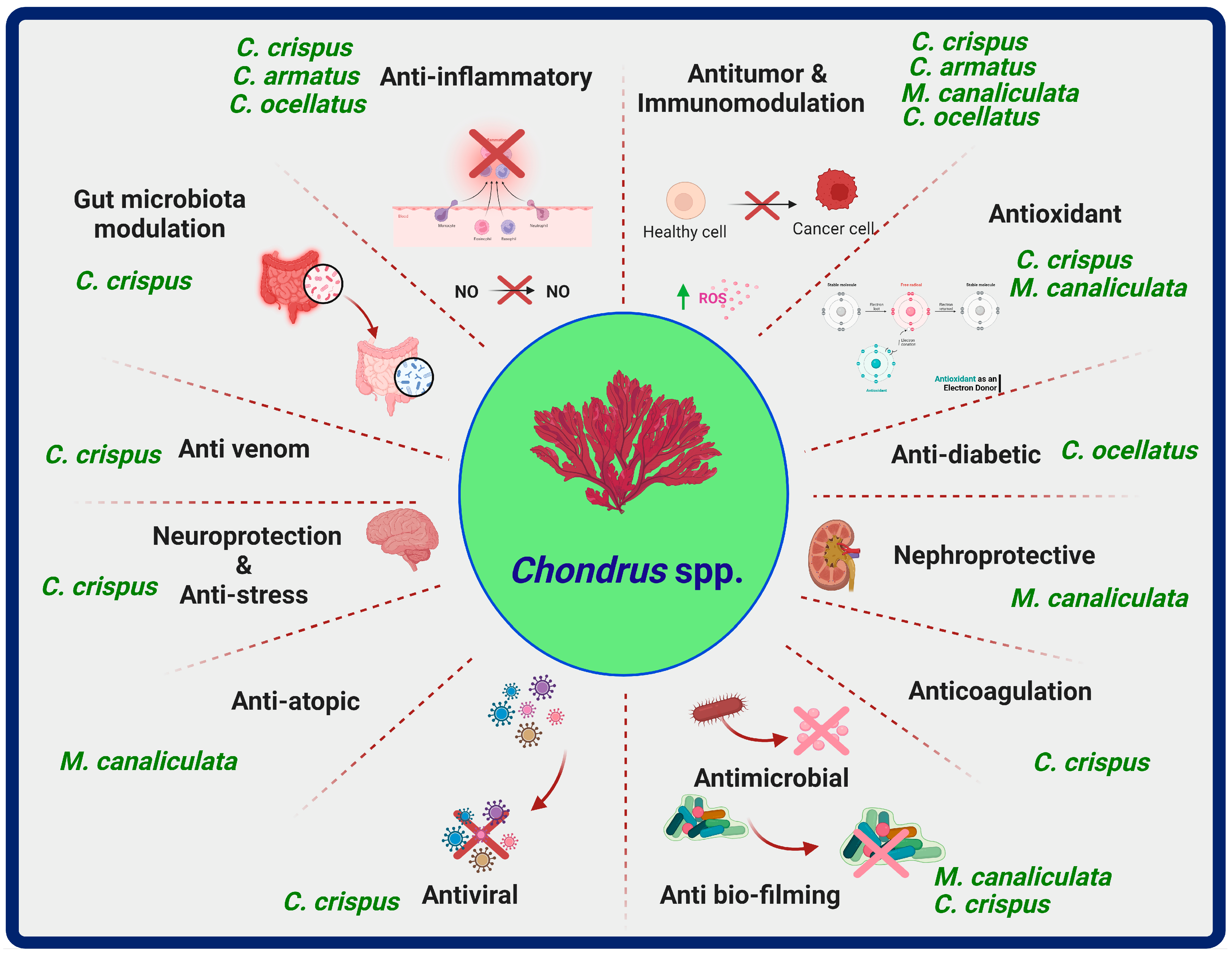
An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus
Abstract
1. Introduction
2. Search Strategy
3. Rhodophyta (Red Seaweeds)
4. General Information on the Genus Chondrus
5. Chondrus Species, Chemical Compounds, and Biological Properties
5.1. Chondrus crispus
5.1.1. Antimicrobial Activity
5.1.2. Anti-Stress and Immunomodulation Activity
5.1.3. Nitric Oxide Inhibition
5.1.4. Neuroprotection
5.1.5. Improvement of Gut Health
5.1.6. Antiproliferative Activity
5.1.7. Anti-Oxidant Activity
5.1.8. Antiviral Activity
5.1.9. Anti-Coagulation Activity
5.1.10. Antivenom Activity
| Species | Extract Type | Target Models | Biological Activities | Ref. |
|---|---|---|---|---|
| Antimicrobial activity | ||||
| C. crispus | Crude ethanol (95%) Dried and fresh extracts | Marine bacterial species: Pseudoalteromonas elyakovii, Polaribacter irgensii, Vibrio aestuarianus, Shewanella putrefaciens, and Halomonas marina Microalgae species: Chlorarachnion reptans, Chlorarachnion globosum, Exanthemachrysis gayraliae, Cylindrotheca cloisterium, Navicula jeffreyi | Potential antifoul activity Dried extracts had lower MIC than fresh extract. MIC (μg/mL) dried; fresh: P. elyakovii (10; 25), P. irgensii (10; 25), V. aestuarianus, (25; 50), S. putrefaciens, (25; 25), and H. marina (10; >50); C. reptans (10; 25), C. globosum (25; 10), E. gayraliae (10; 10), C. cloisterium (1; 10), and N. jeffreyi (25; 25). | [67] |
| C. crispus | Crude ethanolic extract of dried algae | Cobetia marina (50–200 ppm), Marinobacter hydrocarbonoclasticus (100–200 ppm) | Anti-biofilming effect | [68] |
| C.crispus | Methanol, ethanol and acetone (60%) | L. monocytogenes, P. aeruginosa, Salmonella abony, and E. faecalis | (%inhibition) (methanol; ethanol; acetone): L. monocytogenes (−3.88; 50.27; 56.13), S. abonya (−10.70; 0.80; 4.70), E. faecalis (−66.08; 100.00 ± 0.38; −89.74), P. aeruginosa (−31.72; 61.51; 81.74) | [69] |
| C. crispus | Ethyl acetate | S. enteridis, E. coli, P. aeruginosa, L. innocua, B. cereus, S. aureus, L. brevis. E. faecalis, Candida sp.from both wild and IMTA regimes | Ethyl acetate: inhibitory activity in both regimes; best in IMTA | [70] |
| Diethyl ether | Diethylether extract: (1) IMTA regime: no effect against S. aureus (CI and FI), L. brevis and E. faecalis (2) wild regime: no effect against S. enteritidis | |||
| Methanol:Water (1:1) | Methanol:Water extract: IMTA: no effect against Candida sp. Wild: no effect against S. enteritidis and S. aureus (CI) | |||
| C. crispus | Acidified ethanol | Bacterial species: Bacillus cereus, Micrococcus flavus, and Staphylococcus aureus, Proteus mirabilis, Salmonella Typhimurium Yeast species: Candida albicans C. tropicalis, and C. krusei | Anti-bacterial (mg/mL) (MIC, MBC): B. cereus (0.045, 0.06), M. flavus (0.09, 0.12), S. aureus (0.06, 0.1), P. mirabilis (0.045, 0.06), and S. Typhimurium (0.06, 0.12). Anti-fungal (mg/mL) (MIC, MFC): C. albicans (0.045, 0.06), C. tropicalis (0.045, 0.06), and C. krusei (0.06, 0.12). | [71] |
| Anti-stress and immunomodulation activity | ||||
| C. crispus | Methanol extract (CCME) | Juglone (300 or 500 μmol)-induced Caenorhabditis elegans + CCME (1 mg/mL) | Anti-stress activity ↓ROS, stress response genes: ↑sod3, ↑hsp16.2, ↑skn1, ↑transcription factor daf16 | [75] |
| C. crispus | Aqueous CCWE and K-CGN | Caenorhabditis elegans + infection with Pseudomonas aeruginosa PA14 CCWE (0, 250, 500, or 750 μg/mL) or K-CGN (200 μg/mL) | ↑Survival rates CCWE = 500 μg/mL (optimum dose) and K-CGN Immune gene expression: ↑hsf-1, ↑irg-1, ↑irg-2, ↑F56D6.2, ↑F49F1.6, ↑K05D8.5, ↑C29F3.7, ↑ZK6.7, ↑abf-1 ↑F28D1.3, ↑F38A1.5, ↑lys-1, and ↑spp-1. Repressed QS (lasI/R and rhlI/R) systems. Virulene factor genes: ↓sbe, ↓hcnC, ↓aroE, ↓rpoN, and ↓sodB. Inhibited biofilm formation | [79] |
| Nitric oxide inhibition | ||||
| C. crispus | Methanol extract of cultivated alga | RAW264.7 cells + LPS (1 µg/mL), extract (25, 50, and 100 µg/mL), 24 h | Dose-dependent NO inhibition | [64] |
| Neuroprotection | ||||
| C. crispus | Methanol extract; organic fraction | C. elegans CL4176 + β amyloid toxicity organic fraction (0.1–1.0 mg/mL) | ↓amyloid species deposition ↑antioxidant activity Stress response genes: ↑sod3, ↑hsp16.2, and ↑skn1 | [82] |
| Improvement of gut health | ||||
| C. crispus | Cultivated alga and FOS | Male Sprague Dawley rats (1) Basal diet (control) (2) the basal diet + 2.5% or 0.5% (dry w/w) of cultivated seaweed (3) basal diet + 2.5% or 0.5% of FOS (dry w/w) | No change in body and organs weights Cultivated alga extract (0.5%): ↑IgA, ↑IgG Alga extract (2.5%): Modulated gut microbiota ↑Bifidobacterium, ↑Legionella, ↑Sutterella, ↑Blautia, ↑Holdemania, ↑Shewanella, ↑Agarivorans and ↓Streptococcus ↑SCFAs | [85] |
| Antiproliferative activity | ||||
| C. crispus | Methanolic extract Wild (high-UV and low-UV) and cultivated | HeLa cells and U-937 cells: 0.125, 0.5, 1,2, and 4 mg/mL, 24 h | Showed antiproliferative effect on HeLa cells: (upto 1 mg/mL): wild low-UV > wild high-UV > cultivated. (at 2 and 4 mg/mL) cultivated > wild low-UV > wild high-UV. U-937 cells: (upto 1 mg/mL): wild high-UV > wild low-UV > cultivated. Cutlivated extract in HeLa cells: ↑caspase-3,↑caspase-7; cell cycle arrest at Sub G1 (apoptotic) Antioxidant: reducing ability: cultivated > wild low-UV > wild high-UV ORAC: cultivated > wild low-UV > wild high-UV | [61] |
| C. crispus | Dried algal powder, 80% methanol | HepG2 cells, MCF7 cells, Caco-2 cells, and A549 cells, (0.01, 0.1, 1.0, 10, and 100 µg/mL), 24 h control (Sorafenib) | % inhibition (IC50 µg/mL): HepG2, control (1.32, 2.23); MCF7 cells, control (179, 4.0); Caco-2 cells, control (8.24, 2.88); A549 cells, control (7.90, 2.55) | [50] |
| C. crispus | Carrageenen fraction | A-2780, A-549, HT-29, and Hela-229 (0.1 mg/mL) | IC50 (mg/mL) = A-2780 (0.0080); A-549 (0.0099); HT-29 (0.0211); Hela-229 (0.0492) | [49] |
| Antioxidant activity | ||||
| C. crispus | Dried algal powder 80% methanol | DPPH and ABTS assay: at 50, 100, 150, and 200 μg/mL TAC: at 100, 200, 300, and 400 μg/mL | DPPH (% inhibition): 50 μg/mL (25.0), 100 μg/mL (37.8), 150 μg/mL (47.3), and 200 μg/mL (84.2), BHT (μg) 91.5 ABTS (% inhibition): 50 μg/mL (82.0), 100 μg/mL (98.0), 150 μg/mL (108.9), and 200 μg/mL (120.3), Trolox (μg) (100.2). TAC: 100 μg/mL (88.48), 200 μg/mL (94.77), 300 μg/mL (136.88), and 400 μg/mL (235.81), vitamin C (μg) 243.46 | [50] |
| C. crispus | Extract with UAE treatments | DPPH and ABTS @ 4 treatments (A) Probe 20 min; (B) Probe 40 min; (C) Bath 20 min; (D) Bath 40 min. Superoxide radical @ 2 treatments anion (C) and (D), 0.5, 1, and 2 mg/mL) | EC50 (mg/mL) DPPH: (A) ND, (B) 6.3, (C) ND, (D) 7.1 EC50 (mg/mL) ABTS: (A) 3.1, (B) 2.4, (C) 4.6, (D) 2.5 % inhibition (2 mg/mL): (C) 21.1 (D) 27.3 | [65] |
| C. crispus | UAE extracted soluble extract | ABTS (1 mL) + 10 mL (soluble extract) or Trolox | Trolox equivalent antioxidant capacity (TEAC) = 182.4 mg/g | [49] |
| Antiviral activity | ||||
| C. crispus | Enzymatic hydrolysates (P1, C1, C2, and C3) | African green monkey kidney cell line and HSV-1 (wild-type strain 17), hydrolysates (1–200 μg/mL (50 μL)) | EC50 (μg/mL): 77.6 –126.8 μg/mL P1 (neutrase) = 77.6; C1 (cellulase) = 103.3; C2 (β-glucanase) = 126.8; C3 (ultaflo) = 109.3 | [44] |
| Anticoagulation activity | ||||
| C. crispus | Crude aqueous extract | 0.125, 1.25, 12.5, and 125 μg/mL | Residual Xa activity * = C. crispus: 253; Heparin: 0.20; Lovenox®: 0.15; Residual IIa activity * = C. crispus: 194; Heparin: 0.25; Lovenox®: 1.8; APTT ** = C. crispus: 21; Heparin: 0.7; Lovenox®: 4.5; PT ** = C. crispus: >415; Heparin: 16; Lovenox®: 495; TT ** = C. crispus: <125; Heparin: 0.45; Lovenox®: 1.82 | [45] |
| Antivenom activity | ||||
| C. crispus | Lambda-carrageenan | In vitro assays Hemolytic/proteolytic activity: human erythrocytes and egg yolk emulsion (substrate) B. jararaca or B. jararacussu venom (20 μg/mL) + polysaccharide (200 μg/mL), 30 min at 25 °C Coagulation: venoms (10 μg/mL), + polysaccharide (200 μg/mL) | Inhibited hemolytic activity (60%) for B. jararaca Inhibited proteolytic activity: B. jararaca (65%) B. jararacussu (100%) Impaired plasma coagulation | [97] |
| Balb/c mice Antihemorrhagic activity (a) Inhibtion protocol: incubation—polysaccharide (150 μg/mouse) or antivenon was mixed with 2 MHD of venom of B. jararacussu (50 μg/mouse) or B. jararaca (30 μg/mouse), 30 min, 25 °C. S.C. route (b) Treatment protocol: venoms (30 μg/mL, S.C.) + λ carrageenan 30 min later (S.C., samesite), or I.V. (c)venoms injection (S.C.), 30 min later (one injection) or 30 + 30 min (two injections) of polysaccharide (I.V.) | Inhibition protocol C. crispus polysaccharide inhibited venom’s hemorrhage: B. jararaca (40%) and B. jararacussu (100%). Treatment protocol C. crispus polysaccharide inhibited venom’s hemorrhage: B. jararaca (20%) and B. jararacussu (40%). C. crispus polysaccharide (one injection: two injections) inhibited venom’s hemorrhage: B. jararaca (20%:50%) and B. jararacussu (40%:60%). | |||
| Antihemorrhagic activity of gel (a) Prevention protocol: Venoms dose (25 μg/mouse) (S.C.), + polysaccharide gel (100 μg) topically applied 15 or 30 min later, + antivenom single injection (I.V.) (b) Treatment protocol: gel containing λ polysaccharide (100 μg/mL) topical application + venoms (S.C., 25 μg/mouse) 15 or 30 min later. | Inhibition of hemorrhage after topical application of polysaccharide based gel, irrespective of protocol Hemorrhage of B. jararacussu was inhibited fully in a 30 min topical application before venom injection. Hemorrhage of B. jararacussu inhibited 30% in a 15 min topical application before venom injection | |||
| Antidematogenic activity single, sub-plantar injection into the right paw (50 μL), after 1 h, the paw’s amputation. λ carrageenan (150 μg/mouse) or antivenom + venoms (25 μg/mouse), 30 min at 25 °C, injected (50 μL of the mixture) (S.C.) Protocol of treatment: venoms (S.C.), λ polysaccharide or venom 15 or 30 min later, (I.V.) | Inhibition (%) of edema (~30%) λ polysaccharide mixed with venom inhibited edema (45%) λ polysaccharide is more effective against B. jararacussu than B. jararaca | |||
| Antilethal activity Protocol of incubation: venoms (130 μg/mouse) + λ polysaccharide (100 μg/mouse) or antivenom, 30 min at 25 °C (I.P. injection) Protocol of treatment: I.P. injection of venoms, 30 min later, λ polysaccharide or antivenon (I.V.). | Polysaccharide + venom protected the mice (both treatments) | |||
| Antimyotoxic activity Protocol of incubation: B. jararacussu venom (50 μg/mouse) + polysaccharide (150 μg/mouse), saline or antivenon, 30 min at 25 °C, 100 μL injection. Protocol of treatment: B. jararacussu venom (I.M.), polysaccharide, antivenom, or polysaccharide + antivenom, 30 min later (I.V.) | Inhibited myotoxic activity. Slight protection was observed. | |||
| Antitumor and immunomodulation | ||||
| C. ocellatus | EA polysaccharides: PC1, PC2, PC3, PC4, and PC5 | ICR mice, S180 and H22 tumor cells (subcutaneous implatation), 0.2 mL of each extract (200 mg/kg/day) for 7 days | Inhibited tumor growth (%) PC1, PC2, PC3, PC4, and PC5 S180: (57.58,37.64, 44.35,50.52, and 66.15) H22: (57.03,61.90, 23.22,68.97, and56.90) ↑spleen weights, ↑NK cell activity, ↑lymphocyte proliferation | [98] |
| C. ocellatus | Polysaccharide PC5 | ICR mice, S180 (subcutaneous implatation) (1) 5-FU:25 mg/kg (2) PFp1 PC5:100 mg/kg (3) PFp2 PC5:50 mg/kg (4) PF1 PC5+5-FU:100 + 25 mg/kg (5) PF2 PC5+5-FU 50 + 25 mg/kg | Inhibited tumor growth (%): 5-FU: (37.30), PFp1: PC5 (32.08), PFp2: PC5 (26.03), PF1: PC5+5-FU (63.87), PF2: PC5+5-FU (55.40) ↑TNFα ↑lymphocyte proliferation ↑spleen weights | [99] |
| C. ocellatus | Polysaccharide PC4 | ICR mice, H22 (subcutaneous implatation) (1) 5-FU:25 mg/kg (2) HFp1 PC4:100 mg/kg (3) HFp2 PC4:50 mg/kg (4) HF1 PC4+5-FU:100 + 25 mg/kg (5) HF2 PC4+5-FU 50 + 25 mg/kg | Inhibited tumor growth (%) 5-FU: (30.76), HFp1 PC4: (43.97), HFp2 PC4: (35.37), HF1 PC4+5-FU: (51.73), HF2 PC4 + 5-FU (47.01) ↑lymphocyte proliferation ↑TNFα ↑spleen weights | [100] |
| Anti-inflammatory activity | ||||
| C. ocellatus Holmes | Ethanol extract (COHEE) | LPS +RAW 264.7 cells, (0.1, 1, 10, 50, and 100 μg/mL), 22 h | No cytotoxic effects ↓NO, ↓IL-6, ↓TNF-α, ↓IL-1β, ↓iNOS, ↓COX-2, ↓NF-κB p65, ↓p-MAPK | [101] |
| Croton oil-induced mouse ear edema model, COHEE (10, 50, and 250 mg/kg BW), 200 μL, croton oil (2.5%, 20 μL/ear), 1 h before treatment | ↓Mouse ear edema at a higher dose ↓Mast cell number | |||
| Antidiabetic activity | ||||
| C. ocellatus | Ethanol extract (61%) | In vitro assay | Anti-radical activity: DPPH = (13.11–25.77%), ABTS = (43.48–53.50%) H2O2 inhibition (IC50) = 18.4–85.6 U/mL α-glycosidase inhibition: IC50 = 16.92 mg/mL α-glucosidase inhibition: 375.3 mg/mL | [102] |
| Cytoprotective and antimicrobial activity | ||||
| Mazzaella canaliculata | Methanol extract | In vitro assays | DPPH radical scavenging: IC50 = 0.25 mg/mL Antimicrobial activity: Salmonella Typhimurium, Klebsiella pneumoniae, Listeria monocytogenes, Actinomyces sp., Enterococcus faecalis, Enterobacter sp., and Micrococcus luteus | [103] |
| Wistar female rats (1) Non-treated rats (positive control). (2) maneb (MB I.P.)/kg (300 mg). (3) 300 mg/kg of MB (I.P.) + algal extract (150 mg/kg, oral) (4) algal extract (150 mg/kg), positive control group, 7 days | MB-treated group: ↓body weight, ↓RBC, ↓WBC, ↓viability, ↑DNA damage, ↑platelet rates Co-treated: ↑body weight, ↑RBC, ↑WBC, ↑viability; ↓DNA damage, lowered platelet rates compared to the MB-treated group. Improved mineral levels in blood, bone, and urine In co-treated erythrocyte and bone: ↓MDA, ↓AOPP, ↑SOD, ↑GSH, and ↑GPx | |||
| Mazzaella canaliculata | Polysaccharide (CCP) | 2, 4, 6, 8, 10, and 12 mg/mL | DPPH (2 mg/mL) has strong antiradical activity comparable to gallic acid. Protective effect against β-Carotene bleaching inhibition assay (10 mg/mL = 39.90%) Ferrous ion chelation: 10 mg/mL = 96.37% Ferric-reducing activity: 10 mg/mL = 2.16 Protection against hydroxyl radical-induced DNA damage | [104] |
| Wistar female rats Group 1: control group (saline) Group 2: MB (300 mg/kg, I.P.) Group 3: MB (300 mg/kg, I.P.) + CCP (100 mg/kg), Group 4: MB (300 mg/kg, I.P.) + CCP (200 mg/kg), Group 5: CCP (100 mg/kg, I.P.) (positive control) Group 6: CCP (200 mg/kg, I.P.) (positive control) | Dose-dependent significant improvement in MB’s oxidative and histological injuries. In plasma: ↓urea, ↓creatinine, ↓albumin Co-treatment: ↓MDA, ↓AOPP, ↑SOD, ↑GSH, and ↑GPx Co-treatment: ↑RBCs, ↑WBCs, ↑iron, ↑MCV, ↑MCH and ↑MCHC ↓apoptosis | |||
| Anti-atopic activity | ||||
| Mazzaella canaliculata | Ethanolic extract (CCEE) | BALB/c mouse 1% (w/v) DNCB, 3 times a day; after 1 week, apply 0.3% (w/v) DNCB to the same area once a day (200 μL) DNCB + CCEE | ↓IFNγ, ↓IL-4 ↓clinical severity score | [105] |
| Anti-inflammatory activity | ||||
| C. armatus | LMW and HMW carrageenan | Acetic acid-induced colitis in male Swiss mice + oral pretreatment (carrageenan in H2O) (5, 10, 50 mg/kg) | HMW: ↓ colon damage, ↓MPO. Effective dose: 5 mg/kg LMW: no protective effect. | [106] |
| Antitumor and immunomodulation activity | ||||
| C. armatus | Native κ- and λ-carrageenans LMW κ- and λ-carrageenans degradation products | FLO1, KYSE30, and human dermal fibroblast cell lines Treatments: 50, 100, and 400 μg/mL, 24 or 48 hr PBMC + test polysaccharides (1, 10, or 100 μg/mL) or LPS (0.1 μg/mL), 24 h | ↓ FLO1 and KYSE30 viability. All polysaccharides showed anti-metabolic activity. In FLO1: LMW κ- and λ-carrageenan were more effective (at 400 μg/mL): LMW κ (%): 48; HMW κ (%): 97.7. LMW λ (%) 61.9; HMW λ (%) 79.1. In KYSE30, naïve κ- and λ-carrageenans were more effective: κ-carrageenans (%) 47.5;. λ-carrageenans (%) 55.1. All carrageenans: induce monocytes to produce cytokines: IL1β, IL6, IL18, and TNFα. LMW λ-carrageenan only: IL10 | [107] |
| C. armatus | κ- and λ-carrageenans Native or HMW. LMWDPs | In vitro: murine peritoneal macrophages Control, LPS, HMW-κ, LMWDPs-k, HMW-λ, LMWDPs-λ (1, 10, and 100 μg/mL) or LPS (0.1 μg/mL) | ↓phagocytic activity by molecular weight and chemical structure-dependent manner. Anti-phagocytic efficacy = κ-carrageenan > λ-carrageenan At 100 μg/mL: LMWDPs-κ, HMW- λ, no effect on phagocytosis. HMW-κ reduced by 34% | [108] |
| In vivo: male C57BL/6 mice Control, LPS, stress, HMW-κ and λ, LMWDPs-κ and λ, 100 μg/kg/day, 7 days | No significant change in body weight or internal organs. Total leucocyte counts = not affected except for κ-carrageenan. Cell motility: LMWDPs (κ): no effect; LMWDPs (λ): 24% reduction. HMW (λ): ↑peritoneal macrophages (40%). | |||
| C. armatus | κ- and λ-carrageenan | KYSE-30, FLO-1, HCT-116, RKO, and RPE-1 cell lines | Cytotoxicity (IC50 values) of κ- and λ-carrageenan: KYSE-30: 394, 392; FLO-1: 405, 184; HCT-116: 347, 206; RKO: 350.6, 248.3; RPE-1: 728, 615. Delayed cell cycle at different stages. λ-carrageenan in RKO: ↓CDK2, ↓E2F2, ↓cyclin E. Induction of apoptosis | [109] |
5.2. Chondrus ocellatus
5.2.1. Anti-Tumor and Immunomodulation
5.2.2. Anti-Inflammatory Activity
5.2.3. Anti-Diabetic and Antioxidant Activities
5.3. Mazzaella canaliculata (C. Agardh) Arakaki & M. E. Ramírez 2021 (Formerly Known as Chondrus canaliculatus)
5.3.1. Antioxidant and Antimicrobial Activities
5.3.2. Nephro-Protective Activity
5.3.3. Anti-Atopic Activity
5.4. Chondrus armatus
5.4.1. Anti-Inflammatory Activity
5.4.2. Anti-Tumor and Immunomodulation Activity
6. Other Species and Biological Applications
7. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Guiry, M.; Guiry, G. Algaebase [Online]; Worldwide Electronic Publication, National University of Ireland: Galway, Ireland, 2020. [Google Scholar]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent advances in industrial applications of seaweeds. Crit. Rev. Food Sci. Nutr. 2023, 63, 4979–5008. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive metabolites from marine algae as potent pharmacophores against oxidative stress-associated human diseases: A comprehensive review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for Human and Animal Nutrition; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed minerals as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 371–390. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.E.; Kulshreshtha, G.; Humayun, S.; Darko, C.N.S.; Rjabovs, V.; Hammami, R.; Critchley, A.T.; Tuvikene, R.; Hincke, M.T. Polysaccharides from red seaweeds: Effect of extraction methods on physicochemical characteristics and antioxidant activities. Food Hydrocoll. 2023, 147, 109307. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Clerici, M.T.P.S. Brown algae and their multiple applications as functional ingredient in food production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Shahrukh, S.; Jain, N.; Vambhurkar, G.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Multifaceted applications of ulvan polysaccharides: Insights on biopharmaceutical avenues. Int. J. Biol. Macromol. 2023, 234, 123669. [Google Scholar] [CrossRef]
- Nova, P.; Gomes, A.M.; Costa-Pinto, A.R. It comes from the sea: Macroalgae-derived bioactive compounds with anti-cancer potential. Crit. Rev. Biotechnol. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A. Seaweeds and microalgae: An overview for unlocking their potential in global aquaculture development. FAO Fish. Aquac. Circ. 2021, 1229, 48. [Google Scholar]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Lee, R.E. Phycology; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Yoon, H.S.; Nelson, W.; Lindstrom, S.C.; Boo, S.M.; Pueschel, C.; Qiu, H.; Bhattacharya, D. Rhodophyta. In Handbook of the Protists, 2nd ed.; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Margulis, L., Melkonian, M., Chapman, D.J., Corliss, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 89–133. [Google Scholar]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Stévant, P.; Schmedes, P.S.; Le Gall, L.; Wegeberg, S.; Dumay, J.; Rebours, C. Concise review of the red macroalga dulse, Palmaria palmata (L.) Weber & Mohr. J. Appl. Phycol. 2023, 35, 523–550. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.; Lee, W.W.; Jeon, Y.-J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 1–11. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Baello, O.Q.; Dimatangal, J.M.; Dumelod, B.D. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar]
- Yermak, I.M.; Barabanova, A.O.B.; Aminin, D.L.; Davydova, V.N.; Sokolova, E.V.; Solov’eva, T.F.; Kim, Y.H.; Shin, K.S. Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities. Carbohydr. Polym. 2012, 87, 713–720. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Pujol, C.A.; Damonte, E.B.; Stortz, C.A. Partial and total C-6 oxidation of gelling carrageenans. Modulation of the antiviral activity with the anionic character. Carbohydr. Polym. 2015, 128, 199–206. [Google Scholar] [CrossRef]
- Lewis, J.; Stanley, N.; Guist, G.G. Commercial production and applications of algal hydrocolloids. In Algae and Human Affairs; Lembi, C.A., Waaland, J.R., Eds.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef]
- Michel, G.; Nyval-Collen, P.; Barbeyron, T.; Czjzek, M.; Helbert, W. Bioconversion of red seaweed galactans: A focus on bacterial agarases and carrageenases. Appl. Microbiol. Biotechnol. 2006, 71, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Verlecar, X.N. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 65, pp. 115–217. [Google Scholar]
- Arakaki, N.; Ramírez, M.E. Mazzaella canaliculata comb. nov. based on Chondrus canaliculatus (Gigartinaceae, Rhodophyta) from Peru and Chile. Phytotaxa 2021, 497, 211–228. [Google Scholar] [CrossRef]
- Taylor, A.R.A.; Chen, L.C.M. Chondrus Stackhouse. In Biology of Economic Algae; Akatsuka, I., Ed.; SPB Academic Publishing: Hague, The Netherlands, 1994; pp. 35–76. [Google Scholar]
- Borg, M.; Krueger-Hadfield, S.A.; Destombe, C.; Collén, J.; Lipinska, A.; Coelho, S.M. Red macroalgae in the genomic era. New Phytol. 2023, 240, 471–488. [Google Scholar] [CrossRef]
- Yang, M.Y.; Kim, M.S. Phylogeography of the economic seaweeds Chondrus (Gigartinales, Rhodophyta) in the northwest Pacific based on rbcL and COI-5P genes. Algae 2022, 37, 135–147. [Google Scholar] [CrossRef]
- Lamont, T.; McSweeney, M. Consumer acceptability and chemical composition of whole-wheat breads incorporated with brown seaweed (Ascophyllum nodosum) or red seaweed (Chondrus crispus). J. Sci. Food Agric. 2021, 101, 1507–1514. [Google Scholar] [CrossRef]
- Andersen, S.; Noahsen, P.; Rex, K.F.; Florian-Sørensen, H.C.; Mulvad, G. Iodine in edible seaweed, its absorption, dietary use, and relation to iodine nutrition in Arctic people. J. Med. Food 2019, 22, 421–426. [Google Scholar] [CrossRef]
- Correa, J.A.; McLachlan, J.L. Endophytic algae of Chondrus crispus (Rhodophyta). IV. Effects on the host following infections by Acrochaete operculata and A. heteroclada (Chlorophyta). Mar. Ecol. Prog. Ser. 1992, 81, 73–87. [Google Scholar] [CrossRef]
- Correa, J.A.; McLachlan, J.L. Endophytic algae of Chondrus crispus (Rhodophyta). V. Fine structure of the infection by Acrochaete operculata (Chlorophyta). Eur. J. Phycol. 1994, 29, 33–47. [Google Scholar] [CrossRef]
- Collén, J.; Roeder, V.; Rousvoal, S.; Collin, O.; Kloareg, B.; Boyen, C. An expressed sequence tag analysis of thallus and regenerating protoplasts of Chondrus crispus (Gigartinales, Rhodophyceae). J. Phycol. 2006, 42, 104–112. [Google Scholar] [CrossRef]
- Collen, J.; Cornish, M.L.; Craigie, J.; Ficko-Blean, E.; Hervé, C.; Krueger-Hadfield, S.A.; Leblanc, C.; Michel, G.; Potin, P.; Tonon, T. Chondrus crispus–A present and historical model organism for red seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 53–89. [Google Scholar]
- Lipinska, A.P.; Collén, J.; Krueger-Hadfield, S.A.; Mora, T.; Ficko-Blean, E. To gel or not to gel: Differential expression of carrageenan-related genes between the gametophyte and tetasporophyte life cycle stages of the red alga Chondrus crispus. Sci. Rep. 2020, 10, 11498. [Google Scholar] [CrossRef]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2022, 22, 1509–1540. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.T.; Hafting, J.T.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-assisted extraction of bioactive material from Chondrus crispus and Codium fragile and its effect on herpes simplex virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Adrien, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Evaluation of the anticoagulant potential of polysaccharide-rich fractions extracted from macroalgae. Nat. Prod. Res. 2017, 31, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Guerreiro, I.; Magalhães, R.; Coutinho, F.; Couto, A.; Sousa, S.; Delerue-Matos, C.; Domingues, V.F.; Oliva-Teles, A.; Peres, H. Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J. Appl. Phycol. 2019, 31, 2115–2124. [Google Scholar] [CrossRef]
- Naseri, A.; Holdt, S.L.; Jacobsen, C. Biochemical and nutritional composition of industrial red seaweed used in carrageenan production. J. Aquat. Food Prod. Technol. 2019, 28, 967–973. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Chondrus crispus treated with ultrasound as a polysaccharides source with improved antitumoral potential. Carbohydr. Polym. 2021, 273, 118588. [Google Scholar] [CrossRef]
- Alkhalaf, M.I. Chemical composition, antioxidant, anti-inflammatory and cytotoxic effects of Chondrus crispus species of red algae collected from the Red Sea along the shores of Jeddah city. J. King Saud Univ.-Sci. 2021, 33, 101210. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Pomin, V.H. Seaweed: Ecology, Nutrient Composition, and Medicinal Uses; Nova Science: Hauppauge, NY, USA, 2012. [Google Scholar]
- Collén, J.; Davison, I.R. Stress tolerance and reactive oxygen metabolism in the intertidal red seaweeds Mastocarpus stellatus and Chondrus crispus. Plant Cell Environ. 1999, 22, 1143–1151. [Google Scholar] [CrossRef]
- Lohrmann, N.L.; Logan, B.A.; Johnson, A.S. Seasonal acclimatization of antioxidants and photosynthesis in Chondrus crispus and Mastocarpus stellatus, two co-occurring red algae with differing stress tolerances. Biol. Bull. 2004, 207, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Young, E.G.; Smith, D.G. Amino acids, peptides, and proteins of Irish moss, Chondrus crispus. J. Biol. Chem. 1958, 233, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Laycock, M.V.; Craigie, J.S. The occurrence and seasonal variation of gigartinine and L-citrullinyl-L-arginine in Chondrus crispus Stackh. Can. J. Biochem. 1977, 55, 27–30. [Google Scholar] [CrossRef]
- Karsten, U.; Franklin, L.A.; Lüning, K.; Wiencke, C. Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 1998, 205, 257–262. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Trevor, R.; Pettitt, A.; Jones, L.; Harwood, J.L. Lipids of the marine red algae, Chondrus crispus and Polysiphonia lanosa. Phytochemistry 1989, 28, 399–405. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Maia, M.L.; Vieira, E.F.; Grosso, C.; Lopes, G.; Vasconcelos, V.; Hilliou, L.; Delerue-Matos, C. Integrated approach applying ultrasound-assisted extraction to recover bioactive material from Chondrus crispus. LWT 2023, 188, 115344. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Chambers, L.D.; Hellio, C.; Stokes, K.R.; Dennington, S.P.; Goodes, L.R.; Wood, R.J.K.; Walsh, F.C. Investigation of Chondrus crispus as a potential source of new antifouling agents. Int. Biodeterior. Biodegrad. 2011, 65, 939–946. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Dennington, S.P.; Stoodley, P.; Stokes, K.R. Anti-biofilm performance of three natural products against initial bacterial attachment. Int. J. Mol. Sci. 2013, 14, 21757–21780. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Mendes, M.; Pereira, R.; Pinto, I.S.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Carpena, M.; Caleja, C.; Pereira, E.; Pereira, C.; Ćirić, A.; Soković, M.; Soria-Lopez, A.; Fraga-Corral, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; et al. Red seaweeds as a source of nutrients and bioactive compounds: Optimization of the extraction. Chemosensors 2021, 9, 132. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. The short-term stress response–Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Upadhyay, A. Natural compounds in the regulation of proteostatic pathways: An invincible artillery against stress, ageing, and diseases. Acta Pharm. Sin. B 2021, 11, 2995–3014. [Google Scholar] [CrossRef]
- Sangha, J.S.; Fan, D.; Banskota, A.H.; Stefanova, R.; Khan, W.; Hafting, J.; Craigie, J.; Critchley, A.T.; Prithiviraj, B. Bioactive components of the edible strain of red alga, Chondrus crispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1180–1190. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef]
- Strugstad, M.; Despotovski, S. A summary of extraction, synthesis, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manag. 2012, 13. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hafting, J.; Critchley, A.T.; Banskota, A.H.; Prithiviraj, B. Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegans to Pseudomonas aeruginosa through the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 2013, 79, 7343–7350. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.B.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Sangha, J.S.; Wally, O.; Banskota, A.H.; Stefanova, R.; Hafting, J.T.; Critchley, A.T.; Prithiviraj, B. A cultivated form of a red seaweed (Chondrus crispus), suppresses β-amyloid-induced paralysis in Caenorhabditis elegans. Mar. Drugs 2015, 13, 6407–6424. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Sharma, N.; Kang, D.-K.; Paik, H.-D.; Park, Y.-S. Beyond probiotics: A narrative review on an era of revolution. Food Sci. Biotechnol. 2023, 32, 413–421. [Google Scholar] [CrossRef]
- Liu, J.; Kandasamy, S.; Zhang, J.; Kirby, C.W.; Karakach, T.; Hafting, J.; Critchley, A.T.; Evans, F.; Prithiviraj, B. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement. Altern. Med. 2015, 15, 279. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with antioxidant activity from marine macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef]
- Hussain, W.; Haleem, K.S.; Khan, I.; Tauseef, I.; Qayyum, S.; Ahmed, B.; Riaz, M.N. Medicinal plants: A repository of antiviral metabolites. Future Virol. 2017, 12, 299–308. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Lombardo, M.E.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M. Efficacy of a nasal spray containing iota-carrageenan in the postexposure prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. HIT: Lessons learned. Pathophysiol. Haemost. Thromb. 2006, 35, 50–57. [Google Scholar] [CrossRef]
- Krichen, F.; Ghlissi, Z.; Amor, I.B.; Sayari, N.; Kallel, R.; Gargouri, J.; Sahnoun, Z.; Boudawara, T.; Bougatef, A. In vitro and in vivo anti-coagulant activity and toxicological studies of marine sulfated glycosaminoglycans. Exp. Toxicol. Pathol. 2017, 69, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Neglected Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef] [PubMed]
- Adrião, A.A.; Dos Santos, A.O.; de Lima, E.J.; Maciel, J.B.; Paz, W.H.; da Silva, F.; Pucca, M.B.; Moura-da-Silva, A.M.; Monteiro, W.M.; Sartim, M.A. Plant-derived toxin inhibitors as potential candidates to complement antivenom treatment in snakebite envenomations. Front. Immunol. 2022, 13, 842576. [Google Scholar] [CrossRef]
- Da Silva, A.C.R.; Ferreira, L.G.; Duarte, M.E.R.; Fujii, M.T.; Sanchez, E.F.; Noseda, M.D.; Fuly, A.L. Protective effect of the sulfated agaran isolated from the red seaweed Laurencia aldingensis against toxic effects of the venom of the snake, Lachesis muta. Mar. Biotechnol. 2016, 18, 619–629. [Google Scholar] [CrossRef]
- Da Silva, A.C.R.; Pereira, K.K.G.; Critchley, A.T.; Sanchez, E.F.; Fuly, A.L. Potential utilization of a lambda carrageenan polysaccharide, derived from a cultivated, clonal strain of the red seaweed Chondrus crispus (Irish moss) against toxic actions of venom of Bothrops jararaca and B. jararacussu snakes. J. Appl. Phycol. 2020, 32, 4309–4320. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.P.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular λ-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef]
- Bae, N.-Y.; Kim, M.-J.; Kim, K.-B.-W.-R.; Park, J.-H.; Park, S.-H.; Sung, N.-Y.; Byun, E.-H.; Ahn, D.-H. Anti-Inflammatory Effect of Chondrus ocellatus Holmes Ethanol Extract on Lipopolysaccharide-induced Inflammatory Responses in RAW 264.7 Cells. Microbiol. Biotechnol. Lett. 2016, 44, 268–277. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, W.; Kong, L.; Zhou, B.; Hua, Y.; Han, Y.; Li, J.; Ji, J.; Fu, M.; Liu, W.; et al. Optimum conditions of ultrasound-assisted extraction and pharmacological activity study for phenolic compounds of the alga Chondrus ocellatus. J. Food Process. Preserv. 2022, 46, e16400. [Google Scholar] [CrossRef]
- Jaballi, I.; Saad, H.B.; Bkhairia, I.; Cherif, B.; Kallel, C.; Boudawara, O.; Droguet, M.; Magné, C.; Hakim, A.; Amara, I.B. Cytoprotective effects of the red marine alga Chondrus canaliculatus against Maneb-Induced hematotoxicity and bone oxidative damages in adult rats. Biol. Trace Elem. Res. 2018, 184, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Kang, W.-S.; Jeong, S.-M.; Ryu, S.-H.; Xu, X.; Lee, J.-E.; Ahn, D.-H. Anti-atopic effect of DNCB-induced mouse in Chondrus canaliculatus ethanol extracts. J. Korean Soc. Food Sci. Nutr. 2020, 49, 653–658. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Marcov, P.A.; Anastyuk, S.D.; Barabanova, A.O.B.; Glazunov, V.P.; Popov, S.V.; Ovodov, Y.S.; Yermak, I.M. Gelling polysaccharide from Chondrus armatus and its oligosaccharides: The structural peculiarities and anti-inflammatory activity. Carbohydr. Polym. 2015, 115, 768–775. [Google Scholar] [CrossRef]
- Cicinskas, E.; Begun, M.A.; Tiasto, V.A.; Belousov, A.S.; Vikhareva, V.V.; Mikhailova, V.A.; Kalitnik, A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. Part A 2019, 108, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Cicinskas, E.; Begun, M.A.; Vikhareva, V.V.; Karetin, Y.A.; Kalitnik, A.A. Immunological effects of Chondrus armatus carrageenans and their low molecular weight degradation products. J. Biomed. Mater. Res. Part A 2021, 109, 1136–1146. [Google Scholar] [CrossRef]
- Tiasto, V.A.; Goncharov, N.V.; Romanishin, A.O.; Zhidkov, M.E.; Khotimchenko, Y.S. κ-and λ-carrageenans from marine alga Chondrus armatus exhibit anticancer in vitro activity in human gastrointestinal cancers models. Mar. Drugs 2022, 20, 741. [Google Scholar] [CrossRef]
- Akatsuka, I. Biology of Economic Algae; SPB Academic Publishing: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Mikami, H. A Systematic Study of the Phyllophoraceae and Gigartinaceae from Japan and Its Vicinity; Scientific Papers of the Institute of Algological Research; Faculty of Science, Hokkaido University: Sapporo, Japan, 1965; Volume 5, pp. 181–285. [Google Scholar]
- Brodie, J.; Guiry, M.D.; Masuda, M. Life history, morphology and crossability of Chondrus ocellatus forma ocellatus and C. ocellatus forma crispoides (Gigartinales, Rhodophyta) from the north-western Pacific. Eur. J. Phycol. 1993, 28, 183–196. [Google Scholar] [CrossRef]
- Li, X.; Zhao, P.; Wang, G.; Li, D.; Wang, J.; Duan, D. Effects of temperature and irradiance on early development of Chondrus ocellatus Holm (Gigartinaceae, Rhodophyta). Chin. J. Oceanol. Limnol. 2010, 28, 508–513. [Google Scholar] [CrossRef]
- Kang, J. Illustrated Encyclopedia of Fauna & Flora of Korea. In Marine Algae; Ministry of Education: Seoul, Republic of Korea, 1968; Volume 8, p. 465. [Google Scholar]
- Jiang, F.; Zhang, Y. The Dictionary of Chinese Oceanic Medicine; China Ocean Press: Beijing, China, 1993. [Google Scholar]
- Song, Y.; Wang, H.; Wang, X.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Comparative lipidomics study of four edible red seaweeds based on RPLC–Q-TOF. J. Agric. Food Chem. 2023, 71, 2183–2196. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, H.K.; Lee, H.J. Carbon dots for the treatment of inflammatory diseases: An appraisal of in vitro and in vivo studies. Oxidative Med. Cell. Longev. 2023, 2023, 3076119. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Bjarnason, I.; Hayllar, J.; Macpherson, A.J.; Russell, A.S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993, 104, 1832–1847. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Nah, J.-W.; Jeon, Y.-J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- DasNandy, A.; Virge, R.; Hegde, H.V.; Chattopadhyay, D. A review of patent literature on the regulation of glucose metabolism by six phytocompounds in the management of diabetes mellitus and its complications. J. Integr. Med. 2023, 21, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pandita, G.; Bhosale, Y.K. Anthocyanin: Potential tool for diabetes management and different delivery aspects. Trends Food Sci. Technol. 2023, 140, 104170. [Google Scholar] [CrossRef]
- Tripathy, B.; Sahoo, N.; Sahoo, S.K. Trends in diabetes care with special emphasis to medicinal plants: Advancement and treatment. Biocatal. Agric. Biotechnol. 2021, 33, 102014. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gao, L.; Zhou, H.; Ai, C.; Huang, X.; Wang, M.; Zhang, Y.; Zhao, C. Opportunities and challenges of algal fucoidan for diabetes management. Trends Food Sci. Technol. 2021, 111, 628–641. [Google Scholar] [CrossRef]
- Agarwal, S.; Singh, V.; Chauhan, K. Antidiabetic potential of seaweed and their bioactive compounds: A review of developments in last decade. Crit. Rev. Food Sci. Nutr. 2023, 63, 5739–5770. [Google Scholar] [CrossRef] [PubMed]
- Edding, M.; Fonck, E.; Acuna, R.; Tala, F. Cultivation of Chondrus canaliculatus (C. Agardh) Greville (Gigartinales, Rhodophyta) in controlled environments. Aquac. Int. 2008, 16, 283–295. [Google Scholar] [CrossRef]
- Sharma, A.; Patnai, P.; Mehta, N.; Dalwadi, P. Effect of coenzyme Q10 alone and it’s combination with pentoxifylline in cisplatin-induced nephrotoxicity in rats. J. Pharm. Sci. Biosci. Res. 2016, 6, 790–800. [Google Scholar]
- Yang, C.; Yang, B. Acute kidney injury in China: A neglected truth and perspective. Asian J. Urol. 2016, 3, 4–5. [Google Scholar] [CrossRef]
- Basist, P.; Parveen, B.; Zahiruddin, S.; Gautam, G.; Parveen, R.; Khan, M.A.; Krishnan, A.; Shahid, M.; Ahmad, S. Potential nephroprotective phytochemicals: Mechanism and future prospects. J. Ethnopharmacol. 2022, 283, 114743. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, C.; Smogorzewski, J. Update on atopic dermatitis. Adv. Pediatr. 2023, 70, 157–170. [Google Scholar] [CrossRef]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on atopic dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-R.; Hsu, K.-T.; Hsu, W.-H.; Lee, B.-H.; Li, T.-L.; Chan, Y.-L.; Wu, C.-J. Immunomodulation and mechanisms of fucoidan from Cladosiphon okamuranus ameliorates atopic dermatitis symptoms. Int. J. Biol. Macromol. 2021, 189, 537–543. [Google Scholar] [CrossRef]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2, 4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef] [PubMed]
- Luan, R.; Zhang, S. Studies on the genus Chondrus (Gigartinaceae) from Dalian, China. J. Syst. Evol. 1998, 36, 268–272. [Google Scholar]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Arisawa, M.; Hayashi, K.; Nikaido, T.; Koike, K.; Fujita, D.; Nunomura, N.; Tanaka, M.; Sasaki, T. Screening of Some Marine Organism Extracts for cAMP Phosphodiesterase Inhibition, Cytotoxicity, and Antiviral Activity against HSV-1. Int. J. Pharmacogn. 1997, 35, 6–11. [Google Scholar] [CrossRef]
- Kim, M.-J.; Bae, N.-Y.; Kim, K.-B.-W.-R.; Park, J.-H.; Park, S.-H.; Jang, M.-R.; Ahn, D.-H. Anti-inflammatory effect of Chondrus nipponicus Yendo ethanol extract on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Korean Soc. Food Sci. Nutr. 2016, 45, 194–201. [Google Scholar] [CrossRef]
- He, X.; Yamauchi, A.; Nakano, T.; Yamaguchi, T.; Ochiai, Y. The composition and anti-inflammatory effect of polysaccharides from the red alga Chondrus verrucosus. Fish. Sci. 2019, 85, 859–865. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef]
- Du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Marques, V.; Afonso, M.B.; Rodrigues, C.M.; Pereira, L. Antitumour potential of Gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs 2020, 18, 50. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, X.; Duan, M.; Ai, C.; Song, S.; Chen, X. In vitro fermentation of κ-carrageenan oligosaccharides by human gut microbiota and its inflammatory effect on HT29 cells. J. Func. Foods 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrason. Sonochem. 2021, 78, 105686. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, W.; Qiu, Y.; Wang, S.; Peng, Y.; Zhu, Y.; Zhuang, S. Lipids monitoring in Scenedesmus obliquus based on terahertz technology. Biotechnol. Biofuels 2020, 13, 161. [Google Scholar] [CrossRef]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef]
- Domingo, G.; Marsoni, M.; Álvarez-Viñas, M.; Torres, M.D.; Domínguez, H.; Vannini, C. The role of protein-rich extracts from Chondrus crispus as biostimulant and in enhancing tolerance to drought stress in tomato plants. Plants 2023, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Soares Dias, A.P.; Rijo, B.; Santos, F.; Galhano dos Santos, R.; Frade, T. Overview on biofuels production in a seaweed biorefinery. Sci. Total Environ. 2023, 884, 163714. [Google Scholar] [CrossRef]
- Raikova, S.; Le, C.D.; Beacham, T.A.; Jenkins, R.W.; Allen, M.J.; Chuck, C.J. Towards a marine biorefinery through the hydrothermal liquefaction of macroalgae native to the United Kingdom. Biomass Bioenergy 2017, 107, 244–253. [Google Scholar] [CrossRef]
- Thomas, I.; Siew, L.Q.; Watts, T.J.; Haque, R. Seaweed allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 714–715. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Sharma, A.; Lee, H.-J. An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus. Mar. Drugs 2024, 22, 47. https://doi.org/10.3390/md22010047
Park S-J, Sharma A, Lee H-J. An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus. Marine Drugs. 2024; 22(1):47. https://doi.org/10.3390/md22010047
Chicago/Turabian StylePark, Seon-Joo, Anshul Sharma, and Hae-Jeung Lee. 2024. "An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus" Marine Drugs 22, no. 1: 47. https://doi.org/10.3390/md22010047
APA StylePark, S.-J., Sharma, A., & Lee, H.-J. (2024). An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus. Marine Drugs, 22(1), 47. https://doi.org/10.3390/md22010047








