new anthraquinone, asperquinone A (
237), from the mangrove endophytic fungus
Aspergillus sp. 16-5C [
65]. Xin Qi et al. isolated a new glyoxylate-containing benzene derivative, 2-(4-hydroxy-3-(3′-methyl-2′-butenyl)phenyl)-2-oxoacetate (
238), from the marine alga-derived fungus
Aspergillus sp. SCSIO 41,304 [
66]. The structures of compounds
215–
238 are shown in
Figure 15.
The sources and biological activities of compounds
131–
238 are summarized in
Table 2.
2.3. Terpenoids
Terpenoids are naturally occurring organic compounds widely distributed in nature. Composed of covalently linked isoprene units, they form unique molecular skeletons with remarkable structural diversity; they are further classified by the number of isoprene units into categories such as monoterpenes, sesquiterpenes, and diterpenes. These compounds are primarily derived from plants, though some are also biosynthesized by microorganisms and marine organisms. Endowed with unique properties and broad application prospects, terpenoids have become a research hotspot across multiple fields and are expected to drive the advancement of future pharmaceuticals [
67].
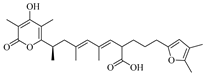
Xu-Meng Ren et al. isolated two new terpenoid derivatives, (1
S,6
R,7
S)-hydrobenzosydowic acid (
239) and (1
R,6
S,7
S)-hydrobenzosydowic acid (
240), from the deep-sea-derived fungus
Aspergillus sydowii DFFSCS007 [
49]. Guang-Ping Cao et al. identified a new compound, millmerranones G (
241), from the mangrove-derived fungus
Aspergillus sp. GXIMD 03004. This fungus was isolated from the leaves of the mangrove plant Acanthus ilicifolius L. collected from the Beibu Gulf, China. Evaluation of anti-Vibrio activity showed that compound
241 exhibited weak activity against Vibrio harveyi [
68].
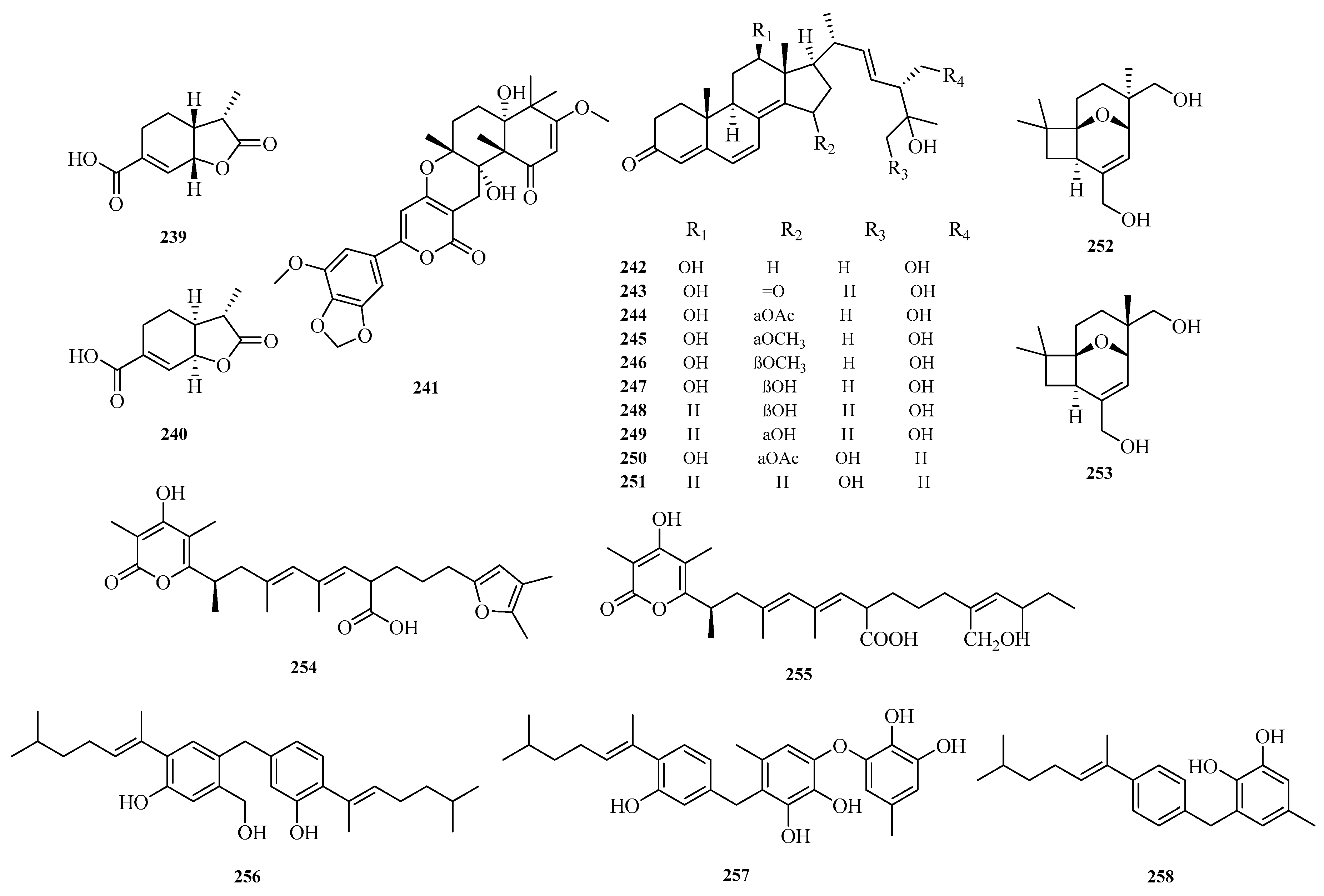
Zhen Zhang et al. isolated 10 new ergot derivatives (
242–
251) from the deep-sea-derived fungus
Aspergillus terreus YPGA10. Compound
242 exhibited cytotoxicity against the human colon cancer SW620 cell line with an IC
50 value of 8.4 μM. It also showed cytotoxicity against five human leukemia cell lines (CCRF-CEM, Jurkat, THP-1, U937, and K562) with IC
50 values ranging from 5.0 to 9.0 μM. Compound
250 displayed weak inhibitory activity against RSL3-induced ferroptosis in U937 cells, with an EC
50 value of 30.0 μM [
69]. Jun Zhang et al. discovered two new compounds with a very rare structural skeleton, asperporonins A (
252) and B (
253), from the deep-sea fungus
Aspergillus terreus SCSIO 41,202 [
70].
Yiwei Hu et al. isolated two new 6-alkenyl pyrone polyketides, alternapyrones G-H (
254–
255), from the marine-derived fungal strain
Arthrinium arundinis. Activity assays revealed that alternapyrone G (
254) not only inhibited M1 polarization in lipopolysaccharide (LPS)-stimulated BV2 microglia but also promoted dendritic regeneration and neuronal survival after Aβ treatment. This indicates that compound
254 has the potential to serve as a privileged scaffold for the development of anti-Alzheimer’s disease drugs [
71]. Hao-Yu Yu et al. isolated a rare dimeric aromatic bisabolane sesquiterpenoid, aspergol A (
256), and two undescribed phenolic bisabolane sesquiterpenoids, expansol H and aspergol B (
257–
258), from the marine sediment-derived
Aspergillus sp. MCCC 3A00392 [
24]. The structures of compounds
239–2
58 are shown in
Figure 16.
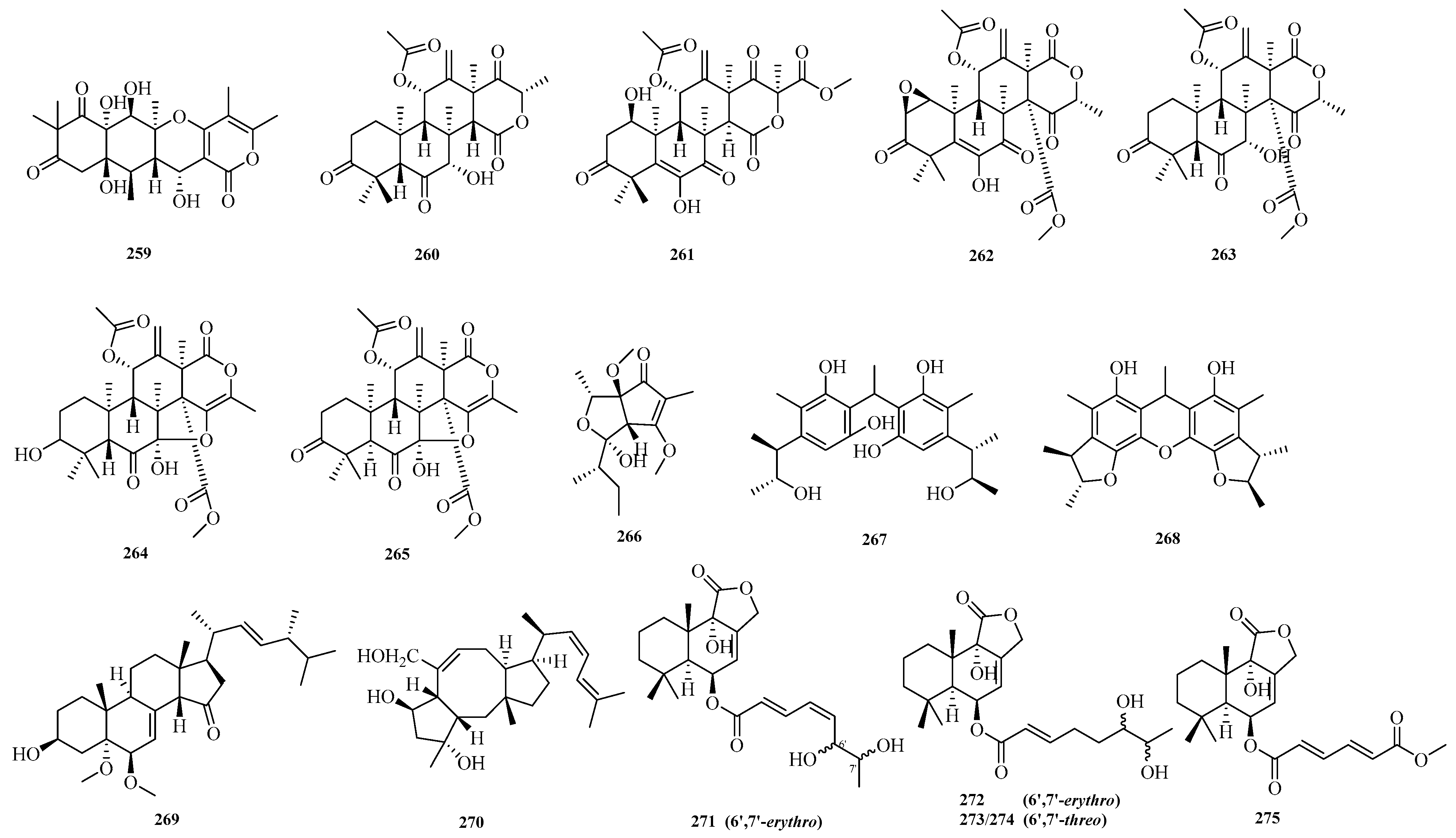
Cangzhu Sun et al. focusing on the significance of fungi as a source of novel bioactive natural products, isolated 12
S-aspertetranone D (
259) from the Mariana Trench-related
Aspergillus sp. SY2601. The new compound
259 exhibited antibacterial activity against both methicillin-resistant
Staphylococcus aureus and
Escherichia coli, with MIC values of 3.8 μM and 5.0 μM, respectively [
27]. Hui Cui et al. isolated six previously undescribed salinene-type terpenoids, aspertermeroterpenes A–F (
260–
265), from the marine-derived fungus
Aspergillus terreus GZU-31-1. During the bioactivity assay, it was found that aspertermeroterpene B (
261) effectively inhibited the activation of hepatic stellate cells at a concentration of 5.0 μM by targeting the Nrf2 signaling pathway. This is the first report that aspertermeroterpene B, as a newly discovered carbon skeleton of meroterpenoids, possesses anti-hepatic fibrosis activity [
72].
Ying Chen et al. conducted a study on the coral-derived fungus
Aspergillus austwickii SCSIO41227 from the Beibu Gulf, obtaining asperpentenone B (
266) [
57]. Shui-Hua Lin et al. performed a systematic chemical investigation on the deep-sea-derived fungus
Aspergillus versicolor 170217, isolating two new dimeric citrinin analogs, dicitrinones K–L (
267–
268) [
32]. Hui-Min Wen et al. isolated a new compound, 3
β-hydroxy-5
α,6
β-methoxyergosta-7,22-dien-15-one (
269), from the crude extract of a marine sponge-derived
Aspergillus sp. Antibacterial activity evaluation showed that compound
269 exhibited antibacterial activity against Staphylococcus aureus [
73].
Sheng-Tao Fang et al. conducted a chemical investigation on the marine alga-derived strain
Aspergillus sp. RR-YLW-12, identifying six new terpenoids: 21-Deoxo-21-hydroxyophiobolin U (
270) and ustusolates K–O (
271–
275). The growth inhibitory effects of all compounds against five species of harmful marine microalgae were evaluated. The new compounds exhibited significant to moderate inhibitory effects on all tested microalgal species, with IC
50 values ranging from 5.8 to 54.5 μM [
74]. The structures of compounds
259–
275 are shown in
Figure 17.
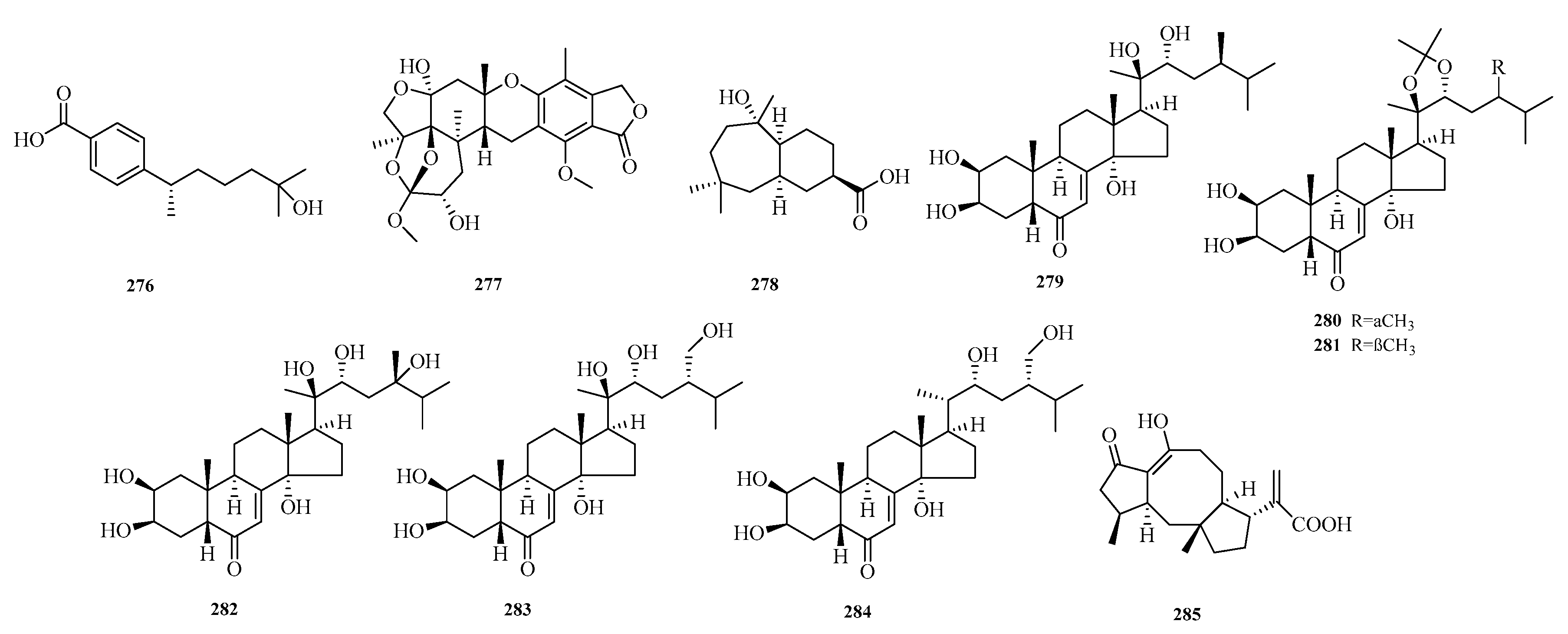
Xinjun Zhang et al. identified a new bisabolane-type sesquiterpenoid, named (+)-8-dehydroxylaustrosene (
276), from the fungus
Aspergillus sydowii (BTBU20213012) isolated from marine sediment samples in the Western Pacific Ocean [
75]. Mohamed S. Elnaggar et al. isolated a new thioterpenoid, austalide Z (
277), from a soft coral-associated fungus. In vitro cytotoxicity evaluation against the Caco-2 cancer cell line using the MTT assay showed that compound
277 exhibited weak to moderate activity, with a half-maximal inhibitory concentration (IC
50) of 51.6 μM [
76].
Siwen Niu et al. isolated a new sesquiterpenoid, malfilanol C (
278), from the deep-sea-derived fungus
Aspergillus puniceus A2. Compound
278 exhibited weak antibacterial activity against
Staphylococcus aureus ATCC 29,213 [
77]. Zhong-Hui Huang et al. isolated six previously undescribed compounds, punicesterones B–G (
279–
284), from the deep-sea-derived fungal strain
Aspergillus puniceus SCSIO z021. Punicesterones B and C (
279–
280) showed cytotoxicity and could reduce intracellular lipid accumulation. Additionally, antibacterial activity assays indicated that compounds
279–
280 exhibited moderate antibacterial activity against five bacterial strains [
45].
Xiuli Xu et al. isolated a new compound, aspergillusneoic acid (
285), from the marine-derived fungus
Aspergillus brunneoviolaceus MF180246. Antibacterial activity testing against
Staphylococcus aureus showed that compound
285 exhibited antibacterial activity, with a minimum inhibitory concentration (MIC) of 200.0 μM [
78]. The structures of compounds
276–
285 are shown in
Figure 18.
The sources and biological activities of compounds
239–
285 are summarized in
Table 3.
2.4. Lignan-like Compounds
Lignan-like compounds are a class of natural organic compounds formed by the linkage of two phenylpropanoid derivatives through the β-carbon atoms of their side chains. They feature uniquely diverse structures and are widely distributed in plants including
Schisandra chinensis and
Forsythia suspensa [
79]. Notably, their distinctive dibenzocyclooctene core structure endows them with a spectrum of biological and pharmacological activitie.
Zheng-Biao Zou et al. isolated 13 new minor furanones, including 5 pairs of enantiomers, namely (±)-nigenolides A–E (
286–
295) and racemic nigenolides F–H (
296–
298), from the deep-sea-derived
Aspergillus niger 3A00562. Activity assays revealed that compounds
288,
292–
293, and
297 could inhibit RSL3-induced ferroptosis. Among them, compound
297 exhibited half-maximal effective concentrations (EC
50) of 0.8 μM and 0.7 μM against ferroptosis in A375 and 786-O cells, respectively. Studies indicated that compound
297 is a potential iron chelator and free radical-scavenging antioxidant, and it exerts its ferroptosis-inhibiting effect by downregulating the expression of the TXNIP gene [
80].
Xinwan Zhang et al. investigated the secondary metabolites of the marine-derived fungal strain
Aspergillus terreus BTBU20211037, which was isolated from the coastal area of Qinhuangdao. A new compound, butyrolactone J (
299), was isolated and identified therefrom. Activity testing against
Staphylococcus aureus ATCC 25,923 showed that compound
299 exerted an inhibitory effect on it, with a minimum inhibitory concentration of 12.5 μM [
81]. Guang-Yu Zhang et al. identified asperteretals L and M (
300–
301) from the coral-derived fungus
Aspergillus terreus. Asperteretal M (
301) exhibited cytotoxic activity against HCT-116 cells with an IC
50 value of 30 μM [
52]. Yufeng Jiang et al. isolated asperbutenolide A (
302) from the marine fungus
Aspergillus terreus. The results of its bioactivity assay showed that asperbutenolide A (
302) had a MIC of 4.0–8.0 μM against methicillin-resistant
Staphylococcus aureus (MRSA), indicating its potential as a novel antibacterial agent [
82]. The structures of compounds
286–
302 are shown in
Figure 19.
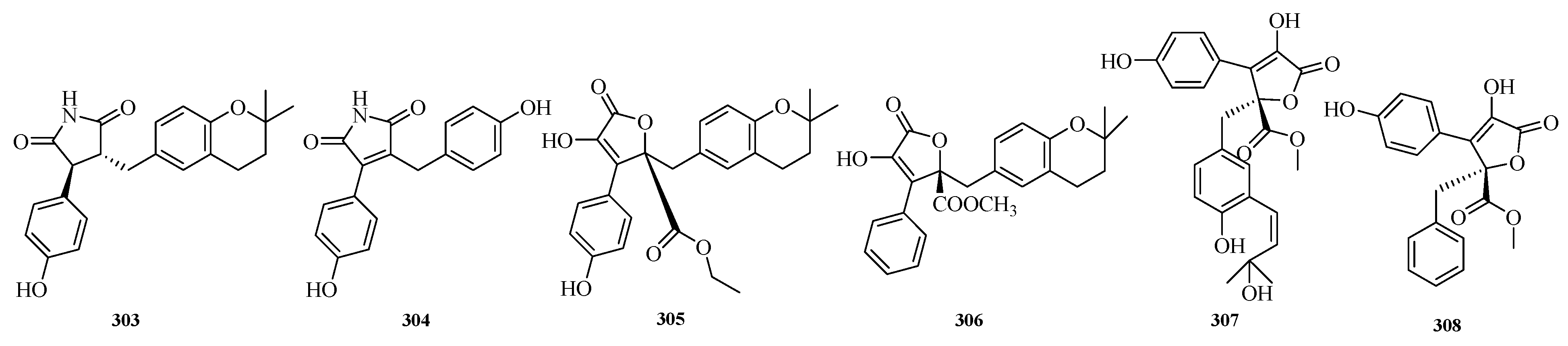
Hao Fan et al. isolated aspergteroids G–H (
303–
304) and aspergteroids I–J (
305–
306) from the fermented extract of the soft coral-associated fungus
Aspergillus terreus EGF7-0-1. In myocardial protection assays, compounds
303 and
304 exhibited protective effects against tert-butyl hydroperoxide (TBHP)-induced apoptosis in H9c2 (rat cardiomyocyte) cells at low concentrations. Based on protein–protein interaction (PPI) network and Western blotting analyses, compound
303 may inhibit apoptosis and inflammatory responses in cardiomyocytes induced by TBHP, while enhancing the antioxidant capacity of cardiomyocytes [
83].
Yuwei Zhou et al. successfully discovered two new butyrolactone derivatives (
307–
308) from
Aspergillus terreus GZU-31-1. The researchers tested all the isolated compounds for their anti-inflammatory effects on lipopolysaccharide-induced nitric oxide production in microglial cells (RAW 264.7 cells). The results showed that compound
307 exhibited potent anti-inflammatory activity with a half-maximal inhibitory concentration (IC
50) of 16.3 μM, which was superior to that of the positive control indomethacin (IC
50 = 24.0 μM) [
84]. The structures of compounds
303–
308 are shown in
Figure 20.
The sources and biological activities of compounds
268–
308 are summarized in
Table 4.
2.5. Cyclopeptides
Cyclopeptides are cyclic molecules formed by amino acids linked end-to-end through peptide bonds and are characterized by structural stability and diverse bioactivities. Widely distributed across animals, plants, and microorganisms, these compounds owe their unique properties to their cyclic framework: notably, this structure endows them with resistance to protease degradation and confers upon them a range of pharmacological activities.
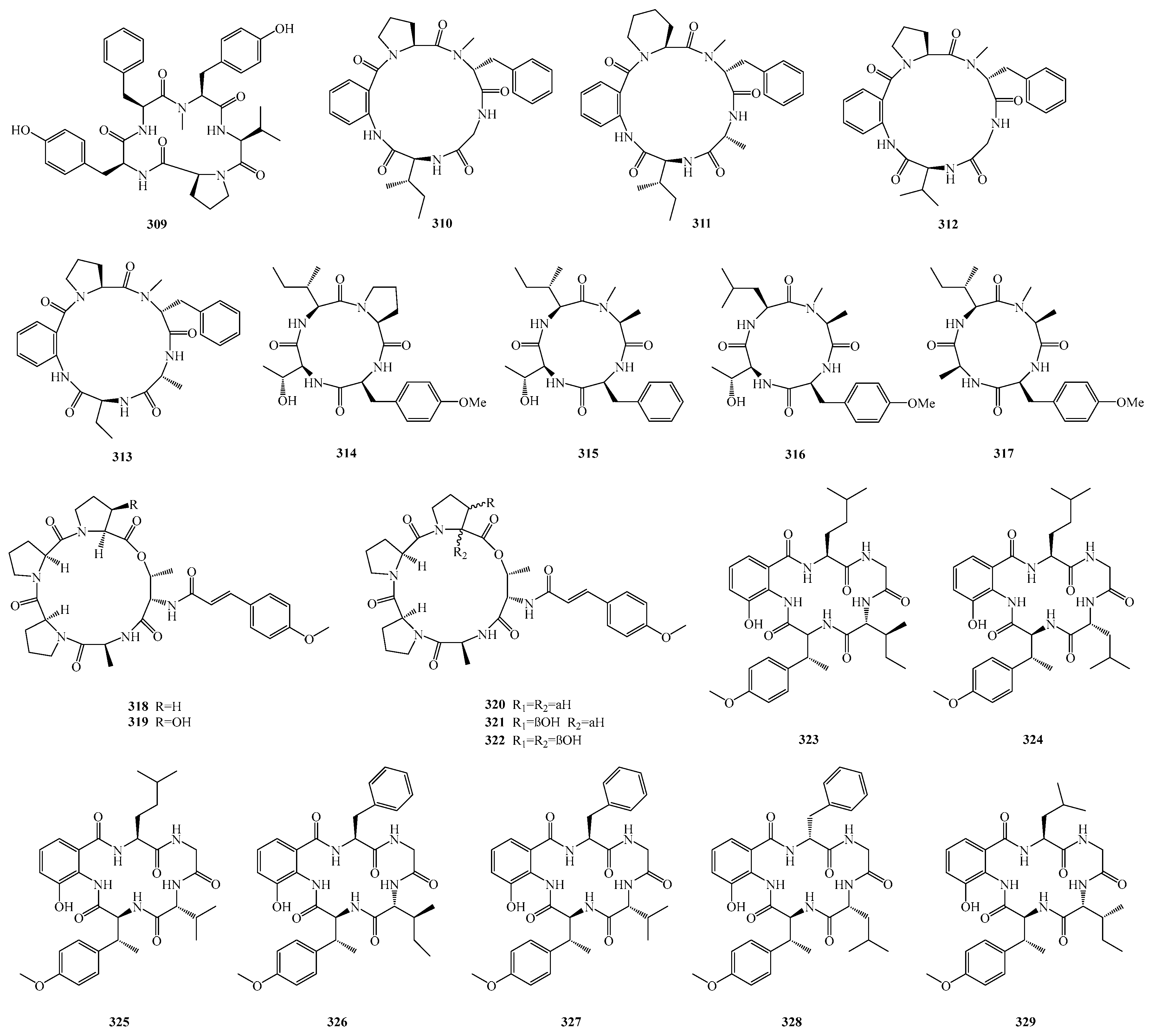
Maokun Zheng et al. isolated a new cyclopentapeptide, cotteslosin D (
309), from the culture of the sponge-derived fungus
Aspergillus versicolor 2-18. Antibacterial activity testing of compound
309 showed that it exhibited weak antibacterial activity against
Escherichia coli and
Staphylococcus aureus [
85].
Yu Wang et al. isolated four new cyclic pentapeptides, avellanins D–G (
310–
313), from the mangrove-derived fungus
Aspergillus fumigatus GXIMD 03099. Activity screening results showed that compound
311 exhibited insecticidal activity against newly hatched Culex quinquefasciatus larvae, with a half-lethal concentration (LC
50) of 86.6 μM; compound
313 showed weak activity against
Vibrio harveyi, with a minimum inhibitory concentration (MIC) of 5.9 μM [
86].
Qin Li et al. identified four new cyclic tetrapeptides, violaceotides B–E (
314–
317), from the sponge-associated fungus
Aspergillus insulicola IMB18-072. Activity assay results showed that compounds
315 and
316 exhibited selective antibacterial activity against aquatic pathogens
Edwardsiella tarda and
E. ictaluri (
Edwardsiella ictaluri). Furthermore, at a concentration of 10.0 μM, compounds
314–
317 inhibited the expression of the inflammatory mediator interleukin-6 (IL-6) in lipopolysaccharide (LPS)-induced RAW264.7 cells [
87].
Lu-Ping Chi et al. isolated the pentapeptides aspertides A–E (
318–
322) from the marine fungi
Aspergillus tamarii MA-21 and
Aspergillus insuetus SD-512. In bioactivity assays, compounds
321 and
322 exhibited antibacterial activity against various aquatic pathogens, including
Edwardsiella tarda,
Vibrio alginolyticus,
Vibrio anguillarum,
Vibrio vulnificus, and
Staphylococcus aureus, with minimum inhibitory concentrations (MICs) ranging from 8.0 to 32.0 μM [
88].
Wenjuan Ding et al. discovered seven new cyclopentapeptides, namely pseudoviridinutans A–F (
323–
329), from the marine-derived fungus
Aspergillus pseudoviridinutans TW58-5. Bioassays revealed that compounds
323–
329 possess anti-inflammatory potential; in particular, compound
328 can inhibit the production of nitric oxide (NO), a key inflammatory mediator, in lipopolysaccharide (LPS)-induced RAW264.7 murine macrophage cells by regulating the expression levels of NLRP3 and inducible nitric oxide synthase (iNOS) [
89]. The structures of compounds
309–
329 are shown in
Figure 21.
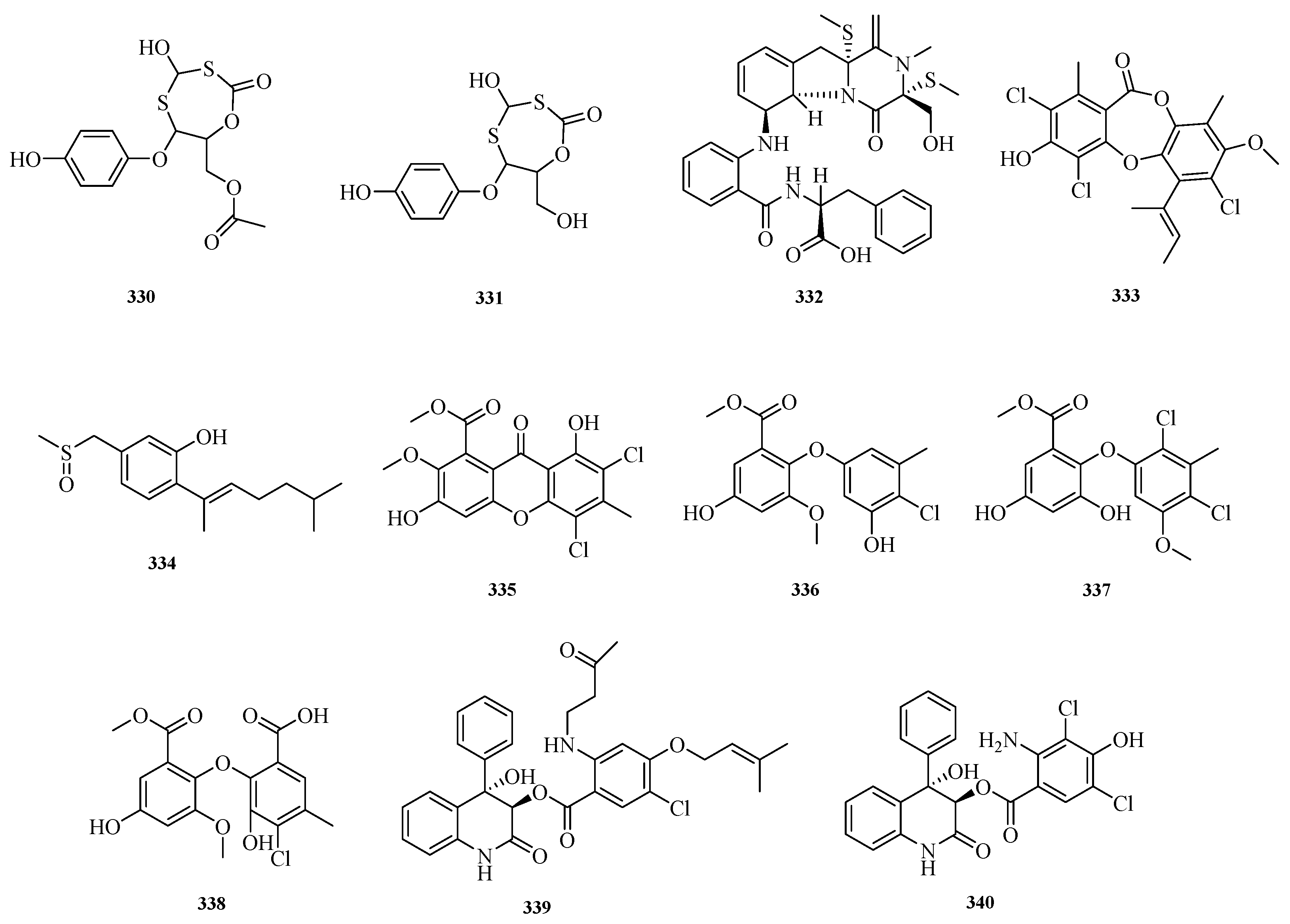
The sources and biological activities of compounds
309–
329 are summarized in
Table 5.