Ascophyllum nodosum (Linnaeus) Le Jolis from Arctic: Its Biochemical Composition, Antiradical Potential, and Human Health Risk
Abstract
1. Introduction
2. Results and Discussion
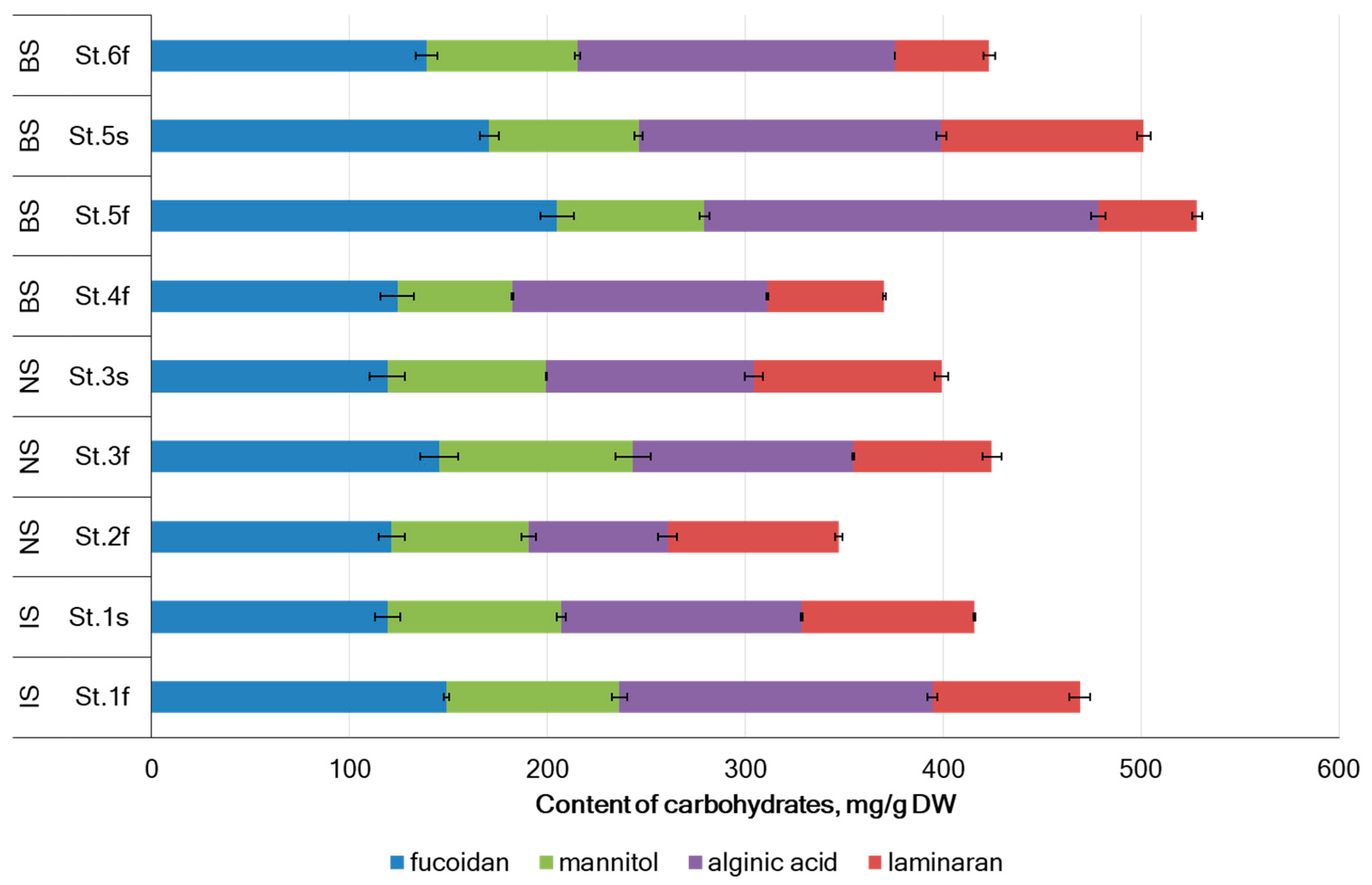
2.1. Carbohydrates Composition
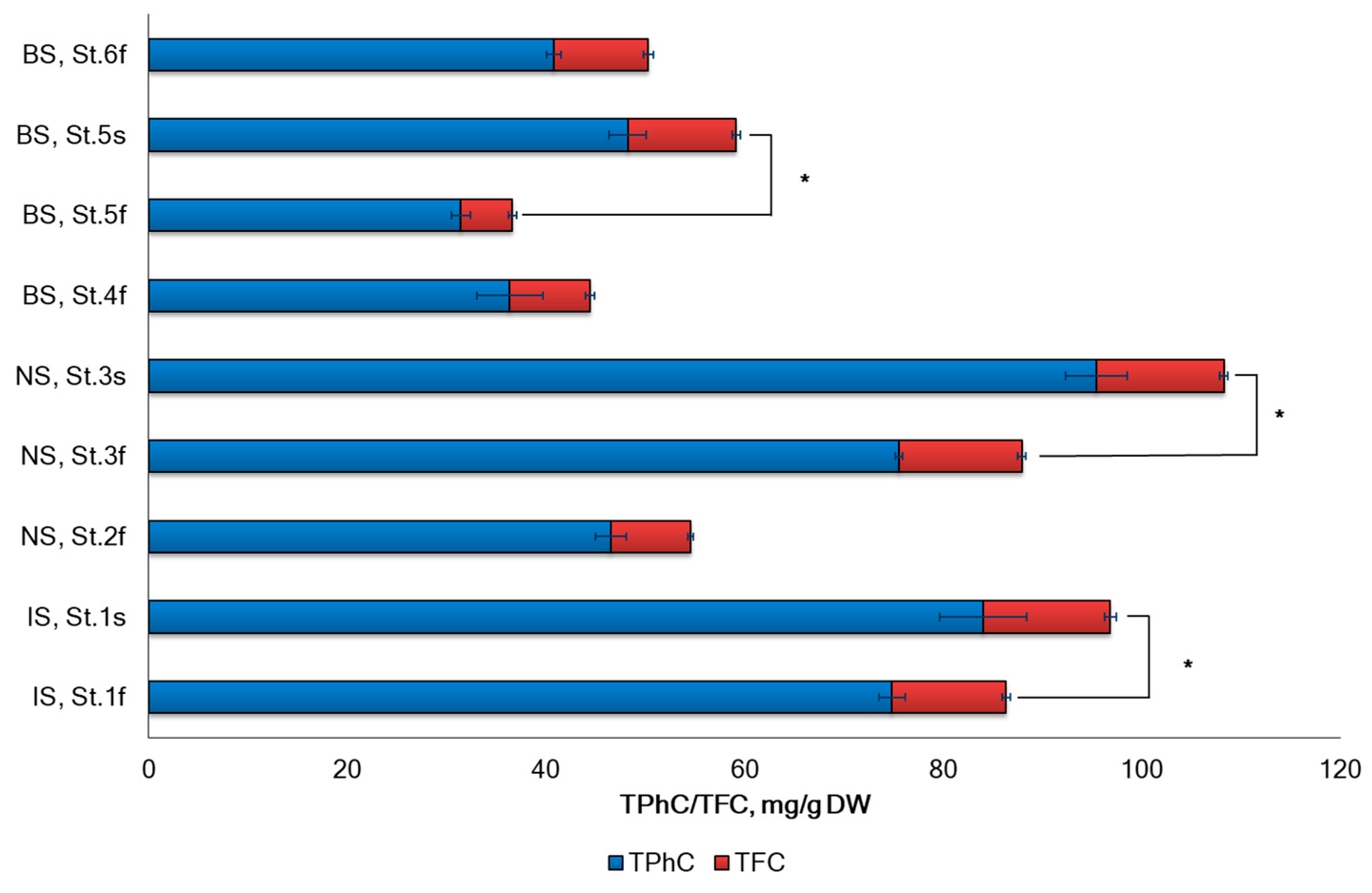
2.2. Polyphenols and Flavonoids Content
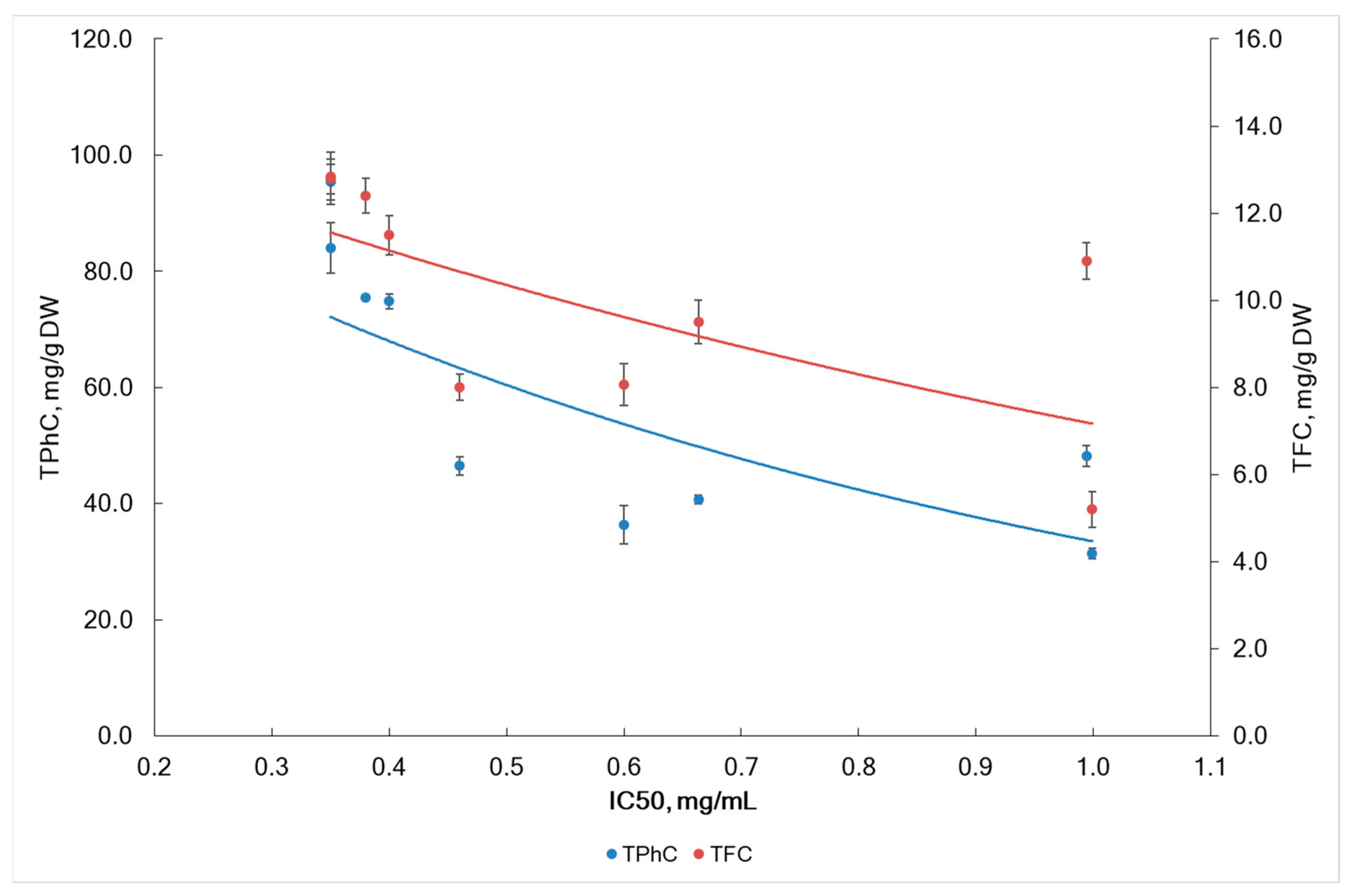
2.3. DPPH Radical Scavenging Activity
2.4. Contents of Elements
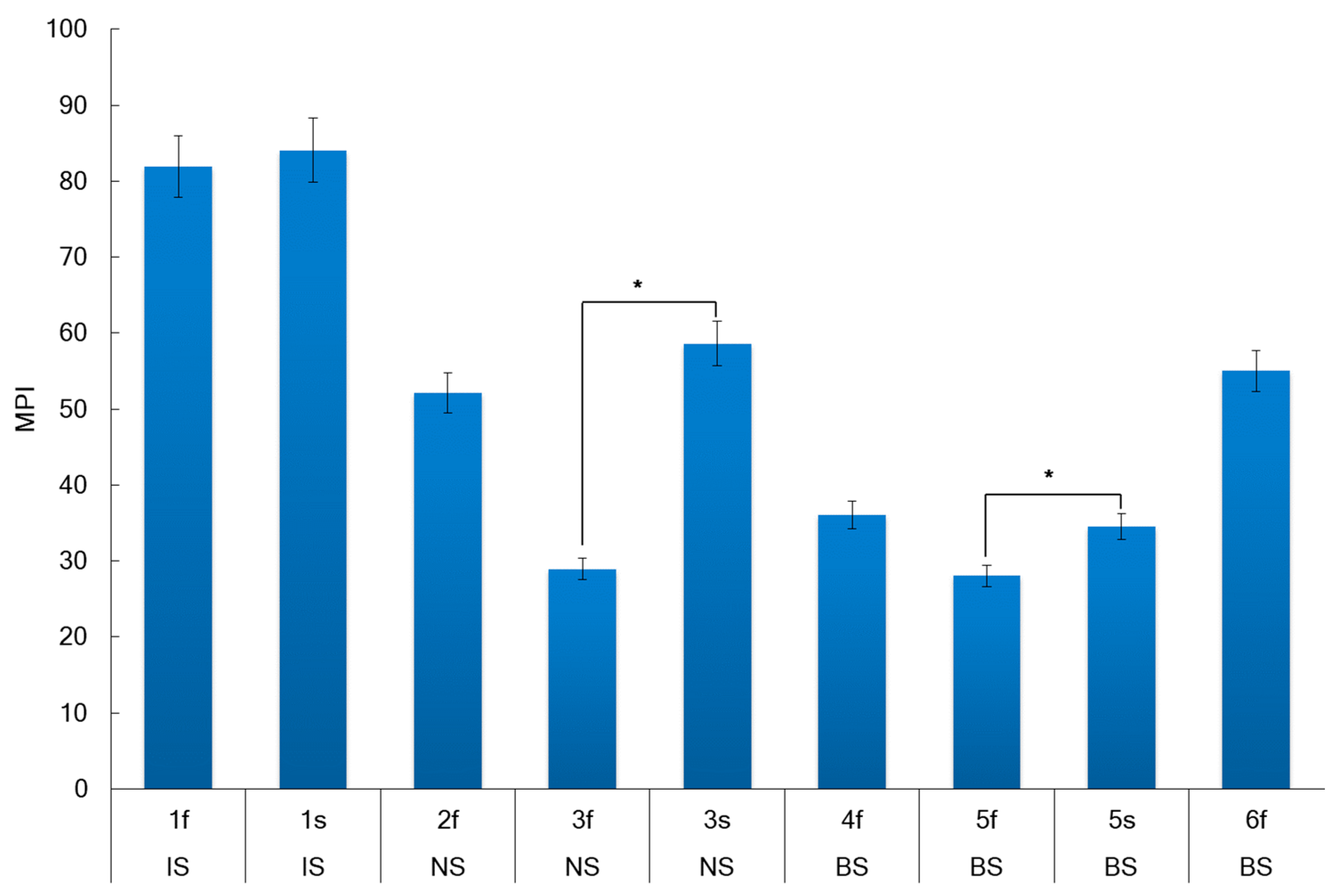
2.5. Metal Pollution Index
2.6. Human Health Risk
3. Materials and Methods
3.1. Samples Collection
3.2. Chemicals and Reagents
3.3. Carbohydrates Composition
3.4. Analysis of Total Phenolic, Total Flavonoids, and Antiradical Activity
3.5. Element Analysis
3.6. Metal Pollution Index
3.7. Assessments of Human Health Risk
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Ireland, Galway, 2023; Available online: https://www.algaebase.org (accessed on 31 October 2023).
- WoRMS Editorial Board. World Register of Marine Species. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=145541 (accessed on 31 October 2023).
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Tropin, I.V.; Radzinskaya, N.V.; Voskoboinikov, G.M. The Influence of salinity on the rate of dark respiration and structure of the cells of brown algae thalli from the Barents Sea littoral. Biol. Bull. 2003, 30, 40–47. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. I. Estimation of absolute polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1977, 30, 209–221. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polymers 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Moreira, R.; Sineiro, J.; Chenlo, F.; Arufe, S.; Díaz-Varela, D. Aqueous extracts of Ascophyllum nodosum obtained by ultrasound-assisted extraction: Effects of drying temperature of seaweed on the properties of extracts. J. Appl. Phycol. 2017, 29, 3191–3200. [Google Scholar] [CrossRef]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a Pivotal Biostimulant toward Sustainable Agriculture: A Comprehensive Review. Agriculture 2023, 13, 1179. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave assisted step-by-step process for the production of fucoidan, alginate sodium, sugars and biochar from Ascophyllum nodosum through a biorefinery concept. Bioresour. Technol. 2015, 198, 819–827. [Google Scholar] [CrossRef]
- Tabakaeva, O.V.; Razgonova, M.P.; Tabakaev, A.V.; Kapusta, S.V.; Zinchenko, Y.N. Qualitative and quantitative composition of carotenoids in extracts of the brown alga Ascophyllum nodosum. Chem. Nat. Compd. 2023, 59, 999–1001. [Google Scholar] [CrossRef]
- Ali, J.; Jan, I.; Ullah, H.; Ahmed, N.; Alam, M.; Ullah, R.; El-Sharnouby, M.; Kesba, H.; Shukry, M.; Sayed, S.; et al. Influence of Ascophyllum nodosum extract foliar spray on the physiological and biochemical attributes of okra under drought stress. Plants 2022, 11, 790. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Shen, P.; Gu, Y.; Zhang, C.; Sun, C.; Qin, L.; Yu, C.; Qi, H. Metabolomic approach for characterization of polyphenolic compounds in Laminaria japonica, Undaria pinnatifida, Sargassum fusiforme and Ascophyllum nodosum. Foods 2021, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Garcia-Vaquero, M.; Poojary, M.M.; Lund, M.N.; O'Donnell, C.; Zhang, Z.; Tiwari, B.K. Green extraction of proteins, umami and other free amino acids from brown macroalgae Ascophyllum nodosum and Fucus vesiculosus. J. Appl. Phycol. 2021, 33, 4083–4091. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Pete, R.; Küpper, F.C.; Glombitza, K.-W.; König, G.M. Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 2009, 44, 331–338. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D'Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro anti-inflammatory activities of fucoidans from five species of brown seaweeds Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural characterization and in vivo anti-inflammatory activity of fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Wang, J.C.; Liao, W.I.; Hsu, Y.J.; Lin, C.Y.; Liao, M.T.; Huang, P.H.; Lin, S.J. Fucoidan attenuates angiotensin II-induced abdominal aortic aneurysms through the inhibition of c-Jun N-terminal kinase and nuclear factor κB activation. J. Vasc. Surg. 2018, 68, 72S–81S. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Colliec-Jouault, S. 5-Skin tissue engineering using functional marine biomaterials. In Functional Marine Biomaterials; Kim, S.-K., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 69–90. [Google Scholar] [CrossRef]
- Jiménez, J.T.; O’Connell, S.; Lyons, H.; Bradley, B.; Hall, M. Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. Chem. Pap. 2010, 64, 434–442. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant potential of extracts obtained from macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Dutot, M.; Fagon, R.; Hemon, M.; Rat, P. Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl. Biochem. Biotechnol. 2012, 167, 2234–2240. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.-F.; Coiffard, L.; C´erantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Kim, S.-K.; Pangestuti, R. Biological properties of cosmeceuticals derived from marine algae. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–10. [Google Scholar]
- Gisbert, M.; Franco, D.; Sineiro, J.; Moreira, R. Antioxidant and antidiabetic properties of phlorotannins from Ascophyllum nodosum seaweed extracts. Molecules 2023, 28, 4937. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Wang, X.; Jiang, H.; Cai, C.; Hao, J.; Yu, G. Fucoidan from Ascophyllum nodosum suppresses postprandial hyperglycemia by inhibiting Na+/Glucose cotransporter 1 activity. Mar. Drugs 2020, 18, 485. [Google Scholar] [CrossRef]
- Ponce, N.M.; Stortz, C.A. A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, A.; Falshaw, R.; Itzhaki, R. Anti-HSV-1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Shikov, A.N. Biochemical composition, antiradical potential and human health risk of the Arctic edible brown seaweed Fucus spiralis L. J. Appl. Phycol. 2023, 35, 365–380. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.; Shevchenko, N.M.; Sukhoverkhov, S.V. Influence of the season on the composition and structural characteristics of brown algae polysaccharides. Chem. Nat. Compd. 2009, 6, 661–665. [Google Scholar]
- Honya, M.; Mori, H.; Anzai, M.; Araki, Y.; Nisizawa, K. Monthly changes in the content of fucans, their constituent sugars and sulphate in cultured Laminaria japonica. Hydrobiologia 1999, 398, 411–416. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Shahrestani, R.G. Innovative strategies for engineering mannitol production. Trends Food Sci. Technol. 2009, 20, 263–270. [Google Scholar] [CrossRef]
- Manns, D.; Nielsen, M.M.; Bruhn, A.; Saake, B.; Meyer, A.S. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J. Appl. Phycol. 2017, 29, 1493–1506. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D. Comparative chemical composition of the Barents Sea brown algae. Appl. Biochem. Microbiol. 2008, 44, 305–309. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Parshina, A.; Ivanchenko, N.; Polomarchuk, D. Seasonal variations in the chemical composition of Arctic brown macroalgae. Algal. Res. 2023, 72, 103112. [Google Scholar] [CrossRef]
- Klindukh, M.P.; Obluchinskaya, E.D.; Matishov, G.G. Seasonal changes in the mannitol and proline contents of the brown alga Fucus vesiculosus L. on the Murman coast of the Barents Sea. Dokl. Biol. Sci. 2021, 441, 373–376. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Hrólfsdóttir, A.Þ.; Arason, S.; Sveinsdóttir, H.I.; Gudjónsdóttir, M. Added value of Ascophyllum nodosum side stream utilization during seaweed meal processing. Mar. Drugs 2022, 20, 340. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Klochkova, N.G. Polysaccharides of algae: 55. Polysaccharide composition of several brown algae from Kamchatka. Russ. J. Bioorg. Chem. 2001, 27, 395–399. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O'Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J. Appl. Phycol. 2016, 28, 533–543. [Google Scholar] [CrossRef]
- Wiencke, C.; Gómez, I.; Dunton, K. Phenology and seasonal physiological performance of polar seaweeds. Bot. Mar. 2009, 52, 585–592. [Google Scholar] [CrossRef]
- Gómez, I.; Wiencke, C. Seasonal changes in C, N and major organic compounds and their significance to morpho-functional processes in the endemic Antarctic brown alga Ascoseira mirabilis. Polar Biol. 1998, 19, 115–124. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Åberg, P.; Pavia, H. Temporal and multiple scale spatial variation in juvenile and adult abundance of the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 1997, 58, 111–119. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef]
- Apostolidis, E.; Karayannakidis, P.D.; Kwon, Y.I.; Lee, C.M.; Seeram, N.P. Seasonal variation of phenolic antioxidant-mediated α-glucosidase inhibition of Ascophyllum nodosum. Plant Foods Hum. Nutr. 2011, 66, 313–319. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Kraberg, A.C.; Norton, T.A. Effect of epiphytism on reproductive and vegetative lateral formation in the brown, intertidal seaweed Ascophyllum nodosum (Phaeophyceae). Phycol. Res. 2007, 55, 17–24. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Breton, F.; Cérantola, S.; Ar Gall, E. Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). J. Exp. Mar. Biol. Ecol. 2011, 399, 167–172. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Valentão, P.; Trindade, P.; Gomes, D.; Guedes de Pinho, P.; Mouga, T.; Andrade, P.B. Codium tomentosum and Plocamium cartilagineum: Chemistry and antioxidant potential. Food Chem. 2010, 119, 1359–1368. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Andrade, L.R.; Salgado, L.T.; Farina, M.; Pereira, M.S.; Mourao, P.A.; Amado Filho, G.M. Ultrastructure of acidic polysaccharides from the cell walls of brown algae. J. Struct. Biol. 2004, 145, 216–225. [Google Scholar] [CrossRef]
- Mariani, P.; Tolomio, C.; Braghetta, P. An ultrastructural approach to the adaptive role of the cell wall in the intertidal alga Fucus virsoides. Protoplasma 1985, 128, 208–217. [Google Scholar] [CrossRef]
- De Andrade, L.R.; Farina, M.; Amado Filho, G.M. Role of Padina gymnospora (Dictyotales, Phaeophyceae) cell walls in cadmium accumulation. Phycologia 2002, 41, 39–48. [Google Scholar] [CrossRef]
- Jensen, A.; Larsen, B.; Indegaard, M. Trondheimsfjorden; Sakshaug, E., Sneli, J.A., Eds.; Tapir Academic Press: Trondheim, Norway, 2000; pp. 157–168. [Google Scholar]
- Ronan, J.M.; Stengel, D.B.; Raab, A.; Feldmann, J.; O'Hea, L.; Bralatei, E.; McGovern, E. High proportions of inorganic arsenic in Laminaria digitata but not in Ascophyllum nodosum samples from Ireland. Chemosphere 2017, 186, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lin, L.; Wu, M.; Yu, H.; Shang, T.; Zhang, T.; Zhao, M. Total and inorganic arsenic contents in seaweeds: Absorption, accumulation, transformation and toxicity. Aquaculture 2018, 497, 49–55. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- Güven, K.C.; Akyüz, K.; Yurdun, T. Selectivity of heavy metal binding by algal polysaccharides. Toxicol. Environ. Chem. 1995, 47, 65–70. [Google Scholar] [CrossRef]
- Ragan, M.A.; Smidsrød, O.; Larsen, B. Chelation of divalent metal ions by brown algal polyphenols. Mar. Chem. 1979, 7, 265–271. [Google Scholar] [CrossRef]
- Riget, F.; Johansen, P.; Asmund, G. Baseline levels and natural variability of elements in three seaweed species from West Greenland. Mar. Pollut. Bull. 1997, 34, 171–176. [Google Scholar] [CrossRef]
- Fuge, R.; James, K.H. Trace metal concentrations in Fucus from the Bristol Channel. Mar. Pollut. Bull. 1974, 5, 9–12. [Google Scholar] [CrossRef]
- Villares, R.; Puente, X.; Carballeira, A. Seasonal variation and background levels of heavy metals in two green seaweeds. Environ. Pollut. 2002, 119, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Oaten, J.F.P.; Gibson, M.C.; Hudson, M.D.; Jensen, A.C.; Williams, I.D. Metal accumulation and metallothionein response in Fucus spiralis. Int. J. Environ. Pollut. Remed. 2017, 5, 1–14. [Google Scholar] [CrossRef][Green Version]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Nova, P.; Pimenta-Martins, A.; Maricato, É.; Nunes, C.; Abreu, H.; Coimbra, M.A.; Freitas, A.C.; Gomes, A.M. Chemical Composition and Antioxidant Potential of Five Algae Cultivated in Fully Controlled Closed Systems. Molecules 2023, 28, 4588. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Chen, F.; Du, M.; Blumberg, J.B.; Chui, K.K.H.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association among dietary supplement use, nutrient intake, and mortality among US adults: A cohort study. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef]
- Costello, R.B.; Rosanoff, A.; Dai, Q.; Saldanha, L.G.; Potischman, N.A. Perspective: Characterization of dietary supplements containing calcium and magnesium and their respective ratio—Is a rising ratio a cause for concern? Adv. Nutr. 2021, 12, 291–297. [Google Scholar] [CrossRef]
- Molvær, J.; Knutzen, J.; Magnusson, J.; Rygg, B.; Skei, J.; Sørensen, J. SFT TA-1467/1997; Classification of Environmental Quality in Fjords and Coastal Waters. A Guide Norwegian Pollution Control Authority. Norwegian Institute for Water Research: Oslo, Norway, 1997; p. 36.
- European Union Commission Regulation (EC) No 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2008, 173, 6–9.
- Morrison, L.; Baumann, H.A.; Stengel, D.B. An assessment of metal contamination along the Irish coast using the seaweed Ascophyllum nodosum (Fucales, Phaeophyceae). Environ Pollut. 2008, 152, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, L119, 103–157.
- World Health Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- JECFA. Safety Evaluation of Certain Food Additives and Contaminants. 67th Joint FAO/WHO Expert Committee on Food Additives; WHO: Rome, Italy, 2006; Available online: https://www.fao.org/3/at874e/at874e.pdf (accessed on 2 May 2023).
- Norms of Physiological Needs Energy and Nutrients for Various Groups of the Population of the Russian Federation. In Methodical Recommendations MR 2.3.1.2432−08; Federal Center for Hygiene and Epidemiology of Rospotrebnadzor: Moscow, Russia, 2009.
- SanPiN 2.3.2.1078-01; Food Raw Materials and Food Products Hygiene Requirements for Safety and Nutritional Food. Sanitary-Epidemiological Rules and Standards: Moscow, Russia, 2002.
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Lee, C.M. Brown seaweed—Derived phenolic phytochemicals and their biological activities for functional food ingredients with focus on Ascophyllum nodosum. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 356–370. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; Science Publishers: Boca Raton, FL, USA, 2016; p. 448. [Google Scholar]
- Vafina, L.K.; Podkorytova, A.V. New products of functional nutrition on the basis of bioactive substances from brown algae. Izv. TINRO 2009, 156, 348–356. [Google Scholar]
- World Health Organization. Report of the Expert Meeting on Food Safety for Seaweed—Current Status and Future Perspectives, Rome, Italy, 28–29 October 2021; Food Safety and Quality Series No. 13; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- CEVA. Edible Seaweed and Microalgae—Regulatory Status in France and Europe (19 March 2019). 2019. Available online: https://www.ceva-algues.com/wp-content/uploads/2020/03/CEVA-Edible-algae-FR-and-EU-regulatory-update-2019.pdf (accessed on 27 November 2023).
- EFSA European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals. February 2006. Available online: www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 27 November 2023).
- SCF. Scientific Committee for Food of the European Communities. 2011. Available online: http://ec.europa.eu/food/fs/sc/scf/index_en.html (accessed on 2 May 2023).
- Moon, K.A.; Oberoi, S.; Barchowsky, A.; Chen, Y.; Guallar, E.; Nachman, K.E.; Rahman, M.; Sohel, N.; D’Ippoliti, D.; Wade, T.J.; et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 2017, 46, 1924–1939. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Z.; Lin, Y.; Zhang, D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: A meta-analysis. J. Epidemiol. Community Health 2014, 68, 176–184. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C. In Arsenic, Metals, Fibres, and Dusts; WHO: Geneva, Switzerland, 2012; Available online: https://publications.iarc.fr/120 (accessed on 27 November 2023).
- Sartal, C.G.; Alonso, M.C.B.; Barrera, P.B. Arsenic in seaweed: Presence, bioavailability and speciation. In Seafood Science: Advances in Chemistry Technology and Applications; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2014; pp. 276–351. [Google Scholar]
- Chien, L.C.; Hung, T.C.; Choang, K.Y.; Yeh, C.Y.; Meng, P.J.; Shieh, M.J.; Ha, B.C. Daily intake of TBT, Cu, Zn, Cd and as for fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar] [CrossRef]
- US EPA. EPA/600/R-06/013F; Concepts, Methods and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document. U.S. Environmental Protection Agency: Washington, DC, USA; National Center for Environmental Assessment: Cincinnati, OH, USA, 2007.
- Khandaker, M.U.; Chijioke, N.O.; Heffny, N.A.B.; Bradley, D.A.; Alsubaie, A.; Sulieman, A.; Faruque, M.R.I.; Sayyed, M.I.; Al-mugren, K.S. Elevated concentrations of metal(loids) in seaweed and the concomitant exposure to humans. Foods 2021, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Integrated Risk Information System (IRIS) Database; National Centre for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2000; Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0550_summary.pdf (accessed on 27 October 2023).
- Obluchinskaya, E.; Zakharova, L. Metal concentrations in three species of Fucus L. on the Murmansk coast of the Barents Sea. Polar Sci. 2021, 28, 100646. [Google Scholar] [CrossRef]
- Dische, Z.; Shettles, L.B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. Biol. Chem. 1948, 175, 595–603. [Google Scholar] [CrossRef]
- Obluchinskaya, E.; Daurtseva, A. Effects of air drying and freezing and long-term storage on phytochemical composition of brown seaweeds. J. Appl. Phycol. 2020, 32, 4235–4249. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]
- Huggett, A.S.G.; Nixoh, D.A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet 1957, 273, 368–370. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown alga Durvillaea Antarctica. Dry Technol. 2020, 38, 1915–1928. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Vargas, N.; Pasten, A.; Rodríguez, K.; Ah-Hen, K.S. Phytochemical components and amino acid profile of brown seaweed Durvillaea antarctica as affected by air drying temperature. J. Food Sci. Technol. 2018, 55, 4792–4801. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Botić, V.; Crnjac, A.; Smoljo, M.; Soldo, B.; Ljubenkov, I.; Čagalj, M.; Skroza, D. Bioactive phenolic metabolites from Adriatic brown algae Dictyota dichotoma and Padina pavonica (Dictyotaceae). Foods 2021, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant capacity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Flores, É.M.D.M.; Barin, J.S.; Paniz, J.N.G.; Medeiros, J.A.; Knapp, G. Microwave-assisted sample combustion: A technique for sample preparation in trace element determination. Anal. Chem. 2004, 76, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Usero, J.; Morillo, J.; Gracia, I. Heavy metal concentrations in molluscs from the Atlantic coast of southern Spain. Chemosphere 2005, 59, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Human Health Risk Assessment; Environmental Protection Agency: Washington, DC, USA, 2020; Available online: https://www.epa.gov/risk/human-health-risk-assessment (accessed on 27 November 2023).
- Siddique, M.A.M.; Hossain, M.S.; Islam, M.M.; Rahman, M.; Kibria, G. Heavy metals and metalloids in edible seaweeds of Saint Martin's Island, Bay of Bengal, and their potential health risks. Mar. Pollut. Bull. 2022, 181, 113866. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Reference Dose (RfD): Description and Use in Health Risk Assessments, Background Document 1a. 1993. Available online: https://www.epa.gov/iris/reference-doserfd-description-and-usehealth-risk-assessments (accessed on 27 November 2023).
- US EPA. Guidelines for Carcinogen Risk Assessment (epa/630/p-03/001f). 2005. Available online: https://www.epa.gov/risk/guidelines-carcinogen-risk-assessment (accessed on 27 November 2023).



| Sea, Station | Reproductive Phase | Fucose, mg/g DW | Xylose, mg/g DW | Fucose:Xylose Proportion |
|---|---|---|---|---|
| IS, St. 1 | Fertile | 74.4 ± 1.2 * | 12.4 ± 0.3 * | 1.0:0.2 * |
| Sterile | 59.6 ± 2.9 | 15.9 ± 1.2 | 1.0:0.3 | |
| NS, St. 2 | Fertile | 60.6 ± 0.7 | 15.8 ± 0.3 | 1.0:0.3 |
| NS, St. 3 | Fertile | 72.6 ± 2.9 * | 12.0 ± 0.9 * | 1.0:0.1 * |
| Sterile | 59.5± 1.8 | 15.8 ± 1.1 | 1.0:0.3 | |
| BS, St. 4 | Fertile | 62.1 ± 1.3 | 16.7 ± 1.0 | 1.0:0.3 |
| BS, St. 5 | Fertile | 102.4 ± 0.8 * | 22.3 ± 0.4 | 1.0:0.2 * |
| Sterile | 85.3 ± 2.5 | 24.5 ± 2.0 | 1.0:0.3 | |
| BS, St. 6 | Fertile | 69.4 ± 0.4 | 16.6 ± 0.4 | 1.0:0.2 |
| Element | LOQ | Mean ± SD | Range (Min–Max) | IS, St. 1 | NS, St. 2 | NS, St. 3 | BS, St. 4 | BS, St. 5 | BS, St. 6 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fertile | ∆ | Fertile | Fertile | ∆ | Fertile | Fertile | ∆ | Fertile | ||||
| Sterile | Sterile | Sterile | ||||||||||
| Al | 1.6 | 60.2 ± 55.6 | 16.3–168.6 | 137.7 ± 21.7 | ↑ | 33.6 ± 5.4 | 16.3 ± 10.5 | * | 36.8 ± 1.5 | 22.0 ± 1.2 | – | 36.1 ± 0.1 |
| 168.6 ± 43.5 | 70.5 ± 11.6 | ↑ | 20.0 ± 1.2 | |||||||||
| As | 6.3 | 32.2 ± 7.9 | 21.5–46.6 | 26.7 ± 2.0 | ↑ | 46.6 ± 1.8 | 28.1 ± 2.4 | * | 36.0 ± 1.3 | 21.5 ± 0.8 | * | 34.0 ± 0.7 |
| 30.8 ± 2.5 | 40.5 ± 4.8 | ↑ | 25.2 ± 1.2 | ↑ | ||||||||
| Ba | 0.016 | 6.6 ± 1.3 | 5.2–8.6 | 5.23 ± 0.11 | * | 6.4 ± 0.3 | 5.3 ± 1.6 | – | 6.3 ± 0.2 | 5.6 ± 0.3 | * | 7.4 ± 0.1 |
| 8.55 ± 0.06 | ↑ | 6.1 ± 0.2 | 8.6 ± 0.1 | ↑ | ||||||||
| Ca | 1.9 | 15,595 ± 5760 | 10,973–26,916 | 17,591 ± 544 | * | 12,256 ± 195 | 10,973 ± 106 | * | 12,377 ± 29 | 12,445 ± 56 | * | 11,453 ± 233 |
| 26,916 ± 813 | ↑ | 23,173 ± 199 | ↑ | 13,202 ± 27 | ↑ | |||||||
| Cd | 0.23 | <LOQ | <LOQ | <LOQ | <LOQ | < LOQ | < LOQ | < LOQ | <LOQ | |||
| Co | 0.12 | 0.70 ± 0.38 | 0.31–1.50 | 1.50 ± 0.04 | * | 0.64 ± 0.08 | 0.60 ± 0.05 | ↑ | 0.93 ± 0.09 | 0.31 ± 0.01 | – | 0.35 ± 0.05 |
| 0.84 ± 0.02 | ↑ | 0.77 ± 0.12 | 0.33 ± 0.04 | |||||||||
| Cr | 0.13 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |||
| Cu | 0.37 | 3.17 ± 2.61 | <LOQ–7.31 | 7.31 ± 0.90 | * | 0.67 ± 0.03 | <LOQ | * | <LOQ | <LOQ | <LOQ | <LOQ |
| 2.33 ± 0.02 | ↑ | 1.59 ± 0.82 | ↑ | <LOQ | ||||||||
| Fe | 0.098 | 90.0 ± 47.7 | 23.5–179.7 | 179.7 ± 25.2 | 89.1 ± 4.2 | 23.5 ± 10.1 | * | 83.9 ± 1.7 | 53.7 ± 1.7 | * | 88.9 ± 0.5 | |
| 149.1 ± 48.6 | ↓ | 77.7 ± 5.0 | ↑ | 64.1 ± 3.2 | ↑ | |||||||
| Mg | 1.7 | 9969 ± 933 | 8804–11,709 | 11,709 ± 101 | * | 9867 ± 37 | 9159 ± 57 | * | 9252 ± 27 | 8804 ± 199 | * | 10,598 ± 23 |
| 10,091 ± 14 | ↓ | 9434 ± 80 | ↑ | 10,810 ± 615 | ↑ | |||||||
| Mn | 0.058 | 14.0 ± 8.8 | 7.8–33.4 | 33.4 ± 0.1 | * | 12.6 ± 0.2 | 8.2 ± 3.2 | – | 8.4 ± 0.1 | 7.8 ± 0.1 | * | 10.7 ± 0.5 |
| 24.1 ± 0.6 | ↓ | 10.9 ± 1.2 | 9.9 ± 0.9 | ↑ | ||||||||
| Ni | 0.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |||
| Pb | 4.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |||
| Rb | 0.55 | 19.2 ± 6.2 | 10.0–28.6 | 18.2 ± 1.5 | ↑ | 28.6 ± 0.9 | 16.9 ± 1.6 | * | 10.0 ± 0.6 | 11.5 ± 1.8 | * | 25.7 ± 0.4 |
| 20.8 ± 0.5 | 19.7 ± 1.8 | ↑ | 24.0 ± 2.9 | ↑ | ||||||||
| Sr | 0.026 | 723 ± 131 | 564–984 | 743 ± 10 | * | 759 ± 14 | 570 ± 31 | * | 616 ± 6 | 565 ± 20 | * | 736 ± 9 |
| 984 ± 19 | ↑ | 729 ± 39 | ↑ | 805 ± 12 | ↑ | |||||||
| Zn | 0.17 | 35.2 ± 14.1 | 25.8–71.7 | 25.8 ± 0.7 | * | 32.0 ± 2.0 | 34.4 ± 5.1 | – | 33.2 ± 0.6 | 26.3 ± 1.0 | ↑ | 28.4 ± 1.1 |
| 71.7 ± 0.9 | ↑ | 34.0 ± 2.0 | 30.9 ± 2.6 | |||||||||
| Ca/Mg ratio | - | 1.56 | - | 1.5 | * | 1.24 | 1.2 | * | 1.34 | 1.41 | ↓ | 1.08 |
| 2.67 | ↑ | 2.46 | ↑ | 1.22 | ||||||||
| Element | Sampling Site with a Maximum Concentration | Mean–Max Concentration (mg/kg) | Single Dose for 3.3 g Consumption (mg/Day) | Daily Dose for 12.5 g Consumption (mg/Day) | Daily Dose from Risk Estimators | Daily Nutritional Requirements |
|---|---|---|---|---|---|---|
| Al | IS, St. 1 | 60.2–168.6 | 0.20–0.56 | 0.75–2.11 | 70 1 | 10 6 |
| As (total) | NS, St. 2 | 32.2–46.6 | 0.11–0.15 | 0.40–0.58 | 0.15 1 (inorganic) 40 2 (total) | 5.0 7 |
| Ba | IS, St. 1 | 6.6–8.6 | 0.02–0.03 | 0.08–0.11 | 200 | 0.75 6 |
| Ca | IS, St. 1 | 15,595–26,916 | 51–89 | 195–337 | 2500 3 | 1000 4 |
| Co | IS, St. 1 | 0.7–1.5 | 0.0023–0.0049 | 0.0087–0.0187 | 30 6 | 10 6 |
| Cu | IS, St. 1 | 2.4–7.3 | 0.0079–0.0241 | 0.0299–0.0914 | 5 3,6 | 0.9 5/1.0 6 |
| Fe | IS, St. 1 | 90–180 | 0.30–0.59 | 1.12–2.25 | 45 6 | 10 4,6 |
| Mg | IS, St. 1 | 9969–11,709 | 33–39 | 125–146 | 800 6 | 400 6 |
| Mn | IS, St. 1 | 14.0–33.4 | 0.046–0.110 | 0.18–0.42 | 11 6 | 2.7 4/2.0 6 |
| Rb | NS, St. 2 | 19.2–28.6 | 0.063–0.094 | 0.24–0.36 | 200 | 2.2 6 |
| Sr | IS, St. 1 | 723–924 | 2.39–3.35 | 9.0–12.3 | 11 6 | 1.9 6 |
| Zn | IS, St. 1 | 35.1–71.7 | 0.12–0.24 | 0.44–0.90 | 25 3/40 6 | 12 4, 6 |
| Sea Area | Sampling Site | Coordinates | Station | Reproductive Phases | Mean Water Temperature, °C | Range of Salinity, ‰ |
|---|---|---|---|---|---|---|
| Irminger Sea | Fossvogur Bay | 64.120887 N 21.930663 W | St. 1 | Fertile | 13.9 | 29.5–30.1 |
| Sterile | 4.0 | 34.9–35.5 | ||||
| Norwegian Sea | Ringvassøya Island | 69.815097 N 19.027894 E | St. 2 | Fertile | 10.6 | 33.7–34.3 |
| Norwegian Sea | Cape Sydspissen | 69.627168 N 18.912621 E | St. 3 | Fertile | 10.6 | 33.7–34.3 |
| Sterile | 6.3 | 34.8–35.2 | ||||
| Barents Sea | Teriberskaya Bay (Korabelnaya Bay) | 69.173088 N 35.168468 E | St. 4 | Fertile | 11.2 | 14.7–15.5 |
| Barents Sea | Teriberskaya Bay (Zavalishina Bay) | 69.184068 N 35.259487 E | St. 5 | Fertile | 9.1 | 19.9–20.7 |
| Sterile | 4.2 | 24.8–26.0 | ||||
| Barents Sea | Dalnezelenetskaya Bay | 69.117150 N 36.070790 E | St. 6 | Fertile | 10.3 | 31.0–32.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obluchinskaya, E.D.; Pozharitskaya, O.N.; Gorshenina, E.V.; Daurtseva, A.V.; Flisyuk, E.V.; Generalova, Y.E.; Terninko, I.I.; Shikov, A.N. Ascophyllum nodosum (Linnaeus) Le Jolis from Arctic: Its Biochemical Composition, Antiradical Potential, and Human Health Risk. Mar. Drugs 2024, 22, 48. https://doi.org/10.3390/md22010048
Obluchinskaya ED, Pozharitskaya ON, Gorshenina EV, Daurtseva AV, Flisyuk EV, Generalova YE, Terninko II, Shikov AN. Ascophyllum nodosum (Linnaeus) Le Jolis from Arctic: Its Biochemical Composition, Antiradical Potential, and Human Health Risk. Marine Drugs. 2024; 22(1):48. https://doi.org/10.3390/md22010048
Chicago/Turabian StyleObluchinskaya, Ekaterina D., Olga N. Pozharitskaya, Elena V. Gorshenina, Anna V. Daurtseva, Elena V. Flisyuk, Yuliya E. Generalova, Inna I. Terninko, and Alexander N. Shikov. 2024. "Ascophyllum nodosum (Linnaeus) Le Jolis from Arctic: Its Biochemical Composition, Antiradical Potential, and Human Health Risk" Marine Drugs 22, no. 1: 48. https://doi.org/10.3390/md22010048
APA StyleObluchinskaya, E. D., Pozharitskaya, O. N., Gorshenina, E. V., Daurtseva, A. V., Flisyuk, E. V., Generalova, Y. E., Terninko, I. I., & Shikov, A. N. (2024). Ascophyllum nodosum (Linnaeus) Le Jolis from Arctic: Its Biochemical Composition, Antiradical Potential, and Human Health Risk. Marine Drugs, 22(1), 48. https://doi.org/10.3390/md22010048






