Abstract
The slow discovery of new antibiotics combined with the alarming emergence of antibiotic-resistant bacteria underscores the need for alternative treatments. In this regard, fish skin mucus has been demonstrated to contain a diverse array of bioactive molecules with antimicrobial properties, including peptides, proteins, and other metabolites. This review aims to provide an overview of the antimicrobial molecules found in fish skin mucus and its reported in vitro antimicrobial capacity against bacteria, fungi, and viruses. Additionally, the different methods of mucus extraction, which can be grouped as aqueous, organic, and acidic extractions, are presented. Finally, omic techniques (genomics, transcriptomics, proteomics, metabolomics, and multiomics) are described as key tools for the identification and isolation of new antimicrobial compounds. Overall, this study provides valuable insight into the potential of fish skin mucus as a promising source for the discovery of new antimicrobial agents.
Keywords:
marine organisms; fish; skin mucus; extract; antimicrobial; antibacterial; antifungal; antiviral; omics 1. Introduction
The windfall for human and animal health in terms of effectively fighting infectious diseases is being threatened by the resurgence and appearance of dangerous pathogens, which largely outpace the discovery and implementation of new antimicrobials. Growing resistance to current antibiotics [1], antifungals [2], and antivirals [3] is one of the greatest reasons for this. This situation is substantially worsened by the long-lasting drought (with a few recent exceptions [4]) in the discovery of new classes of antibiotics since 1962 [1,5] and the continued scarcity and specificity of antifungals [6] and antivirals [7]. On top of that, factors including population growth, intensive farming, globalization, pollution, and climate change are also contributing notably to this issue by negatively unbalancing the pathogen–host–environment interplay [8].
Thus, in order to address the public health menace posed by both new and “renewed” infectious diseases that are quite often unfortunately associated with considerable morbidity and mortality, it is crucial to expand the arsenal of antimicrobials. This is because, even in an unfavourable scenario of rapid generation of antimicrobial resistance, their availability would at least help to buy time for the development of other countermeasures, such as effective vaccines. In this regard, different antimicrobial search and development strategies with high expectations are being adopted [9,10,11]. However, this is not an easy task.
To paraphrase Spellberg et al., (2007) [1], in this war against microorganisms, humanity has definitely underestimated the power of an enemy army that outnumbers, outweighs, and out-experiences us by several orders of magnitude in all its main divisions (viruses, bacteria, and fungi) (Table 1) [12,13,14]. Furthermore, as mentioned above, society is currently running out of effective ammunition, i.e., antimicrobials, because they have become obsolete in the face of today’s new needs. In this sense, and particularly referring to antibiotics, it should not be disregarded that most of the known antibiotics, as well as their resistance determinants, already existed in nature since ancient times, and humans only discovered them [15]. Indeed, most of them are secondary metabolites or their synthetic derivatives (i.e., natural or semisynthetic antibiotics) that originated primarily from microorganisms, among which the actinobacteria of the genus Streptomyces stands out as having provided about two-thirds of the natural antibiotics currently used clinically [16]. This should not discourage rational design efforts to expand the repertoire of synthetic antibiotics that, while still small (e.g., azoles, sulfones, ethambutol, nitrofurans, phenazines, quinolones, and thioamides), have already shown promise in terms of activity and safety [17,18]. However, there is no doubt that the search for natural antimicrobials in biological allies with far more combat experience against these enemies should continue, and that screening capabilities should be improved by exploiting existing and new technologies [11].

Table 1.
Estimates of number, mass, and time of origin on Earth for taxa relevant to this study.
2. Fish Skin Mucus as a Promising Source of Antimicrobials
In this context, marine ecosystems still remain an option with great potential for the discovery of new compounds, as they are relatively unexplored in this regard. Furthermore, they are the most extensive and ecologically diverse ecosystems, and, therefore, harbor the largest biological and hence biochemical diversity on the planet [28,29,30,31]. As expected, in this highly competitive environment [26,28], microorganisms are currently the fastest growing group of marine producers from which new compounds and antimicrobials are being discovered [32,33].
However, the contribution of higher counterparts, such as algae, plants, and animals, has been, and still is, particularly important [32,34], even considering that many compounds initially attributed to them may actually belong to associated or symbiotic microorganisms [32,35]. Among the animals, the majority of suppliers are invertebrates, mainly (in order of contribution) sponges as the overall top producer of marine natural compounds so far, molluscs, tunicates, coelenterates, echinoderms, and bryozoans [32,34]. In this ranking, the contribution of marine vertebrates, almost entirely represented by fish, is still rather modest (just right after coelenterates) [32,33], but certain factors, which will be commented on next, encourage further research into the potential antimicrobials that they may offer, especially from their skin mucus [36,37,38].
Fish are the oldest living vertebrates, with ancestors dating back to the mid-Cambrian period more than 500 million years ago. Among them, the ray-finned fishes (subclass Actinopterygii) are numerically dominant, with about 30,500 species, representing 95% of all fishes (about 32,000 species) and 50% of all vertebrates (about 60,000 species) (Table 1). They diverged about 385 My ago in the mid-Devonian period. The success of this divergence is represented by their enormous diversity as a consequence of having adapted to nearly any aquatic habitat since then [25,39]. This was made possible by a highly advantageous biological innovation (vertebrate evolution) and great genetic flexibility (gene duplication [40], teleost-specific whole-genome duplication events [41], and deletion of genome parts [42]) in a vast environment with extremely diverse conditions and high biological competition. However, this is also applicable to microorganisms.
Marine ecosystems are regulated by complex interactive fluxes that are primarily controlled by microorganisms due to the predominance of their biomass [43]. With a focus on viruses, bacteria, and fungi, some quantitative studies have estimated the abundance of each of the first two groups in the millions per milliliter of seawater [26,44,45]. The virus is the predominant microorganism in the ocean, accounting for about 1030 particles, about 15 times more than estimated bacteria (and archaea) [26]. There is little information on the quantitative abundance of fungi in aquatic environments, although it is assumed to be relatively high based on data on their enzymatic activity in certain environments compared to bacteria [46]. However, of note is not only their quantity, but also the extremely high diversity observed in all these groups, which also comprise pathogenic microorganisms [26,28,44,46].
As a result, fish have co-evolved under this selective pressure by also developing a complex network of defense mechanisms, such as the adaptive immune system [47,48,49]. However, although they have one of the earliest forms of adaptive immunity, their innate immunity still plays a central role in protecting them from and responding to infection [47,50], especially through a complex system of mucosal barriers responsible for fending off pathogens on first contact [47,50,51]. In fact, leukocyte distribution in fish is more organized in the mucosal tissues of the gut, gills, and skin than in the liver or gonads, for example [47,51]. Besides the cellular immune component, the humoral aspect of these tissues is of special relevance because of its antimicrobial function [52]. Among these major mucosa-associated lymphoid tissues (MALT), i.e., gut (GALT), gills (GIALT), and skin (SALT), mucosal glands are much more numerous in the skin [50,53], which is reasonable considering its continuous and intimate exposure to large amounts of microorganisms [26,44,45,46]. Together with the fact that the skin is the largest tissue of any organism and its mucus can be obtained non-invasively, the use of such fish fluid for biomedical purposes is very promising given its content of antimicrobial factors [54,55,56]. This is particularly true considering the large fish farming industry already established, which can valorize these previously overlooked natural by-products.
To explore the potential of fish mucus as a valuable source of antimicrobials, this review study conducted a search of the scientific databases PubMed, Scopus, and Web of Science. Keywords, such as “fish skin mucus,” in combination with “antimicrobial activity” or “composition,” were used in the search. The search period included studies published from the earliest available records to the present to ensure a thorough investigation. Inclusion criteria for the articles were: (i) published in English; (ii) in peer-reviewed journals; (iii) focused on the in vitro antimicrobial activity of fish skin mucus; and (iv) sufficiently described the mucus extraction method. Studies were selected based on their relevance to the topic and their adherence to predefined criteria.
3. Composition of Fish Skin Mucus in Innate Immunity Antimicrobial Molecules
The mucosal layer is a biochemical matrix that serves as a protective interface between the fish and the external environment [57]. Its multiple functions, which can be summarized as mechanical, physiological, and immunological, are dependent on its molecular components [37,57]. These are secreted by specific cell types (i.e., goblet, club, and sacciform cells) and the overall composition is influenced by developmental, hormonal, environmental, and nutritional factors [37,57,58]. The high-molecular-weight glycoproteins, called “mucins,” are the most representative component of the mucus and provide it with the characteristic gel structure that allows for the correct performance of all the above-mentioned functions [37,50,57,58,59]. The immunologic importance of this mucosal layer and its epithelial scaffold is demonstrated by the fact that its disruption increases the incidence and severity of infections [60,61]. In a recent example study, it was shown that such a disruption caused by the cyprinid herpesvirus 3 (CyHV-3) facilitates the occurrence of secondary infections that are ultimately responsible for exacerbated health complications and even death [61]. Besides the mucins themselves, which have been reported to have direct antimicrobial activity, fish skin mucus also contains a broad repertoire of antimicrobial factors apart from antibodies [36,50,51,58,62]. Mucus has often been recognized as a source of new antimicrobials [38,63,64]. Known compounds are summarized in this section.
3.1. Antimicrobial Peptides (AMPs)
AMPs, also known as Host Defense Peptides (HDPs), are gene-encoded peptides of up to approximately 80 amino acid residues, mostly characterized by a cationic, amphipathic chemical nature and antimicrobial properties. They are ancient innate immune molecules present in all groups of organisms. Their mature forms in eukaryotic cells are often cysteine-rich molecules with multiple intramolecular disulfide bridges. Through conservation or reduction of these bonds, some families of AMPs can modulate their type and/or level of activity [65,66,67,68]. For instance, in defensins (one of the most studied families of AMPs), some reports on a particular group of human beta-defensins indicate that the reduction of such bonds affects their function by disabling their chemotactic activities and triggering their direct antimicrobial ones [67]. Indeed, AMPs are generally known for their microbicidal activity exerted directly on the target microorganism, but they can also be endowed with potent immunomodulatory and receptor-mediated chemotactic activities, which together explain their broad antimicrobial activity against bacteria, protozoa, fungi, and both enveloped and non-enveloped viruses, as well as the difficulty of selecting resistant mutants against them [65,66]. In general, the mechanism of action for their direct antimicrobial activity is based on the affinity and fixation of the peptide to the generally anionic surface membrane of the pathogen and its subsequent destabilization through pore formation or, simply, bilayer disruption [65].
The large number of AMPs discovered (the Antimicrobial Peptide Database (APD) of the University of Nebraska Medical Centers currently counts 3569 and growing [accessed 5 May 2023]) can be classified into different families based on (i) their primary sequence; (ii) the presence and organization of various functional regions, such as the propeptide, which inhibits and protects the region corresponding to the mature peptide until its cleavage and is located at either the C- or N-terminal; (iii) their molecular structure, for which the simplest distinction is between linear and disulfide-stabilized peptides, and, in the latter case, the number, location, and association pattern of their cysteine residues; and (iv) the origin of the mature peptide, which may be produced directly after typical post-translational modifications and minor cleavages of accessory regions as it occurs in most cases, but may also be derived from the cleavage of a larger protein, often with a very different primary function, e.g., peptides derived from histone and ribosomal proteins [65,69,70,71].
Given the importance of these molecules in the innate immune system, they are extremely diverse in fish and include not only families of AMPs found in other animal groups, such as cathelicidins, defensins, hepcidins, and histone-derived peptides, but also exclusive fish AMP families, such as piscidins and pleurocidins [69,70]. Probably also for this reason, the skin mucus is the major source of AMPs in fish, with approximately 70% of all AMPs expressed in the skin compared to 52% and 29% expressed in the gills and the gut, respectively [50,53]. Besides expression, several AMPs were isolated from skin mucus, and their antimicrobial activities were tested. Representatives of several families of AMPs isolated from skin mucus are listed in Table 2.

Table 2.
Examples of AMPs isolated from skin mucus.
Histone-derived AMPs were first described in fish [86] just a few years after their discovery in the Asian toad Bufo bufo gargarizans [87]. Robinette et al., (1998) [86] isolated two histone-like proteins (HLP-1 and HLP-2) in the epidermis of channel catfish that were found to be inhibitory to bacterial and fungal pathogens. Shortly thereafter, several histone-derived peptides were isolated from fish skin mucus [72,73,74,75]. In general, this family of AMPs is thought to be released from cells during infection-induced apoptosis [88]. Oncorhyncin III is a 66-residue N-terminal fragment of the non-histone chromosomal protein H6 from O. mykiss skin mucus and was shown to be active against gram-negative and gram-positive bacteria [80]. Other AMPs derived from larger proteins isolated from skin mucus are the peptidic fragments from the 40S and 60S ribosomal subunits. The AMP 40S ribosomal protein S30 was found in rainbow trout and showed antibacterial activity against gram-positive bacteria [85]. The 60S ribosomal proteins L40, L36A, and L35 were isolated from Atlantic cod (G. morhua) mucus extracts [76].
Other peptides found in fish skin mucus include myxinidin, pardaxin, pelteobagrin, and piscidin, all of which are unique to this group of animals and have been reported to have broad-spectrum antimicrobial activity. Myxinidin is a cationic 12-amino acid peptide isolated from the skin mucus of hagfish (M. glutinosa) [79]. Pardaxin was first isolated from the Red Sea Moses sole (P. marmoratus) and described as a single, helical, monomeric, acidic toxin [82]. Its antibacterial activity against gram-negative and gram-positive bacteria was subsequently demonstrated [89]. Pelteobagrin is a 20-amino acid amphipathic α-helical peptide and was identified in the skin mucus of yellow catfish (P. fulvidraco) [81]. The piscidin family also comprises α-helical peptides, with low molecular weight and cationic charge at physiological pH [90].
3.2. Proteins
Animal mucosa, in a broad sense, is characterized by the presence of mucins, which are glycosylated proteins responsible for providing viscoelastic and rheological properties, as well as trapping pathogens and contributing to cell surface signaling [36]. Additionally, they possess a diverse range of other proteins, including many with antimicrobial/immune-related and structural functions [50,51,57,58]. In this sense, a large number of defense proteins against pathogens have been described in fish mucosa, especially in the skin, besides the immunoglobulins IgG and the teleost-specific IgT [50,51,58].
Other types of glycoproteins have been found in fish skin mucus. For example, Ebran et al., (2000) [91] isolated and characterized glycoproteins from rainbow trout (Oncorhynchus mykiss), European eel (Anguilla anguilla), and tench (Tinca tinca) skin mucus. These proteins possess both α-helix and random coil structures and show antibacterial activity correlated with pore-forming properties. Transferrin glycoprotein has also been isolated from Atlantic cod [92] and Atlantic salmon (Salmo salar) [93] skin mucus. Transferrin is responsible for iron transporting in absorption, storage, and disposal sites in vertebrates. As all organisms require iron for their growth, transferrin plays an important role in the innate defense mechanisms of fish by binding to iron and reducing its availability to pathogens by chelating it [94].
Lectins are a diverse class of highly specific carbohydrate-binding proteins [95]. They have been found in the skin mucus of fish, where they provide an external defense mechanism via the agglutination process to stop pathogen penetration and colonization [96]. There are several types of different lectins depending on their structure; for example, C-type lectins, whose binding is dependent on Ca2+, F-type lectins or fucolectins, which are distinguished by their α-l-fucose recognition domain, galectin family or S-type, which require thiol, and pentraxins or pentameric lectins, or P-type lectins, which target glycoproteins containing mannose 6-phosphate [95]. The isolation of C-type lectins has been described in cichlid (Symphysodon aequifasciata) skin mucus [97]. Another example includes fucose-binding lectin (FBL, F-type lectin), which was identified in European sea bass (Dicentrarchus labrax) skin mucus [98] and is responsible for the agglutination, immobilization, and opsonization of microorganisms and phagocyte activation. Mannose-binding lectin (MBL, P-type lectin) and galectin were also found in Atlantic cod skin mucus [99]. MBL plays a significant role in opsonization and the initiation of the lectin pathway of complement activation, and galectin binds to pathogens and orchestrates several immune processes. Another type of MBL, named “pufflectin,” was reported in pufferfish (Takifugu rubripes) [100]. Pentraxins play an important role in inflammatory responses and pathogen recognition and have been identified in common skate (Dipturus batis) [101] and lumpsucker (Cyclopterus lumpus) [102] skin mucus. C-reactive protein (CRP) belongs to the pentraxin family, and it is part of the innate immune defense system because it has the ability to activate the classical complement pathway [103]. CRP was reported in tilapia (Tilapia mossambica) skin mucus, and its levels were found to increase in response to inflammation and necrosis [104].
Lysozyme (N-acetylmuramide glucanohydrolase or muramidase) is a bacteriolytic enzyme and an important component of the immune system. It has been reported in the skin mucus of several fish species, including mrigal carp (Cirrhinus mrigala), catla (Catla catla), spotted snakehead (Channa punctata), Japanese eel (Anguilla japonica), and Nile tilapia (Oreochromis niloticus) [105,106,107]. Given its ability to hydrolyze the bond between N-acetylmuramic acid and 3-acetyl amino-2-deoxy-D-glucose residues of the mucopolysaccharide found in bacterial cell walls [108], it acts directly on gram-positive bacteria. In gram-negative bacteria, lysozyme can also attack the inner peptidoglycan layer after the disruption of the outer wall by complement and other enzymes [106].
Proteases are enzymes of great importance in the mechanisms of the immune system. Their role is to hydrolyze the peptide bonds of proteins. Proteases can be classified into serine, cysteine, aspartic, and metalloproteases based on their catalytic mechanisms [58]. They are associated with resistance to infection because of their ability to degrade the proteins of pathogens. Proteases, including trypsin (serine protease), cathepsin B and L (cysteine proteases), cathepsin D (aspartic protease), aminopeptidases, and metalloproteases, have been reported in the skin mucus of several species, such as rainbow trout [109], Japanese eel [110], European eel [111], catfish (Parasilurus asotus) [75], and Atlantic salmon [112].
Cytoskeletal proteins with potential antimicrobial activity have also been reported in fish skin mucus. For example, keratin has been identified in skin mucus from lumpsucker [102], Atlantic cod [99], European sea bass [98], and gilthead sea bream (Sparus aurata) [113]. Although keratin is a structural protein, pore-forming properties have also been described in keratin from the skin mucus of rainbow trout [114] and may therefore contribute to host defense against water-borne pathogens. Likewise, actin is a structural protein involved in several roles associated with cellular membranes, such as cell migration, phagocytosis, pinocytosis, cytokinesis, and cytoplasmic streaming [115]. Beta actin has been reported in lumpsucker [102], European sea bass [98], Atlantic cod [99], and gilthead sea bream [113,116]. Increased actin fragmentation by proteases has been linked to stress situations, and actin fragments generated could trigger an immune response [117].
3.3. Other Components
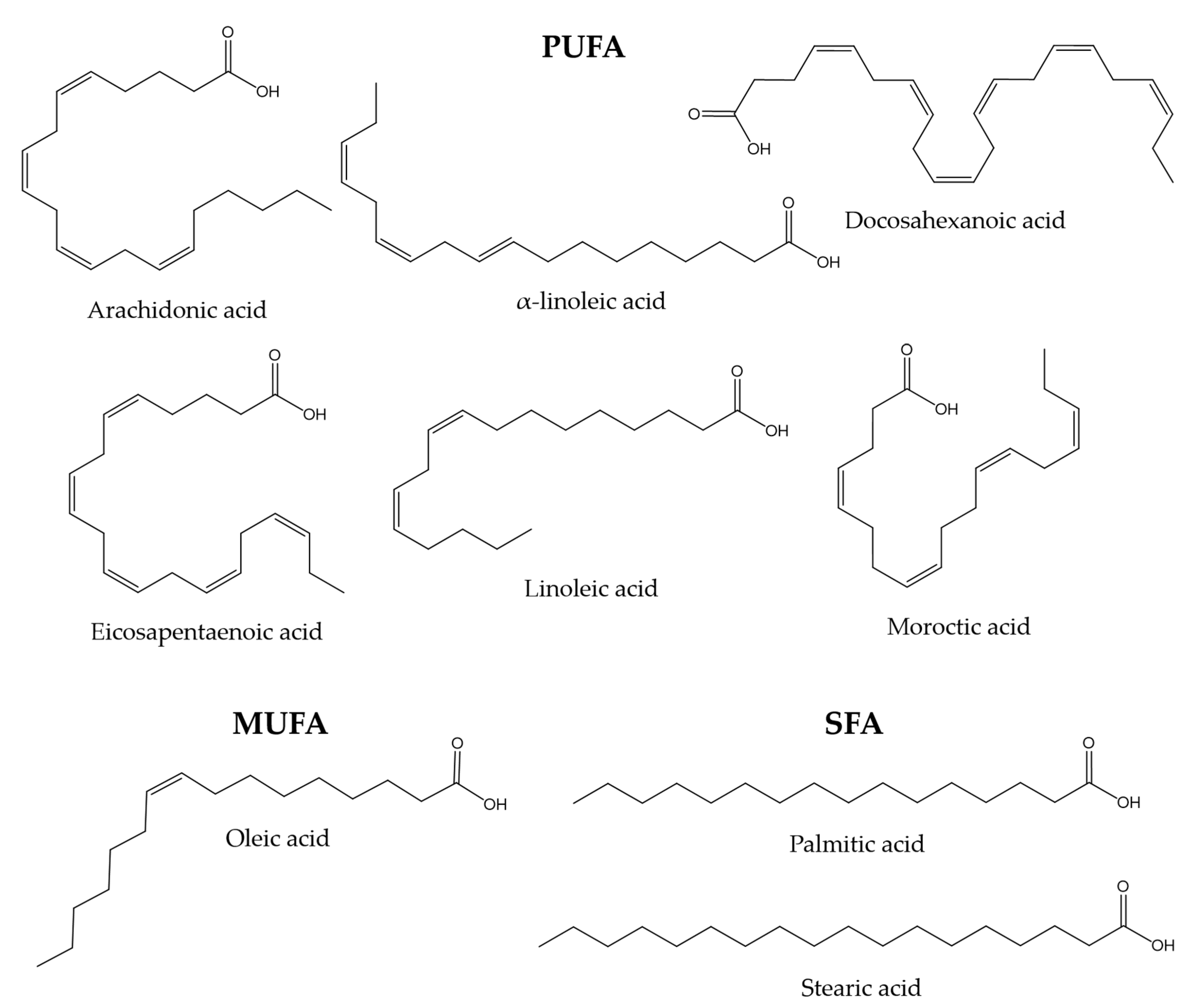
The lipid composition of fish skin mucus has not been studied as thoroughly as other mucous secretions, such as gut mucus. However, some studies show that skin mucus may also be a significant source of lipids. Mono-unsaturated fatty acids (MUFA), such as oleic acid, poly-unsaturated fatty acids (PUFA), such as linoleic, alpha-linoleic, docosahexaenoic, arachidonic, eicosapentaenoic, and moroctic acid, and saturated fatty acids (SFA), such as palmitic and stearic acid, have been reported in gilthead sea bream and flathead grey mullet (Mugil cephalus) [118,119]. Lipids are thought to be involved in maintaining the internal structure of the mucus through interactions with glycoproteins [120]. Figure 1 shows the chemical structures of the compounds mentioned in this paragraph.
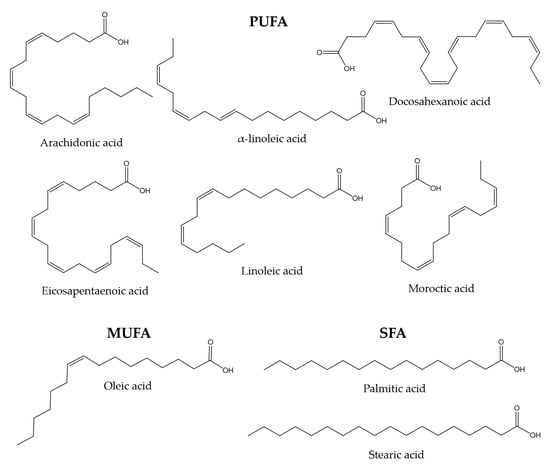
Figure 1.
Chemical structures of some MUFA, PUFA, and SFA molecules found in the skin mucus of gilthead sea bream and flathead grey mullet [118,119].
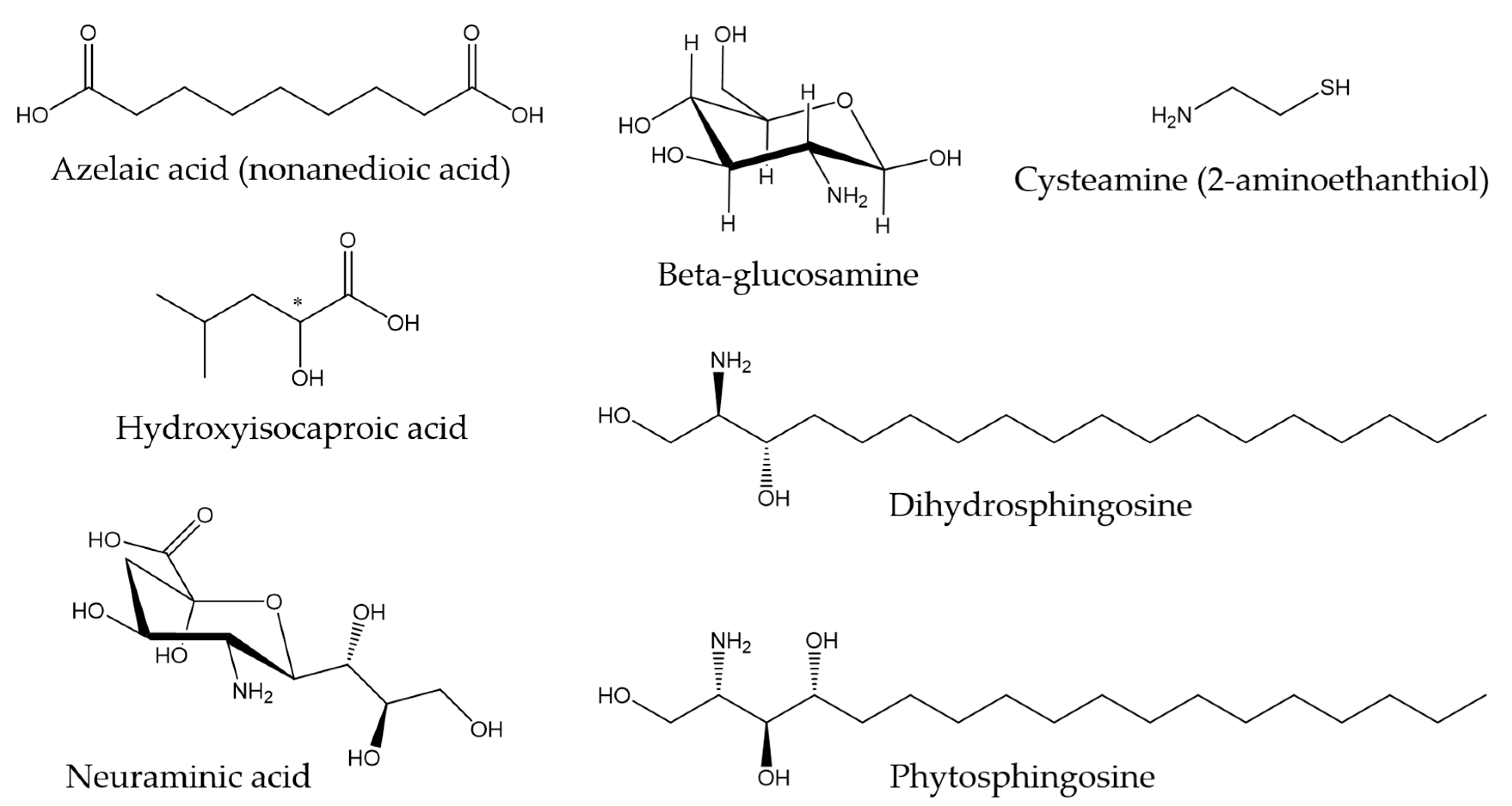
Regarding other types of molecules, Ekman et al. (2015) [121] described the metabolite profile of fathead minnow (Pimephales promelas) skin mucus with the aim of providing a tool for environmental monitoring and surveillance. Some of the metabolites found are associated with antibacterial properties. For instance, azelaic acid has been shown to inhibit bacterial growth by interfering with protein synthesis [122], and hydroxyisocaproic acid has been found to be effective against both bacteria and fungi [123]. In another study conducted by Patel et al., (2020) [124], the metabolic profile of the skin mucus of the pool barb (Puntius sophore) was characterized. In this research, compounds found with proven antimicrobial activity included amino sugars, such as glucosamine and neuraminic acid, cysteamine (organic disulfide), dihydrosphingosine (amino alcohol), and phytosphingosine (sphingolipid), among others. Figure 2 shows the chemical structures of the compounds mentioned in this paragraph.
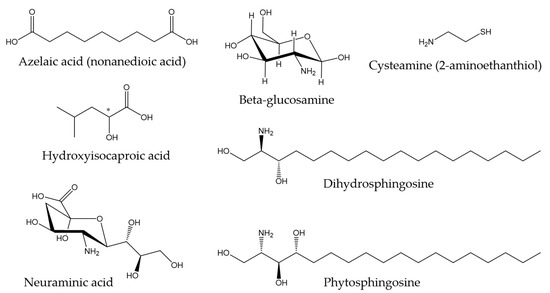
Figure 2.
Chemical structures of other metabolites found in skin mucus of fathead minnow and pool barb [121,124].
4. Antimicrobial Activity of Fish Skin Mucus
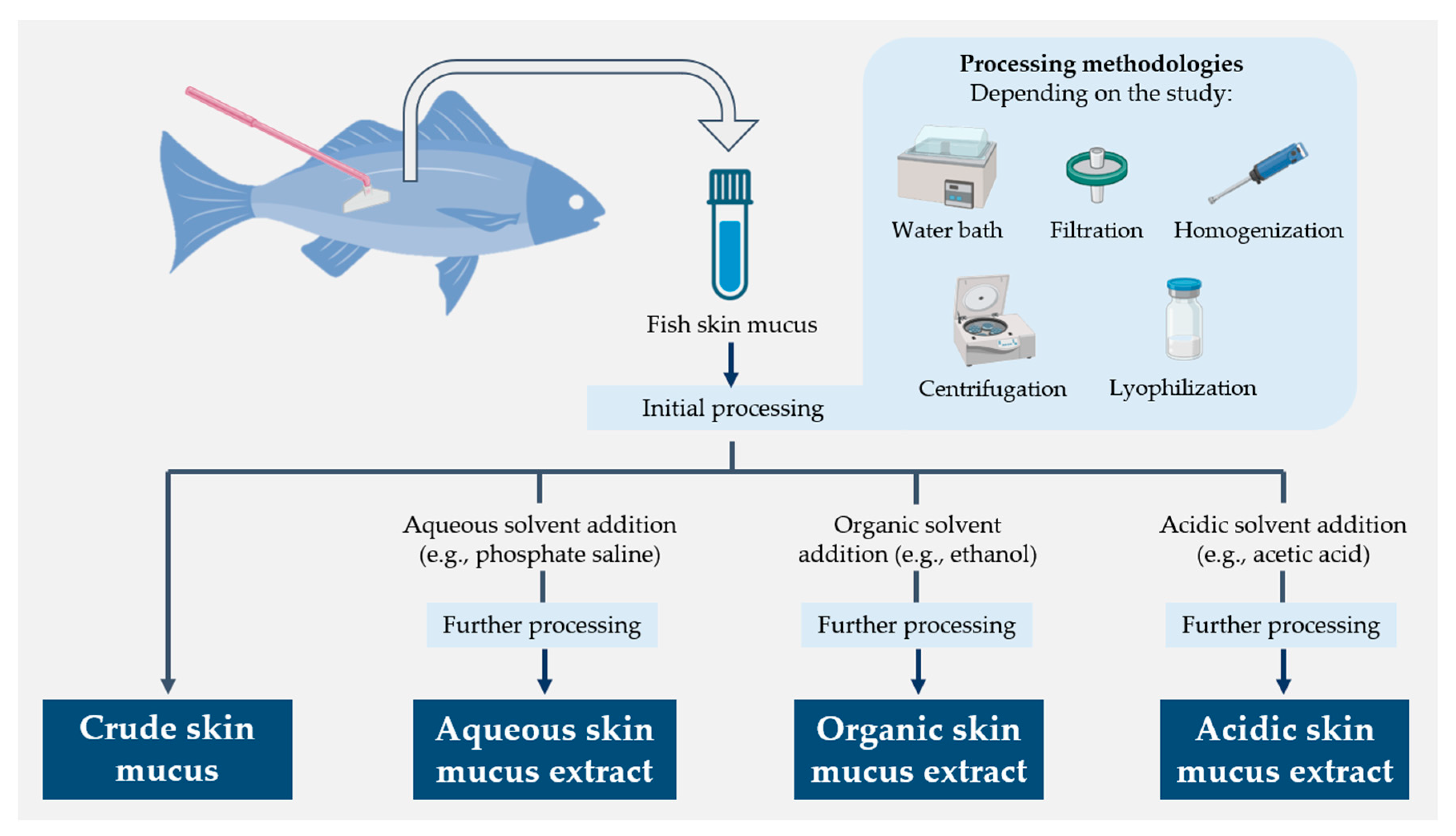
The humoral component of fish skin mucus has been extensively studied for its high content in molecules endowed with antimicrobial properties and, thus, its potential for implementation in biomedical and veterinary applications [38,50,51,58]. Such a variety of compounds has also necessitated the use of different molecular extraction approaches in mucus samples (Figure 3) [63,125]. However, it appears that the amount of functional data is relatively small compared to the overall progress made in researching and developing new methods. This section summarizes the activity demonstrated against different groups of microorganisms by different types of fish skin mucus extracts, mentioning the techniques used to determine this activity when specified in the studies.
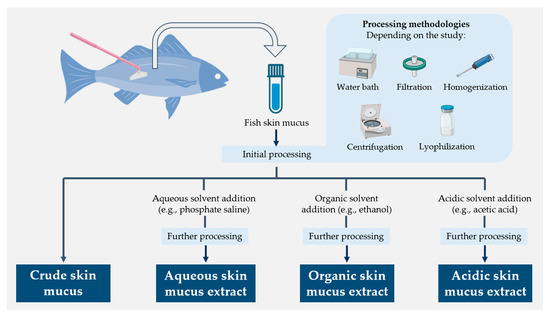
Figure 3.
Schematic diagram summarizing the different molecular extraction approaches used for fish skin mucus samples [63,125].
4.1. Antibacterial Activity of Fish Skin Mucus Extracts
Fish skin mucus has proven to be effective against bacteria that affect not only fish, but humans as well. Table 3 summarizes the results of an extended list of studies on antibacterial activity of fish skin mucus extracts. In total, there are 47 fish species represented, most of which are teleosts (exceptions include Myxine glutinosa (Myxini) and Dasyatis pastinaca (Elasmobranch)). The different extraction methods can be divided almost entirely into aqueous, organic, and acidic extractions. Some authors also used crude mucus with only minor processing steps.

Table 3.
Antibacterial effect observed in extractions of mucus of different fish species.
4.1.1. Aqueous Extractions
Of all the experiments presented in Table 3, the most frequently used extraction method was the aqueous one. The most commonly employed solvents in these studies, listed in order of frequency of use, were physiological saline, water, ammonium bicarbonate, and Tris-buffered saline. Further information about these studies can be found in Table S1.
Although aqueous extraction was the most popular extraction method, it also showed the least antibacterial activity. This was particularly evident in those experiments where different extraction methods were compared. In some experiments, aqueous extracts did not show any antibacterial activity [127,137,138,139,140,141,151]. For example, Subramanian et al., (2008) [140] found antimicrobial agents, such as lysozyme, cathepsin B, and trypsin-like proteases, in the aqueous skin mucus extracts of several fish species, but they did not exert any antimicrobial activity. Al-Rashed et al., (2018) [127] used aqueous and acidic extracts of the skin mucus of the climbing perch (Anabas testudineus), but only found antibacterial activity in the latter. Similarly, Hellio et al., (2002) [138] performed aqueous and organic extractions of the skin mucus of the ballan wrasse (L. bergylta), but only observed antibacterial activity with the organic extracts. Subhashini et al., (2013) [151] did not find antimicrobial activity in the aqueous extract of the skin mucus of tinfoil barb fish (Barbonymus Schwanenfeldii), even if the amount of protein was higher than in the organic extracts obtained in parallel.
The lack of activity of these extracts has been attributed by different authors to several causes: (i) inactivation of enzymes, such as lysozyme, trypsin, or proteases, by the high incubation temperatures and/or low pH conditions used in the procedure [140]; (ii) low concentration of antimicrobial compounds in the media, probably because some of these enzymes regulate their production [161]; and (iii) inter- and intraspecies variability of mucus composition, influenced by both internal (e.g., sex and developmental stage) and external factors (e.g., stress, hyperosmolarity, pH, and infection) [62]. This last point is particularly noticeable in the species studied in several studies performing aqueous extractions. The aqueous skin mucus extract of the common snakehead (Channa striata) has been shown in some studies to be active against gram-negative and gram-positive bacteria [130,132,133]. However, Wei et al., (2010) [131] also tested the antibacterial activity of this extract and found inhibition only against Aeromonas hydrophila but not against other bacteria previously tested (i.e., Bacillus subtilis, Klebsiella pneumoniae, Proteus vulgaris, and Pseudomonas aeruginosa).
In other studies, the aqueous skin mucus extract did inhibit both gram-negative and gram-positive bacteria [130,132,133,134,135,149,152,153,155,156,157]. Guardiola et al., (2014) [134] demonstrated that an aqueous extract of the skin mucus of grouper (Epinephelus marginatus) inhibited gram-positive and gram-negative bacteria. They also found high levels of lysozyme, alkaline phosphatase, esterase, protease, and antiprotease activities, which are associated with defense against bacterial infections. In a study by Kumari et al., (2019), [156] the aqueous skin mucus extract of several carp species exhibited high antibacterial activity against both gram-negative and gram-positive bacteria.
4.1.2. Organic Extractions
For the organic extractions, the most used solvents have been ethanol and dichloromethane. Some authors performed alcoholic extractions and then partitioned distilled water with dichloromethane to obtain aqueous and organic phases [138,151]. High antibacterial activity has been reported for organic fish skin mucus extracts (see Table 3 and Table S2 for further details). Indeed, in some studies, all bacteria tested were inhibited, both gram-positive and gram-negative [76,138,145,151]. The main reasons explaining such activity are that (i) the presence of hydrophobic groups is often a common feature of antimicrobial molecules because of their affinity for membranes and their ability to disrupt them [162]; and (ii) these extracts are enriched in hydrophobic molecules because organic solvents favor their isolation by reducing the interactions between hydrophobic groups, which hinders their aggregation [163]. In fact, Mahadevan et al., (2019) [145] obtained greater inhibitory activity against gram-negative and gram-positive bacteria using organic mucus extracts compared to aqueous ones. Hellio et al., (2002) [138] correlated high antimicrobial activity with low polarity of the solvents used; they also showed that extracts from the dichloromethane phase were more active than those from the aqueous phase. In a study by Bergsson et al., (2005) [76], an organic (acetonitrile (ACN) + 1% trifluoroacetic acid (TFA)) extract of cod skin mucus exhibited high antimicrobial activity against gram-positive and gram-negative bacteria. In these extracts, they also identified four peptides with known antimicrobial activity, i.e., those derived from the histone H2B and the 60S ribosomal proteins L40, L36A, and L35.
In some studies, the same species were used to obtain the organic extracts of skin mucus. García-Marciano et al., (2019) [141] and Wibowo and Maftuch (2015) [143] tested the organic skin mucus of tilapia against V. harveyi, and in both studies, bacterial growth was inhibited. Katra et al., (2016) [139] and Hellio et al., (2002) [138] employed the same methodology to obtain organic skin mucus extracts from ballan wrasse (L. bergylta) and these two studies, no activity against gram-positive bacteria was found. However, Hellio et al., (2002) [138] found antimicrobial activity against gram-negatives, while Katra et al., (2016) [139] did not. These contradictory results were attributed to the possible development of antimicrobial resistance in the strains used, or to seasonal, housing, or dietary differences between the two studies that may have affected the experimental animals.
4.1.3. Acidic Extractions
For acidic extractions, the most common solvent was acetic acid (AA) followed by TFA. In general, acidic extracts showed greater antibacterial activity than other extracts (see Table 3 and Table S3 for further information) [124,127,131,137,140,141,142,150,151]. In most studies using acidic extractions to determine antibacterial capacity, all bacteria tested were inhibited [124,131,137,140,142,154]. This may be due to the presence of cationic peptides and defensive low-molecular-weight proteins. This type of molecule has been shown to be more soluble in mildly acidic solutions [164].
Subramanian et al., (2008) [140] tested the antimicrobial activity of brook trout (Salvelinus fontinalis), haddock (Melanogrammus aeglefinus), and hagfish acidic (AA) skin mucus extracts against gram-negative (A. salmonicida, E. coli, Listonella anguillarum, Salmonella enterica, P. aeruginosa, Yersinia ruckeri) and gram-positive (Staphylococcus epidermidis) bacteria, and all species studied were inhibited. Hagfish mucus was the most active one, and its protein profile showed mainly low-molecular-weight proteins below 20 kDa. This was related to the fact that hagfish are evolutionarily the most primitive of the species studied and they lack essential components of adaptive defense, as well as their presence in muddy ocean bottoms, which requires a greater amount of antimicrobial components for their survival [140].
Nigam et al., (2017) [154] identified the antimicrobial protein histone H2B in acidic (AA and TFA) mrigal carp skin mucus extracts. However, the TFA extract only inhibited two (Salmonella paratyphi, Vibrio cholerae) of the five bacteria tested. Patel et al., (2020) [124] detected some metabolites in the acidic (AA) pool of barb mucus extract that were associated with antimicrobial activity, such as 10-nitro-9Z,12Z-octadecadienoic acid, 3alpha,6beta,7alpha-trihydroxy-5betacholan-24-oic acid, 1-octanoyl-rac-glycerol, dihydrosphingosine, phytosphingosine, 5beta-chol-2-en-24-oic acid, neuraminic acid, glucosamine, and cysteamine. It has been described that antimicrobial lipids probably act by inducing cell wall and membrane destabilization of bacteria [165].
4.1.4. Crude Mucus
Finally, some studies have evaluated the activity of fish skin mucus in its almost raw form, without any type of solvent extraction. Table 3 and Table S4 provide additional information about these studies. Sanahuja et al., (2019) [128] compared the crude skin mucus of gilthead sea bream, European sea bass, and meagre (Argyrosomus regius). In particular, meagre mucus showed biocidal activity against all bacterial species tested, i.e., E. coli, V. anguillarum, and P. anguilliseptica (all gram-negative), which was associated with higher levels of non-specific defenses, such as protease and carboxylesterase activities. Fuochi et al., (2017) [158] studied the antibacterial activity of common stingray (D. pastinaca) crude skin mucus and found that it inhibits the bacterial growth of gram-negative, but not gram-positive, bacteria. This observation was attributed to a strong interaction between the outer membrane (present in gram-negatives only) and the biomolecules present in the mucus. They also demonstrated the presence of chitinase 1, an enzyme involved in the degradation of chitin [158].
Other authors have compared the antibacterial activity between the crude mucus and some of its solvent extracts. Kumari et al., (2019) [156] found that the crude mucus of several carp species shows higher antibacterial activity than its aqueous extract. Wei et al., (2010) [131] compared crude, aqueous, and acidic mucus from common snakehead. Although the crude mucus was found to contain a higher amount of protein than the other extracts, it inhibited only the fish pathogen A. hydrophila. In this work, the crude mucus did not exert any effect against the human bacteria pathogens tested, unlike the other extracts.
4.2. Antifungal Activity of Fish Skin Mucus Extracts
Fish skin mucus has also shown antimicrobial activity against fungal pathogens. However, the number of studies evaluating antifungal activity is much lower than the number of studies evaluating antibacterial activity. Of the 39 selected studies that evaluated antibacterial and antifungal activity, 28 evaluated only antibacterial activity, two evaluated antifungal activity exclusively, and nine evaluated both. The antifungal studies conducted using fish skin mucus are shown in Table 4.

Table 4.
List of antifungal studies using skin mucus from different fish species.
Several studies have produced mixed results using crude fish skin mucus. On the one hand, the antifungal activity of crude skin mucus of catla, mrigal carp, and European eel inhibited the growth of Aspergillus awamori, Colletotrichum falcatum, and Fusarium oxysporum in the study by Pethkar et al., (2017) [146]. Fuochi et al., (2017) [158] also found that the crude skin mucus of the common stingray was active against Candida albicans, Candida glabrata, and Candida tropicalis. On the other hand, Hisar et al., (2014) [159] tested the crude skin mucus of rainbow trout against C. albicans and Candida parapsilosis, but no antifungal activity was observed. Ikram et al., (2013) [167] screened the antifungal activity of crude and aqueous (i.e., PBS and water) skin mucus of Asian swamp eel (Monopterus albus) against C. albicans, Candida krusei, Cryptococcus neoformans, and Fusarium spp., but only the water extract revealed an inhibitory effect, with activity against all the fungi tested and mostly against Fusarium spp.
A few studies only investigated aqueous mucus extractions. For example, walking catfish (Clarias batrachus) aqueous skin mucus inhibited Aspergillus niger, Aspergillus nidulans, Fusarium moniliforme, C. albicans, and Trichoderma koningi in the study by Loganathan et al., (2011) [149]. Balasubramanian et al., (2011) [153] tested the antifungal activity of catla, silver carp (Hypophthalmichthys molitrix), rohu (Labeo rohita), and grass carp (Ctenopharyngodon idella) aqueous skin mucus against Aspergillus flavus, A. niger, C. albicans, Mucor globosus, and Rhizopus arrhizus. The catla and rohu extracts inhibited all the species tested, the silver carp extract inhibited M. globosus, A. flavus, and A. niger, and the grass carp extract inhibited all species except A. niger.
Other studies carried out aqueous and organic or acidic extractions in parallel and compared their activity. Hellio et al., (2002) [138] screened the antifungal activity of the aqueous and organic skin mucus extracts of pollock (Pollachius virens), ballan wrasse, European flounder (Platichthys flesus), common sole (Solea solea), and brill (Scophthalmus rhombus) against Candida brusei, C. albicans, C. tropicalis, Saccharomyces cerevisiae, and Issatchenkia orientalis. Antifungal activity was found only in the aqueous and organic fractions of the organic extraction of brill mucus against C. tropicalis, I. orientalis, and S. cerevisiae. In the study by Subramanian et al., (2008) [140], the aqueous, acidic, and organic skin mucus extracts of arctic char (Salvelinus alpinus), brook trout (Salvelinus fontinalis), Eurasian carp (Cyprinus carpio sub sp. Koi), striped bass (Morone saxatilis), haddock (Melanogrammus aeglefinus), and hagfish were tested against C. albicans. The results showed that the acidic skin mucus of arctic char, brook trout, and hagfish exerted fungicidal activity, and the acidic skin mucus extracts of European carp and striped bass were fungistatic. Mahadevan et al., (2019) [145] showed that the aqueous and organic skin mucus extracts of the giant mudskipper (Periophthalmodon schlosseri) inhibited C. albicans, A. flavus, Mucor sp., and Trichoderma longibriachtin.
Bergsson et al. (2005) [76] extracted the skin mucus of Atlantic cod using 60% ACN containing 1% TFA. They evaluated its activity against C. albicans, which was fully eliminated when Medium E (salt solution) was added to the medium. Without Medium E, only mild inhibition was observed. In another study, al-Arifa et al., (2011) [166] demonstrated that the use of alkali treatments to induce the production of skin mucus in rohu before its collection inactivated its antifungal properties, and they were not restored after the neutralization of its pH. Instead, it favored the growth of C. albicans.
The antifungal activity of mucus components, such as lysozyme [168,169] or chitinases [170], have been demonstrated. Some studies suggest that the lysozyme may disrupt cell wall integrity by hydrolyzing the glycosidic linkages between cell wall proteins and polysaccharides [168]. Other hypotheses include that lysozyme is likely to induce apoptosis in this fungus accompanied by activation of membrane potassium channels [169]. Regarding chitinases, they catalyze the hydrolysis of chitin, a main component of the cell wall in fungi [170].
4.3. Antiviral Activity of Fish Skin Mucus
Information on the antiviral activity of fish skin mucus extracts is scarce to date. Raj et al., (2011) [171] investigated the role of carp epidermal mucus as an innate immune barrier against CyHV-3 entry. They found that skin mucus inhibits CyHV-3 binding on epidermal cells and leads to a significant reduction in the number of viral plaques. This reduction only occurred when cells were pre-incubated with mucus, but not when mucus was added after the incubation period. Most of the studies, however, report antiviral activity of compounds that had been previously isolated from skin mucus. For instance, Valero et al., (2020) [172] reported the presence of NK-lysin in Atlantic salmon skin mucus, and Falco et al., (2019) [173] demonstrated its antiviral activity against spring viremia of carp virus (SVCV) by inhibiting not only the binding of viral particles to host cells, but also the fusion of virus and cell membranes. Beta-defensins, an important factor in the antimicrobial barrier function of the skin [174], have also been shown to have antiviral activity against another rhabdovirus, the viral haemorrhagic septicaemia virus (VHSV) [175]. Furthermore, lysozyme [176] and piscidin (piscidin 1) [177] have antiviral activity. However, the scarcity of systemic and functional data shows that more research on the antiviral role of fish mucus is required to draw clear conclusions and to develop new strategies to treat viral infections.
5. Omics Techniques as a Promising Tool in Fish Skin Mucus Research
The field of fish skin mucus research has generally been limited by the complexity of its composition, as well as the interactions between its components. Omic techniques have recently emerged to provide a holistic approach to cellular components and their interactions, providing an effective tool towards a deeper understanding of marine systems [178]. The progress of these techniques has allowed the field of studies of fish skin mucus to grow quickly in recent years [36]. These techniques have been applied to fish skin mucus research in different topics, such as welfare, health, and nutrition. However, the discovery of antimicrobial agents through these methods is an underexplored opportunity.
Genomics have provided insights into molecular and genetic mechanisms in fish. Ao et al., (2015) [179] sequenced and assembled the genome of Larimichthys crocea using a bacterial artificial chromosome and a whole-genome shotgun hierarchical strategy. They identified 159 genes related to mucin biosynthesis and mucus production based on previous studies in mammals, thus suggesting that the mucin synthetic pathway is conserved between fish and mammals. Carda-Diéguez et al., (2017) [180] searched for genomic and metagenomic evidence in wild eel to discover if fish mucus could constitute an adequate niche for the evolution of mucosal aquatic pathogens in natural environments. The results obtained suggest that skin mucus concentrates in bacteria present in water with abilities to attach, resist innate immunity, and compete with other bacteria, and that it favors the exchange of genes encoding these functions.
Transcriptomics provides information on the RNA transcripts produced by the genome, from protein coding (mRNA) to noncoding RNA. A recent study examined the efficacy of whole-transcriptomic profiling of mahi-mahi epidermal mucus as a method for oil exposure detection using RNASeq [181]. Transcripts involved in immune response, cardiotoxicity, and calcium homeostasis showed differential expression after oil exposure, which indicates that mucus is a promising source for noninvasive monitoring techniques. Parida et al., (2018) [182] examined the transcriptome of immune-relevant genes in the mucus of infected rohu to characterize mucosal immune responses. The results show, in general, the upregulation of immune-related transcripts, such as interleukins, toll-like receptor 22, and lysozyme G, which broadens our knowledge of mucosa-associated molecular events that occur during infections and reinforces the role of mucus as the first line of defense against pathogens.
Proteomics is the characterization of proteins expressed in an organism. It is the most studied omic technique in fish mucus research. Proteomics provides information about the entire effect of the gene expression process and encompasses post-transcriptional and post-translational protein expression regulation [183]. Proteomic profiles of the skin mucus of gilthead seabream [113], Atlantic cod [99], Atlantic salmon [184], mudskipper [185], discus fish (Symphysodon spp.) [186], European sea bass [98], and lumpsucker [102] have been published, allowing for the possibility of comparative studies to better understand the dynamics of fish mucus. Moreover, the proteomic profile of fish mucus subjected to different types of stress has been studied, such as chronic wounds [116], bacterial infection [187,188], parasitic infection [189], artificial stressors [190], and sample collection [191]. These types of studies expand our knowledge of proteomic changes associated with immune processes, and they can be a starting point to develop a powerful tool to identify bioindicators of fish welfare and physiological status via non-invasive methods.
Metabolomics can be defined as the quantitative complement of all low-molecular-weight molecules present in cells in a particular physiological or developmental state [192]. Metabolomics is situated downstream of proteomics, transcriptomics, and genomics, thus making metabolomics extremely useful for understanding organism responses and for biomarker discovery [37]. Analytical methods in metabolomics commonly include mass spectrometry (MS), often in conjunction with gas chromatography (GC) and liquid chromatography (LC), and nuclear magnetic resonance (NMR). Studies on fish metabolomics cover a wide range of fields of knowledge, including fish physiology and development, pollutants’ effects on fish, fish condition and disease, and fish as foodstuff [193]. Ekman et al., (2015) [121] characterized fathead minnow skin mucus metabolites using LC-MS in order to report a minimally invasive sampling method. Results indicate that the metabolome varies between sexes and that it is very sensitive to chemical exposure, suggesting that metabolomics is useful for environmental monitoring and surveillance. Wen et al., (2020) [194] analyzed discus fish skin mucus metabolites using LC-MS/MS-based metabolomics and found changes in metabolite profiles as they entered the stage of parental care; those variations were sex-specific in parental fish. Ivanova et al., (2018) [195] employed LC-MS metabolomics to detect small metabolites using different sample collection techniques. The results suggest that the scraping method to collect mucus is more invasive than other methods due to changes in metabolic profiles.
A combination of different omic techniques pursues the integration of different biological entities to understand their interrelation and the functioning of larger systems, and serves to identify new biomarkers in specific tissues. For instance, multiomic analysis of gilthead sea bream skin mucosa was carried out by measuring the entirety of biomolecules differentially expressed by means of skin transcriptomic analyses and the modification in mucus layer exudation by the analysis of the mucus proteome [196]. Information on fish mucus at genomic, transcriptional, protein, and metabolic levels has significantly moved fish mucus research forward. Applications associated with fish health and welfare, monitoring, food safety, and aquaculture production can be achieved using omic techniques. Important insights can be found not only within those techniques but also through understanding the interactions between them.
6. Conclusions
Besides being a key component in several physiological functions, fish skin mucus provides an effective chemical and physical barrier against pathogens. The performance of this activity is highly dependent on mucus composition. Therefore, the choice of a suitable molecular extraction method is crucial for its antimicrobial use in other applications. Indeed, notable differences in antimicrobial activity have been shown for the different types of extracts reviewed here, which is particularly relevant in those studies comparing different extraction methods on the same samples. In general, acidic extracts, followed by organic ones, showed the highest antimicrobial activity. This may be because these procedures favor the isolation of cationic and/or amphipathic antimicrobial compounds, such as AMPs, their enrichment in the final extracts and, apparently, the minimization of molecular inactivation events.
The general analysis of the studies reviewed here shows that 76% of the authors tested antibacterial activity against both gram-negative and gram-positive bacteria, while the remaining 24% used gram-negative bacteria only. In this sense, E. coli and S. aureus were the most commonly used gram-negative and gram-positive bacteria, respectively. With regard to the origin (or host target) of the pathogens tested, most studies used both human and fish pathogens (57%), while 35% and 8% used only human and fish pathogens, respectively. In some experiments, the (mostly acidic and organic) extracts inhibited the replication of gram-negative, but not gram-positive, bacteria. One of the reasons for this may be the higher affinity of the cationic antimicrobial molecules in the extracts for the negatively charged outer membrane of gram-negative bacteria, which is not present in gram-positive bacteria.
Focusing now on the procedures to quantify antimicrobial activity, and, mostly, antibacterial and antifungal activity, the most common methods employed are, in order, disc diffusion (46%), agar-well diffusion (26%), broth dilution (12%), optical density measurement (7%), and broth dilution recorded by optical measurement (5%). The disc diffusion and agar-well diffusion assays are simple, inexpensive, and intuitive to interpret; however, their low sensitivity makes them unsuitable for comparative studies and for determining precise inhibitory factors, such as the minimum and half-maximal inhibitory concentration (MIC and IC50, respectively). Therefore, and as a preview of the next section, it would be recommended that the methods used to evaluate the antimicrobial activity of compounds in this particular area of research be standardized to allow for appropriate comparisons.
7. Recommendations and Future Perspectives
This review emphasizes the importance of mucus composition for its antimicrobial activity. However, this composition may differ notably depending on several factors, such as species, sex, age, and environment. Therefore, more information on these factors should be included in such studies to improve reproducibility. A practical option could be to focus these studies on animals at harvesting stages in order to normalize the results at the most appropriate time for industrial exploitation. In this sense, it would also be of great interest to develop efficient technologies for the collection of fish skin mucus at harvesting sites.
However, the momentum needed to accelerate progress in these lines of research and their translation into practice requires a substantial increase in the need for these products. The urgency for new antimicrobials noted in the introduction to this review is one such need. However, new ideas that define the targets against which these compounds could already make a difference would greatly accelerate their development. Examples include the use of marine antimicrobials in high ionic strength environments, such as the mucosal tissues of cystic fibrosis patients [197] or food preservation [198]. In this line of research, it would also be interesting to study formats to increase their stability and to improve their delivery; for example, by encapsulating them in micro- or even nanomaterials.
Finally, it is important to reiterate that the study and understanding of the fish skin mucus interactome using omic techniques provides new, unprecedented opportunities for antimicrobial drug discovery. Multiomics may also allow for the discovery of clinically important metabolites, interactions between components, and the mechanisms by which components exert their antimicrobial activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21060350/s1, Table S1: List of antibacterial studies using aqueous skin mucus extracts from different fish species; Table S2: List of antibacterial studies using organic skin mucus extracts from different fish species; Table S3: List of antibacterial studies using acidic skin mucus extracts from different fish species; Table S4: List of antibacterial studies using crude skin mucus from different fish species. References [126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, R.D.-P. and A.F.; methodology, R.D.-P. and R.M.; software, R.D.-P. and A.F.; validation, M.A. and R.M.; formal analysis, R.D.-P., M.A., R.M. and A.F.; investigation, R.D.-P., M.A., R.M. and A.F.; resources, R.M. and A.F.; data curation, R.M. and A.F.; writing—original draft preparation, R.D.-P. and A.F.; writing—review and editing, R.D.-P., M.A., R.M. and A.F.; visualization, R.D.-P., M.A., R.M. and A.F.; supervision, R.M. and A.F.; project administration, R.M. and A.F.; funding acquisition, R.M. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the FEDER/Spanish Ministry of Science, Innovation and Universities—State Agency of Research, grant number RTI2018-101969-J-I00, and the European Maritime, Fisheries and Aquaculture Fund (EMFAF) and the Ministry of Agriculture, Fisheries and Food (MAPA), grant number MetDisFish.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr.; Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Mason, S.; Devincenzo, J.P.; Toovey, S.; Wu, J.Z.; Whitley, R.J. Comparison of antiviral resistance across acute and chronic viral infections. Antivir. Res. 2018, 158, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Merelli, M.; Temperoni, C.; Astilean, A. New antibiotics for bad bugs: Where are we? Ann. Clin. Microbiol. Antimicrob. 2013, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging antifungal targets and strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Pawlowski, A.C.; Johnson, J.W.; Wright, G.D. Evolving medicinal chemistry strategies in antibiotic discovery. Curr. Opin. Biotechnol. 2016, 42, 108–117. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Falco, A.; Adamek, M.; Pereiro, P.; Hoole, D.; Encinar, J.A.; Novoa, B.; Mallavia, R. The Immune System of Marine Organisms as Source for Drugs against Infectious Diseases. Mar. Drugs 2022, 20, 363. [Google Scholar] [CrossRef]
- Breitbart, M.; Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005, 13, 278–284. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Spížek, J.; Sigler, K.; Řezanka, T.; Demain, A. Biogenesis of antibiotics—Viewing its history and glimpses of the future. Folia Microbiol. 2016, 61, 347–358. [Google Scholar] [CrossRef]
- Oloke, J. Activity pattern of natural and synthetic antibacterial agents among hospital isolates. Microbios 2000, 102, 175–181. [Google Scholar] [PubMed]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional therapy and natural compounds with antibacterial activity—A pharmaco-toxicological screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Forterre, P.; Prangishvili, D. The origin of viruses. Res. Microbiol. 2009, 160, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Mojzsis, S.J.; Arrhenius, G.; McKeegan, K.; Harrison, T.; Nutman, A.; Friend, C. Evidence for life on Earth before 3,800 million years ago. Nature 1996, 384, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.-C.; Kumar, S.; Hedges, S.B. Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1999, 266, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Grün, R.; Joannes-Boyau, R.; Steele, T.E.; Amani, F.; Rué, M.; Fernandes, P.; Raynal, J.-P.; Geraads, D.; Ben-Ncer, A. The age of the hominin fossils from Jebel Irhoud, Morocco, and the origins of the Middle Stone Age. Nature 2017, 546, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Eizirik, E.; Johnson, W.E.; Zhang, Y.P.; Ryder, O.A.; O’Brien, S.J. Molecular phylogenetics and the origins of placental mammals. Nature 2001, 409, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Sallan, L.C. Major issues in the origins of ray-finned fish (A. ctinopterygii) biodiversity. Biol. Rev. 2014, 89, 950–971. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Madsen, E.L. Microorganisms and their roles in fundamental biogeochemical cycles. Curr. Opin. Biotechnol. 2011, 22, 456–464. [Google Scholar] [CrossRef]
- Hagström, A.k.; Pommier, T.; Rohwer, F.; Simu, K.; Stolte, W.; Svensson, D.; Zweifel, U.L. Use of 16S ribosomal DNA for delineation of marine bacterioplankton species. Appl. Environ. Microbiol. 2002, 68, 3628–3633. [Google Scholar] [CrossRef]
- Margulis, L.; Chapman, M.J. Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Kong, D.-X.; Jiang, Y.-Y.; Zhang, H.-Y. Marine natural products as sources of novel scaffolds: Achievement and concern. Drug Discov. Today 2010, 15, 884–886. [Google Scholar] [CrossRef]
- Harizani, M.; Ioannou, E.; Roussis, V. The Laurencia paradox: An endless source of chemodiversity. Prog. Chem. Org. Nat. Prod. 2016, 102, 91–252. [Google Scholar]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Svozil, D. An enumeration of natural products from microbial, marine and terrestrial sources. Phys. Sci. Rev. 2020, 5. [Google Scholar] [CrossRef]
- Principe, P.P.; Fisher, W.S. Spatial distribution of collections yielding marine natural products. J. Nat. Prod. 2018, 81, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, M.F. Immune relevant molecules identified in the skin mucus of fish using -omics technologies. Mol. BioSyst. 2016, 12, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Tapissier-Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Biological and Ecological Roles of External Fish Mucus: A Review. Fishes 2018, 3, 41. [Google Scholar] [CrossRef]
- Tiralongo, F.; Messina, G.; Lombardo, B.M.; Longhitano, L.; Li Volti, G.; Tibullo, D. Skin mucus of marine fish as a source for the development of antimicrobial agents. Front. Mar. Sci. 2020, 7, 760. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Martin, A.P. Increasing genomic complexity by gene duplication and the origin of vertebrates. Am. Nat. 1999, 154, 111–128. [Google Scholar] [CrossRef]
- Hurley, I.A.; Mueller, R.L.; Dunn, K.A.; Schmidt, E.J.; Friedman, M.; Ho, R.K.; Prince, V.E.; Yang, Z.; Thomas, M.G.; Coates, M.I. A new time-scale for ray-finned fish evolution. Proc. R. Soc. B Biol. Sci. 2007, 274, 489–498. [Google Scholar] [CrossRef]
- Ravi, V.; Venkatesh, B. Rapidly evolving fish genomes and teleost diversity. Curr. Opin. Genet. Dev. 2008, 18, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Bergh, Ø.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Ducklow, H. Bacterial production and biomass in the oceans. Microb. Ecol. Oceans 2000, 1, 85–120. [Google Scholar]
- Grossart, H.-P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Tafalla, C. Overview of fish immunity. In Mucosal Health in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–54. [Google Scholar]
- Ponnappan, N.; Budagavi, D.P.; Yadav, B.K.; Chugh, A. Membrane-active peptides from marine organisms-antimicrobials, cell-penetrating peptides and Peptide toxins: Applications and prospects. Probiotics Antimicrob. Proteins 2015, 7, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Litman, G.W.; Rast, J.P.; Fugmann, S.D. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 2010, 10, 543–553. [Google Scholar] [CrossRef]
- Esteban, M.Á.; Cerezuela, R. Fish mucosal immunity: Skin. In Mucosal Health in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 67–92. [Google Scholar]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Ellis, A. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Otero-Gonzáiez, A.J.; Magalhaes, B.S.; Garcia-Villarino, M.; López-Abarrategui, C.; Sousa, D.A.; Dias, S.C.; Franco, O.L. Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J. 2010, 24, 1320–1334. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar] [CrossRef]
- Shephard, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Esteban, M.Á. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunol. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Shephard, K.L. Mucus on the epidermis of fish and its influence on drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 403–417. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Liu, Q.; Wang, Q.; Xiao, J.; Zhang, Y. Skin-injured Zebrafish, Danio rerio, are more Susceptible to Vibrio anguillarum Infection. J. World Aquac. Soc. 2015, 46, 301–310. [Google Scholar] [CrossRef]
- Adamek, M.; Matras, M.; Rebl, A.; Stachnik, M.; Falco, A.; Bauer, J.; Miebach, A.-C.; Teitge, F.; Jung-Schroers, V.; Abdullah, M. Don’t Let It Get Under Your Skin!—Vaccination Protects the Skin Barrier of Common Carp From Disruption Caused by Cyprinid Herpesvirus 3. Front. Immunol. 2022, 13, 787021. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar]
- Hussain, A.; Sachan, S.G. Fish Epidermal Mucus as a Source of Diverse Therapeutical Compounds. Int. J. Pept. Res. Ther. 2023, 29, 36. [Google Scholar] [CrossRef]
- Rakers, S.; Niklasson, L.; Steinhagen, D.; Kruse, C.; Schauber, J.; Sundell, K.; Paus, R. Antimicrobial peptides (AMPs) from fish epidermis: Perspectives for investigative dermatology. J. Investig. Dermatol. 2013, 133, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Wu, Z.; Nuding, S.; Groscurth, S.; Marcinowski, M.; Beisner, J.; Buchner, J.; Schaller, M.; Stange, E.F.; Wehkamp, J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 2011, 469, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Haag, A.F.; Kerscher, B.; Dall’Angelo, S.; Sani, M.; Longhi, R.; Baloban, M.; Wilson, H.M.; Mergaert, P.; Zanda, M.; Ferguson, G.P. Role of cysteine residues and disulfide bonds in the activity of a legume root nodule-specific, cysteine-rich peptide. J. Biol. Chem. 2012, 287, 10791–10798. [Google Scholar] [CrossRef]
- Falco, A.; Ortega-Villaizan, M.; Chico, V.; Brocal, I.; Perez, L.; Coll, J.M.; Estepa, A. Antimicrobial peptides as model molecules for the development of novel antiviral agents in aquaculture. Mini Rev. Med. Chem. 2009, 9, 1159–1164. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 2559. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Kemp, G.D.; Molle, M.G.; Smith, V.J. Anti-microbial properties of histone H2A from skin secretions of rainbow trout, Oncorhynchus mykiss. Biochem. J. 2002, 368, 611–620. [Google Scholar] [CrossRef]
- Birkemo, G.A.; Lüders, T.; Andersen, Ø.; Nes, I.F.; Nissen-Meyer, J. Hipposin, a histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.). Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2003, 1646, 207–215. [Google Scholar] [CrossRef]
- Park, I.Y.; Park, C.B.; Kim, M.S.; Kim, S.C. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 1998, 437, 258–262. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, I.Y.; Kim, H.S.; Lee, W.T.; Kim, M.S.; Kim, S.C. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 2002, 16, 429–431. [Google Scholar] [CrossRef]
- Bergsson, G.; Agerberth, B.; Jörnvall, H.; Gudmundsson, G.H. Isolation and identification of antimicrobial components from the epidermal mucus of Atlantic cod (Gadus morhua). FEBS J. 2005, 272, 4960–4969. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Molle, G.; Kemp, G.D.; Smith, V.J. Isolation and characterisation of oncorhyncin II, a histone H1-derived antimicrobial peptide from skin secretions of rainbow trout, Oncorhynchus mykiss. Dev. Comp. Immunol. 2004, 28, 127–138. [Google Scholar] [CrossRef]
- Lüders, T.; Birkemo, G.A.; Nissen-Meyer, J.; Andersen, Ø.; Nes, I.F. Proline conformation-dependent antimicrobial activity of a proline-rich histone h1 N-terminal Peptide fragment isolated from the skin mucus of Atlantic salmon. Antimicrob. Agents Chemother. 2005, 49, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Myxinidin, A Novel Antimicrobial Peptide from the Epidermal Mucus of Hagfish, Myxine glutinosa L. Mar. Biotechnol. 2009, 11, 748. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Saint, N.; Kemp, G.D.; Smith, V.J. Oncorhyncin III: A potent antimicrobial peptide derived from the non-histone chromosomal protein H6 of rainbow trout, Oncorhynchus mykiss. Biochem. J. 2003, 373, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Su, Y. Isolation and identification of pelteobagrin, a novel antimicrobial peptide from the skin mucus of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 158, 149–154. [Google Scholar] [CrossRef]
- Lazarovici, P.; Primor, N.; Loew, L.M. Purification and pore-forming activity of two hydrophobic polypeptides from the secretion of the Red Sea Moses sole (Pardachirus marmoratus). J. Biol. Chem. 1986, 261, 16704–16713. [Google Scholar] [CrossRef] [PubMed]
- Ruangsri, J.; Salger, S.A.; Caipang, C.M.A.; Kiron, V.; Fernandes, J.M.O. Differential expression and biological activity of two piscidin paralogues and a novel splice variant in Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2012, 32, 396–406. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and Characterization of Pleurocidin, an Antimicrobial Peptide in the Skin Secretions of Winter Flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Smith, V.J. A novel antimicrobial function for a ribosomal peptide from rainbow trout skin. Biochem. Biophys. Res. Commun. 2002, 296, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Robinette, D.; Wada, S.; Arroll, T.; Levy, M.G.; Miller, W.L.; Noga, E.J. Antimicrobial activity in the skin of the channel catfish Ictalurus punctatus: Characterization of broad-spectrum histone-like antimicrobial proteins. Cell. Mol. Life Sci. CMLS 1998, 54, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, C.B.; Kim, M.S.; Kim, S.C. cDNA cloning and characterization of buforin I, an antimicrobial peptide: A cleavage product of histone H2A. Biochem. Biophys. Res. Commun. 1996, 229, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; Chaves-Pozo, E.; Meseguer, J.; Esteban, M.; Cuesta, A. Biologial Role of Fish Antimicrobial Peptides. In Antimicrobial Peptides; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 31–60. [Google Scholar]
- Oren, Z.; Shai, Y. A Class of Highly Potent Antibacterial Peptides Derived from Pardaxin, A Pore-Forming Peptide Isolated from Moses Sole Fish Pardachirus marmoratus. Eur. J. Biochem. 1996, 237, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-S.; Jung, J.-M.; An, C.M.; Kim, J.-W.; Hwang, S.D.; Kwon, M.-G.; Park, M.-A.; Kim, M.-C.; Park, C.-I. Piscidin: Antimicrobial peptide of rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol. 2016, 51, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ebran, N.; Julien, S.; Orange, N.; Auperin, B.; Molle, G. Isolation and characterization of novel glycoproteins from fish epidermal mucus: Correlation between their pore-forming properties and their antibacterial activities. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1467, 271–280. [Google Scholar] [CrossRef]
- Easy, R.H.; Trippel, E.A.; Burt, M.D.B.; Cone, D.K. Identification of transferrin in Atlantic cod Gadus morhua epidermal mucus. J. Fish Biol. 2012, 81, 2059–2063. [Google Scholar] [CrossRef]
- Ræder, I.L.U.; Paulsen, S.M.; Smalås, A.O.; Willassen, N.P. Effect of fish skin mucus on the soluble proteome of Vibrio salmonicida analysed by 2-D gel electrophoresis and tandem mass spectrometry. Microb. Pathog. 2007, 42, 36–45. [Google Scholar] [CrossRef]
- Santoso, H.B.; Suhartono, E.; Yunita, R.; Biyatmoko, D. Epidermal mucus as a potential biological matrix for fish health analysis. Egypt. J. Aquat. Biol. Fish. 2020, 24, 361–382. [Google Scholar] [CrossRef]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tasumi, S.; Tsutsui, S.; Okamoto, M.; Suetake, H. Molecular diversity of skin mucus lectins in fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 723–730. [Google Scholar] [CrossRef]
- Chong, K.; Joshi, S.; Jin, L.T.; Shu-Chien, A.C. Proteomics profiling of epidermal mucus secretion of a cichlid (Symphysodon aequifasciata) demonstrating parental care behavior. Proteomics 2006, 6, 2251–2258. [Google Scholar] [CrossRef]
- Cordero, H.; Brinchmann, M.F.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Skin mucus proteome map of European sea bass (Dicentrarchus labrax). Proteomics 2015, 15, 4007–4020. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.; Fernandes, J.M.O.; Caipang, C.M.A.; Kiron, V.; Rombout, J.H.W.M.; Brinchmann, M.F. Proteome reference map of the skin mucus of Atlantic cod (Gadus morhua) revealing immune competent molecules. Fish Shellfish Immunol. 2011, 31, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Tasumi, S.; Suetake, H.; Suzuki, Y. Lectins Homologous to Those of Monocotyledonous Plants in the Skin Mucus and Intestine of Pufferfish, Fugu rubripes. J. Biol. Chem. 2003, 278, 20882–20889. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Yamaguchi, M.; Hirasawa, A.; Nakamura, O.; Watanabe, T. Common Skate (Raja kenojei) Secretes Pentraxin into the Cutaneous Secretion: The First Skin Mucus Lectin in Cartilaginous Fish*. J. Biochem. 2009, 146, 295–306. [Google Scholar] [CrossRef]
- Patel, D.M.; Brinchmann, M.F. Skin mucus proteins of lumpsucker (Cyclopterus lumpus). Biochem. Biophys. Rep. 2017, 9, 217–225. [Google Scholar] [CrossRef]
- Gewurz, H.; Zhang, X.-H.; Lint, T.F. Structure and function of the pentraxins. Curr. Opin. Immunol. 1995, 7, 54–64. [Google Scholar] [CrossRef]
- Ramos, F.; Smith, A.C. The C-reactive protein (CRP) test for the detection of early disease in fishes. Aquaculture 1978, 14, 261–266. [Google Scholar] [CrossRef]
- Lazado, C.C.; Skov, P.V. Secretory Proteins in the Skin Mucus of Nile Tilapia (Oreochromis niloticus) are Modulated Temporally by Photoperiod and Bacterial Endotoxin Cues. Fishes 2019, 4, 57. [Google Scholar] [CrossRef]
- Nigam, A.K.; Kumari, U.; Mittal, S.; Mittal, A.K. Comparative analysis of innate immune parameters of the skin mucous secretions from certain freshwater teleosts, inhabiting different ecological niches. Fish Physiol. Biochem. 2012, 38, 1245–1256. [Google Scholar] [CrossRef]
- Ren, T.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Micheal, F.R.; Uyan, O.; Tung, H.T. Influence of dietary vitamin C and bovine lactoferrin on blood chemistry and non-specific immune responses of Japanese eel, Anguilla japonica. Aquaculture 2007, 267, 31–37. [Google Scholar] [CrossRef]
- Subramanian, S.; MacKinnon, S.L.; Ross, N.W. A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, K.; Christie, M.; Raa, J. Skin mucus protease from rainbow trout, Salmo gairdneri Richardson, and its biological significance. J. Fish Biol. 1983, 23, 13–22. [Google Scholar] [CrossRef]
- Aranishi, F.; Nakane, M. Epidermal proteases of the Japanese eel. Fish Physiol. Biochem. 1997, 16, 471–478. [Google Scholar] [CrossRef]
- Aranishi, F.; Nakane, M. Epidermal Proteinases in the European Eel. Physiol. Zool. 1997, 70, 563–570. [Google Scholar] [CrossRef]
- Firth, K.; Johnson, S.; Ross, N. Characterisation of proteases in the skin mucus of Atlantic Salmon (Salmo salar) infected with the Salmon louse (Lepeophtheirus salmonis) and in whole-body louse homogenate. J. Parasitol. 2001, 86, 1199–1205. [Google Scholar] [CrossRef]
- Jurado, J.; Fuentes-Almagro, C.A.; Guardiola, F.A.; Cuesta, A.; Esteban, M.Á.; Prieto-Álamo, M.-J. Proteomic profile of the skin mucus of farmed gilthead seabream (Sparus aurata). J. Proteom. 2015, 120, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Molle, V.; Campagna, S.; Bessin, Y.; Ebran, N.; Saint, N.; Molle, G. First evidence of the pore-forming properties of a keratin from skin mucus of rainbow trout (Oncorhynchus mykiss, formerly Salmo gairdneri). Biochem. J. 2008, 411, 33–40. [Google Scholar] [CrossRef]
- Hayes, M.J.; Rescher, U.; Gerke, V.; Moss, S.E. Annexin–actin interactions. Traffic 2004, 5, 571–576. [Google Scholar] [CrossRef]
- Cordero, H.; Brinchmann, M.F.; Cuesta, A.; Esteban, M.A. Chronic wounds alter the proteome profile in skin mucus of farmed gilthead seabream. BMC Genom. 2017, 18, 939. [Google Scholar] [CrossRef]
- Easy, R.H.; Ross, N.W. Changes in Atlantic salmon Salmo salar mucus components following short- and long-term handling stress. J. Fish Biol. 2010, 77, 1616–1631. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Gunasekaran, G. Fatty acids and amino acids composition in skin epidermal mucus of selected fresh water fish mugil cephalus. World J. Pharm. Pharm. Sci. 2015, 4, 1275–1287. [Google Scholar]
- Torrecillas, S.; Montero, D.; Domínguez, D.; Robaina, L.; Izquierdo, M. Skin Mucus Fatty Acid Composition of Gilthead Sea Bream (Sparus Aurata): A Descriptive Study in Fish Fed Low and High Fish Meal Diets. Fishes 2019, 4, 15. [Google Scholar] [CrossRef]
- Lewis, R.W. Fish cutaneous mucus: A new source of skin surface lipid. Lipids 1970, 5, 947–949. [Google Scholar] [CrossRef]
- Ekman, D.R.; Skelton, D.M.; Davis, J.M.; Villeneuve, D.L.; Cavallin, J.E.; Schroeder, A.; Jensen, K.M.; Ankley, G.T.; Collette, T.W. Metabolite Profiling of Fish Skin Mucus: A Novel Approach for Minimally-Invasive Environmental Exposure Monitoring and Surveillance. Environ. Sci. Technol. 2015, 49, 3091–3100. [Google Scholar] [CrossRef]
- Holland, K.; Bojar, R. Antimicrobial effects of azelaic acid. J. Dermatol. Treat. 1993, 4, S8–S11. [Google Scholar] [CrossRef]
- Sakko, M.; Moore, C.; Novak-Frazer, L.; Rautemaa, V.; Sorsa, T.; Hietala, P.; Järvinen, A.; Bowyer, P.; Tjäderhane, L.; Rautemaa, R. 2-hydroxyisocaproic acid is fungicidal for Candida and Aspergillus species. Mycoses 2014, 57, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Ashraf, M.S.; Siddiqui, A.J.; Ashraf, S.A.; Sachidanandan, M.; Snoussi, M.; Adnan, M.; Hadi, S. Profiling and Role of Bioactive Molecules from Puntius sophore (Freshwater/Brackish Fish) Skin Mucus with Its Potent Antibacterial, Antiadhesion, and Antibiofilm Activities. Biomolecules 2020, 10, 920. [Google Scholar] [CrossRef]
- Lee, Y.; Bilung, L.M.; Sulaiman, B.; Chong, Y.L. The antibacterial activity of fish skin mucus with various extraction solvents and their in-vitro evaluation methods. Int. Aquat. Res. 2020, 12, 1–21. [Google Scholar]
- Wang, H.; Tang, W.; Zhang, R.; Ding, S. Analysis of enzyme activity, antibacterial activity, antiparasitic activity and physico-chemical stability of skin mucus derived from Amphiprion clarkii. Fish Shellfish Immunol. 2019, 86, 653–661. [Google Scholar] [CrossRef]
- Al-Rasheed, A.; Handool, K.O.; Garba, B.; Noordin, M.M.; Bejo, S.K.; Kamal, F.M.; Daud, H.H.M. Crude extracts of epidermal mucus and epidermis of climbing perch Anabas testudineus and its antibacterial and hemolytic activities. Egypt. J. Aquat. Res. 2018, 44, 125–129. [Google Scholar] [CrossRef]
- Sanahuja, I.; Fernández-Alacid, L.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Ramos, A.; Araujo, R.M.; Ibarz, A. Comparison of several non-specific skin mucus immune defences in three piscine species of aquaculture interest. Fish Shellfish Immunol. 2019, 89, 428–436. [Google Scholar] [CrossRef]
- Leng, W.; Wu, X.; Xiong, Z.; Shi, T.; Sun, Q.; Yuan, L.; Gao, R. Study on antibacterial properties of mucus extract of snakehead (Channa argus) against Escherichia coli and its application in chilled fish fillets preservation. LWT 2022, 167, 113840. [Google Scholar] [CrossRef]
- Dhanaraj, M.; Haniffa, M.A.; Arun Singh, S.V.; Ramakrishnan, C.; Manikandaraja, D.; James Milton, M. Antibacterial Activity of Skin and Intestinal Mucus of Five Different Freshwater Fish Species Viz., Channa striatus, C. micropeltes, C. marulius, C. Punctatus and C. gachua. Malays. J. Sci. 2009, 28, 256–262. [Google Scholar] [CrossRef]
- Wei, O.Y.; Xavier, R.; Marimuthu, K. Screening of antibacterial activity of mucus extract of snakehead fish, Channa striatus. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 675–681. [Google Scholar]
- Ramesh, B.K. Assessment of antimicrobial peptides from mucus of fish. Indian J. Clin. Biochem. 2013, 1, 5–8. [Google Scholar]
- Haniffa, M.A.; Viswanathan, S.; Jancy, D.; Poomari, K.; Manik, S. Antibacterial studies of fish mucus from two marketed air-breathing fishes-Channa striatus and Heteropneustes fossilis. Int. Res. J. Microbiol. 2014, 5, 22–27. [Google Scholar]
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Maricchiolo, G.; Genovese, L.; De Pasquale, F.; Caruso, R.; Denaro, M.G.; Delia, S.; Laganà, P. Comparative Study of Antibacterial and Haemolytic Activities in Sea Bass, European Eel and Blackspot Seabream. Open Mar. Biol. J. 2014, 8, 10–16. [Google Scholar]
- Magariños, B.; Pazos, F.G.; Santos, Y.; Romalde, J.L.; Toranzo, A.E. Response of Pasteurella piscicida and Flexibacter maritimus to skin mucus of marine fish. Dis. Aquat. Org. 1995, 21, 103–108. [Google Scholar] [CrossRef]
- Manikantan, G.; Lyla, S.; Khan, S.A.; Vijayanand, P.; Edward, G.; Jothi, G. Bioactive potency of epidermal mucus extracts from greasy grouper, Epinephelus tauvina (Forsskal, 1775). J. Coast. Life Med. 2016, 4, 510–520. [Google Scholar] [CrossRef]
- Hellio, C.; Pons, A.M.; Beaupoil, C.; Bourgougnon, N.; Gal, Y.L. Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int. J. Antimicrob. Agents 2002, 20, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Katra, N.; Hisar, O.; Karatas, S.; Turgay, E.; Sarvan, C.; KATRA, N. In vitro antimicrobial activities of extracts from ballan wrasse (Labrus bergylta) skin mucus. Mar. Sci. Technol. Bull. 2016, 5, 13–15. [Google Scholar]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Comparison of antimicrobial activity in the epidermal mucus extracts of fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 150, 85–92. [Google Scholar] [CrossRef]
- García-Marciano, M.; Apún-Molina, J.P.; Sainz-Hernández, J.C.; Santamaría-Miranda, A.; Medina-Godoy, S.; Aguiñaga-Cruz, J.A. Antibacterial activity evaluation of the nile tilapia Oreochromis niloticus (Linnaeus, 1758) skin mucus, against vibrio bacteria affecting the white shrimp Penaeus vannamei. Lat. Am. J. Aquat. Res. 2019, 47, 580–585. [Google Scholar] [CrossRef]
- Lirio, G.A.C.; De Leon, J.A.A.; Villafuerte, A.G. Antimicrobial Activity of Epidermal Mucus from Top Aquaculture Fish Species against Medically-Important Pathogens. Walailak J. Sci. Technol. 2018, 16, 329–340. [Google Scholar] [CrossRef]
- Wibowo, A.; Fadjar, M. Utilization of Tilapia Mucus to Inhibit Vibrio harveyi on Vannamei (Litopenaeus vannamei). J. Life Sci. Biomed. 2015, 5, 141–148. [Google Scholar]
- Elavarasi, K.; Ranjini, S.; Rajagopal, T.; Rameshkumar, G.; Ponmanickam, P. Bactericidal proteins of skin mucus and skin extracts from fresh water fishes, Clarias batrachus and Tilapia mossambicus. Thai J. Pharm. Sci. 2013, 37, 194–200. [Google Scholar]
- Mahadevan, G.; Mohan, K.; Vinoth, J.; Ravi, V. Biotic potential of mucus extracts of giant mudskipper Periophthalmodon schlosseri (Pallas, 1770) from Pichavaram, southeast coast of India. J. Basic Appl. Zool. 2019, 80, 13. [Google Scholar] [CrossRef]
- Pethkar, M.R.; Lokhande, M.V. Antifungal activity of skin mucus of three cultivable fish species. Int. J. Zool. Stud. 2017, 2, 1–3. [Google Scholar]
- Manivasagan, P.; Annamalai, N.; Ashokkumar, S.; Sampathkumar, P. Studies on the proteinaceous gel secretion from the skin of the catfish, Arius maculatus (Thunberg, 1792). Afr. J. Biotechnol. 2009, 8, 7125–7129. [Google Scholar]
- Patil, R.; Ingole, J.; Sathe, T.; Jadhav, A. Antibacterial activity of fish mucus from Clarias batrachus (Linn.) against selected microbes. Biolife 2015, 3, 788–791. [Google Scholar]
- Loganathan, K.; Muniyan, M.; Prakash, A.A.; Raja, P.S.; Prakash, M. Studies on the Role of Mucus from Clarias batrachus (Linn) Against Selected Microbes. Int. J. Pharm. Appl. 2011, 2, 202–206. [Google Scholar]
- Kumari, U.; Nigam, A.K.; Mittal, S.; Mittal, A.K. Antibacterial properties of the skin mucus of the freshwater fishes, Rita rita and Channa punctatus. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 781–786. [Google Scholar]
- Subhashini, S.; Lavanya, J.; Jain, S.; Agihotri, T. Screening of Antibacterial and Cytotoxic Activity of Extracts from Epidermis and Epidermal Mucus of Barbonymus Schwanenfeldii (Tinfoil Barb Fish). Int. J. Res. Eng. Technol. 2013, 2, 492–497. [Google Scholar] [CrossRef]
- Islam, M.M.; Hossain, M.M.M.; Islam, M.S.; Khondoker, S.; Khatun, M.A. Competitive antibacterial activity of two Indian major carps and two Chinese carps fish mucus against common pathogenic bacteria at aquaculture pond. Int. J. Fish. Aquat. Stud. 2014, 2, 158–162. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Rani, P.B.; Prakash, A.A.; Prakash, M.; Senthilraja, P.; Gunasekaran, G. Antimicrobial properties of skin mucus from four freshwater cultivable Fishes (Catla catla, Hypophthalmichthys molitrix, Labeo rohita and Ctenopharyngodon idella). Afr. J. Microbiol. Res. 2011, 6, 5110–5120. [Google Scholar]
- Nigam, A.K.; Kumari, U.; Mittal, S.; Mittal, A.K. Evaluation of antibacterial activity and innate immune components in skin mucus of Indian major carp, Cirrhinus mrigala. Aquac. Res. 2017, 48, 407–418. [Google Scholar] [CrossRef]
- Kuppulakshmi, C.; Prakash, M.; Gunasekaran, G.; Manimegalai, G.; Sarojini, S. Antibacterial properties of fish mucus from Channa punctatus and Cirrhinus mrigala. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 149–153. [Google Scholar]
- Kumari, S.; Tyor, A.K.; Bhatnagar, A. Evaluation of the antibacterial activity of skin mucus of three carp species. Int. Aquat. Res. 2019, 11, 225–239. [Google Scholar] [CrossRef]
- Tyor, A.; Kumari, S. Biochemical characterization and antibacterial properties of fish skin mucus of fresh water fish, Hypophthalmichthys nobilis. Int. J. Pharm. Pharm. Sci. 2016, 8, 132–136. [Google Scholar]
- Fuochi, V.; Li Volti, G.; Camiolo, G.; Tiralongo, F.; Giallongo, C.; Distefano, A.; Petronio Petronio, G.; Barbagallo, I.; Viola, M.; Furneri, P.M.; et al. Antimicrobial and Anti-Proliferative Effects of Skin Mucus Derived from Dasyatis pastinaca (Linnaeus, 1758). Mar. Drugs 2017, 15, 342. [Google Scholar] [CrossRef]
- Hisar, O.; Hisar, S.A.; Uyanik, M.H.; Sahin, T.; Cakir, F.; Yilmaz, S. In vitro antimicrobial and antifungal activities of aqueous skin mucus from rainbow trout (Oncorhynchus mykiss) on human pathogens. Mar. Sci. Technol. Bull. 2014, 3, 19–22. [Google Scholar]
- Guardiola, F.A.; Cuartero, M.; del Mar Collado-González, M.; Díaz Baños, F.G.; Cuesta, A.; Moriñigo, M.Á.; Esteban, M.Á. Terminal carbohydrates abundance, immune related enzymes, bactericidal activity and physico-chemical parameters of the Senegalese sole (Solea senegalensis, Kaup) skin mucus. Fish Shellfish Immunol. 2017, 60, 483–491. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, I.Y.; Kim, M.S.; Kim, S.C. Matrix metalloproteinase 2 is involved in the regulation of the antimicrobial peptide parasin I production in catfish skin mucosa. FEBS Lett. 2002, 531, 459–463. [Google Scholar] [CrossRef]
- Yount, N.Y.; Bayer, A.S.; Xiong, Y.Q.; Yeaman, M.R. Advances in antimicrobial peptide immunobiology. Pept. Sci. Orig. Res. Biomol. 2006, 84, 435–458. [Google Scholar] [CrossRef]
- Afkarian, M.; Bhasin, M.; Dillon, S.T.; Guerrero, M.C.; Nelson, R.G.; Knowler, W.C.; Thadhani, R.; Libermann, T.A. Optimizing a proteomics platform for urine biomarker discovery. Mol. Cell. Proteom. 2010, 9, 2195–2204. [Google Scholar] [CrossRef]
- Ming, L.; Xiaoling, P.; Yan, L.; Lili, W.; Qi, W.; Xiyong, Y.; Boyao, W.; Ning, H. Purification of antimicrobial factors from human cervical mucus. Hum. Reprod. 2007, 22, 1810–1815. [Google Scholar] [CrossRef]
- Fischer, C.L. Antimicrobial Activity of Host-Derived Lipids. Antibiotics 2020, 9, 75. [Google Scholar] [CrossRef]
- Al-Arifa, N.; Mughal, M.; Hanif, A.; Batool, A. Effect of alkaline pH on Biozctive Molecules of Epidermal mucus from Labeo rohita. Turk. J. Biochem. 2011, 36, 29–34. [Google Scholar]
- Ikram, M.; Ridzwan, B.H. A preliminary screening of antifungal activities from skin mucus extract of Malaysian local swamp eel (Monopterus albus). Int. Res. J. Pharm. 2013, 3, 1–8. [Google Scholar]
- Nishiyama, Y.; Nakaoka, C.; Hiratani, T.; Abe, S.; Uchida, K.; Yamaguchi, H. Synergy of lysozyme and lanoconazole on the morphology of Candida albicans. J. Electron Microsc. 2001, 50, 41–49. [Google Scholar] [CrossRef]
- Sowa-Jasiłek, A.; Zdybicka-Barabas, A.; Stączek, S.; Wydrych, J.; Skrzypiec, K.; Mak, P.; Deryło, K.; Tchórzewski, M.; Cytryńska, M. Galleria mellonella lysozyme induces apoptotic changes in Candida albicans cells. Microbiol. Res. 2016, 193, 121–131. [Google Scholar] [CrossRef]
- Zarei, M.; Aminzadeh, S.; Zolgharnein, H.; Safahieh, A.; Daliri, M.; Noghabi, K. Characterization of A Chitinase with Antifungal Activity from a Native Serratia. Braz. J. Microbiol. 2011, 42, 1017–1029. [Google Scholar] [CrossRef]
- Raj, V.S.; Fournier, G.; Rakus, K.; Ronsmans, M.; Ouyang, P.; Michel, B.; Delforges, C.; Costes, B.; Farnir, F.; Leroy, B. Skin mucus of Cyprinus carpio inhibits cyprinid herpesvirus 3 binding to epidermal cells. Vet. Res. 2011, 42, 1–10. [Google Scholar] [CrossRef]
- Valero, Y.; Arizcun, M.; Cortes, J.; Ramirez-Cepeda, F.; Guzman, F.; Mercado, L.; Esteban, M.Á.; Chaves-Pozo, E.; Cuesta, A. NK-lysin, dicentracin and hepcidin antimicrobial peptides in European sea bass. Ontogenetic development and modulation in juveniles by nodavirus. Dev. Comp. Immunol. 2020, 103, 103516. [Google Scholar] [CrossRef]
- Falco, A.; Medina-Gali, R.M.; Poveda, J.A.; Bello-Perez, M.; Novoa, B.; Encinar, J.A. Antiviral activity of a Turbot (Scophthalmus maximus) NK-lysin peptide by inhibition of low-pH virus-induced membrane fusion. Mar. Drugs 2019, 17, 87. [Google Scholar] [CrossRef]
- Casadei, E.; Wang, T.; Zou, J.; Vecino, J.L.G.; Wadsworth, S.; Secombes, C.J. Characterization of three novel β-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss). Mol. Immunol. 2009, 46, 3358–3366. [Google Scholar] [CrossRef]
- Falco, A.; Chico, V.; Marroqui, L.; Perez, L.; Coll, J.; Estepa, A. Expression and antiviral activity of a β-defensin-like peptide identified in the rainbow trout (Oncorhynchus mykiss) EST sequences. Mol. Immunol. 2008, 45, 757–765. [Google Scholar] [CrossRef]
- Mai, W.-j.; Wang, W.-n. Protection of blue shrimp (Litopenaeus stylirostris) against the White Spot Syndrome Virus (WSSV) when injected with shrimp lysozyme. Fish Shellfish Immunol. 2010, 28, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guo, N.; Chen, S.; Guo, X.; Liu, X.; Ye, S.; Chai, Q.; Wang, Y.; Liu, B.; He, Q. Antiviral activity of Piscidin 1 against pseudorabies virus both in vitro and in vivo. Virol. J. 2019, 16, 95. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Silva, T.S.; Dias, J.; Jessen, F. PROTEOMICS in aquaculture: Applications and trends. J. Proteom. 2012, 75, 4325–4345. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Mu, Y.; Xiang, L.-X.; Fan, D.; Feng, M.; Zhang, S.; Shi, Q.; Zhu, L.-Y.; Li, T.; Ding, Y.; et al. Genome sequencing of the perciform fish Larimichthys crocea provides insights into molecular and genetic mechanisms of stress adaptation. PLoS Genet. 2015, 11, e1005118. [Google Scholar] [CrossRef]
- Carda-Diéguez, M.; Ghai, R.; Rodríguez-Valera, F.; Amaro, C. Wild eel microbiome reveals that skin mucus of fish could be a natural niche for aquatic mucosal pathogen evolution. Microbiome 2017, 5, 162. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.B.; Andrzejczyk, N.E.; Mager, E.M.; Stieglitz, J.D.; Benetti, D.; Grosell, M.; Schlenk, D. Whole-Transcriptome Sequencing of Epidermal Mucus as a Novel Method for Oil Exposure Assessment in Juvenile Mahi-Mahi (Coryphaena hippurus). Environ. Sci. Technol. Lett. 2019, 6, 538–544. [Google Scholar] [CrossRef]
- Parida, S.; Mohapatra, A.; Kar, B.; Mohanty, J.; Sahoo, P.K. Transcriptional analysis of immune-relevant genes in the mucus of Labeo rohita, experimentally infected with Argulus siamensis. Acta Parasitol. 2018, 63, 125–133. [Google Scholar] [CrossRef]
- Nissa, M.U.; Pinto, N.; Parkar, H.; Goswami, M.; Srivastava, S. Proteomics in fisheries and aquaculture: An approach for food security. Food Control 2021, 127, 108125. [Google Scholar] [CrossRef]
- Minniti, G.; Rød Sandve, S.; Padra, J.T.; Heldal Hagen, L.; Lindén, S.; Pope, P.B.; Arntzen, M.Ø.; Vaaje-Kolstad, G. The Farmed Atlantic Salmon (Salmo salar) Skin-Mucus Proteome and Its Nutrient Potential for the Resident Bacterial Community. Genes 2019, 10, 515. [Google Scholar] [CrossRef]
- Liu, H.-h.; Sun, Q.; Jiang, Y.-t.; Fan, M.-h.; Wang, J.-x.; Liao, Z. In-depth proteomic analysis of Boleophthalmus pectinirostris skin mucus. J. Proteom. 2019, 200, 74–89. [Google Scholar] [CrossRef]
- Chong, K.; Sock Ying, T.; Foo, J.; Toong Jin, L.; Chong, A. Characterisation of proteins in epidermal mucus of discus fish (Symphysodon spp.) during parental phase. Aquaculture 2005, 249, 469–476. [Google Scholar] [CrossRef]
- Rajan, B.; Lokesh, J.; Kiron, V.; Brinchmann, M.F. Differentially expressed proteins in the skin mucus of Atlantic cod (Gadus morhua) upon natural infection with Vibrio anguillarum. BMC Vet. Res. 2013, 9, 103. [Google Scholar] [CrossRef]
- Xiong, Y.; Dan, C.; Ren, F.; Su, Z.; Zhang, Y.; Mei, J. Proteomic profiling of yellow catfish (Pelteobagrus fulvidraco) skin mucus identifies differentially-expressed proteins in response to Edwardsiella ictaluri infection. Fish Shellfish Immunol. 2020, 100, 98–108. [Google Scholar] [CrossRef]
- Fernández-Montero, Á.; Torrecillas, S.; Montero, D.; Acosta, F.; Prieto-Álamo, M.-J.; Abril, N.; Jurado, J. Proteomic profile and protease activity in the skin mucus of greater amberjack (Seriola dumerili) infected with the ectoparasite Neobenedenia girellae—An immunological approach. Fish Shellfish Immunol. 2021, 110, 100–115. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J.; Terova, G.; Simó-Mirabet, P.; Rimoldi, S.; Folkedal, O.; Calduch-Giner, J.A.; Olsen, R.E.; Sitjà-Bobadilla, A. Skin Mucus of Gilthead Sea Bream (Sparus aurata L.). Protein Mapping and Regulation in Chronically Stressed Fish. Front. Physiol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Fæste, C.K.; Tartor, H.; Moen, A.; Kristoffersen, A.B.; Dhanasiri, A.K.S.; Anonsen, J.H.; Furmanek, T.; Grove, S. Proteomic profiling of salmon skin mucus for the comparison of sampling methods. J. Chromatogr. B 2020, 1138, 121965. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004, 22, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, L.M.; Larsson, D.G.J. Contributions from metabolomics to fish research. Mol. BioSyst. 2008, 4, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhou, J.-Q.; Gao, J.-Z.; Chen, H.-R.; Shen, Y.-Q.; Chen, Z.-Z. Sex-dependent changes in the skin mucus metabolome of discus fish (Symphysodon haraldi) during biparental care. J. Proteom. 2020, 221, 103784. [Google Scholar] [CrossRef]
- Ivanova, L.; Tartor, H.; Grove, S.; Kristoffersen, A.B.; Uhlig, S. Workflow for the Targeted and Untargeted Detection of Small Metabolites in Fish Skin Mucus. Fishes 2018, 3, 21. [Google Scholar] [CrossRef]
- Reyes-López, F.E.; Ibarz, A.; Ordóñez-Grande, B.; Vallejos-Vidal, E.; Andree, K.B.; Balasch, J.C.; Fernández-Alacid, L.; Sanahuja, I.; Sánchez-Nuño, S.; Firmino, J.P.; et al. Skin Multi-Omics-Based Interactome Analysis: Integrating the Tissue and Mucus Exuded Layer for a Comprehensive Understanding of the Teleost Mucosa Functionality as Model of Study. Front. Immunol. 2021, 11, 613824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Parente, J.; Harris, S.M.; Woods, D.E.; Hancock, R.E.; Falla, T.J. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 2005, 49, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).