Recovery of Nutrients from Cod Processing Waters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Side-Stream Characterization
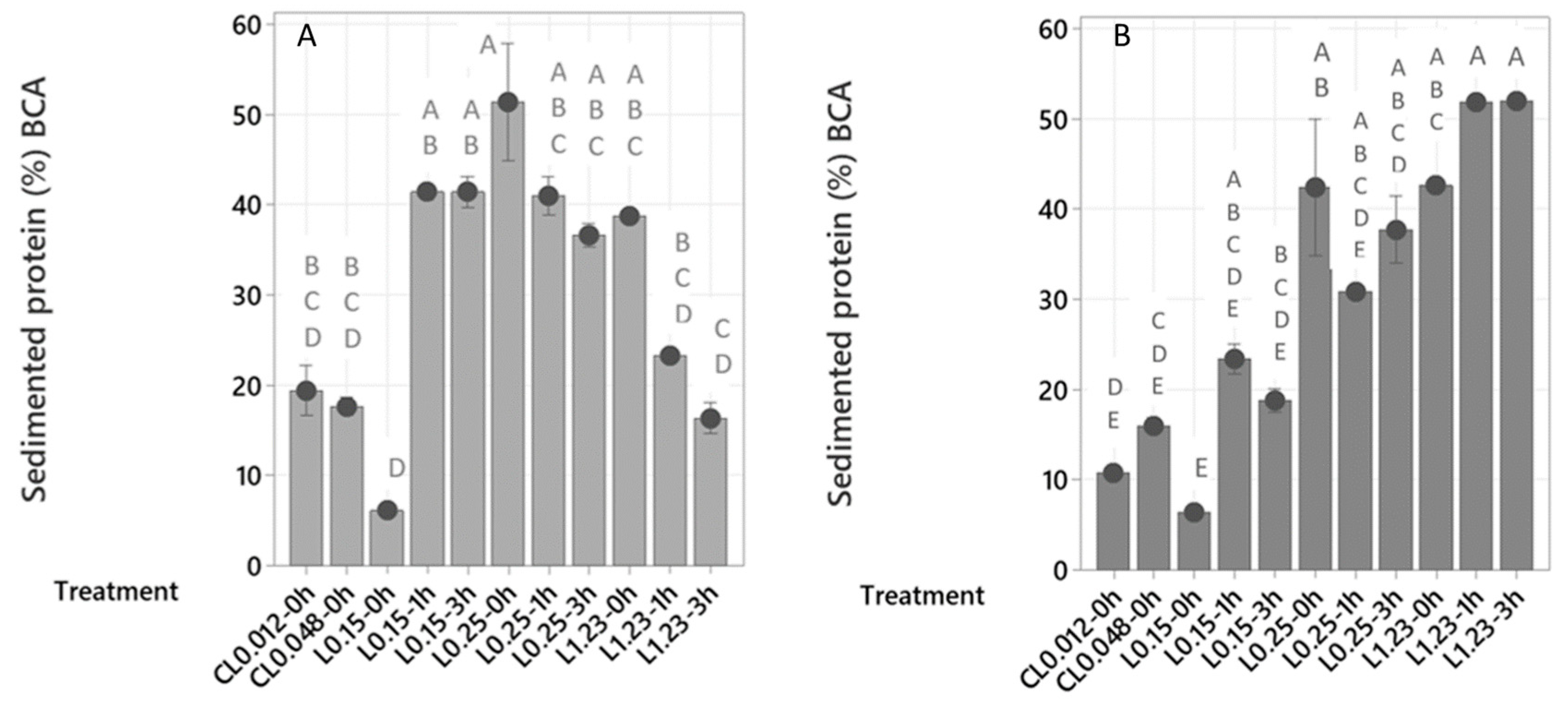
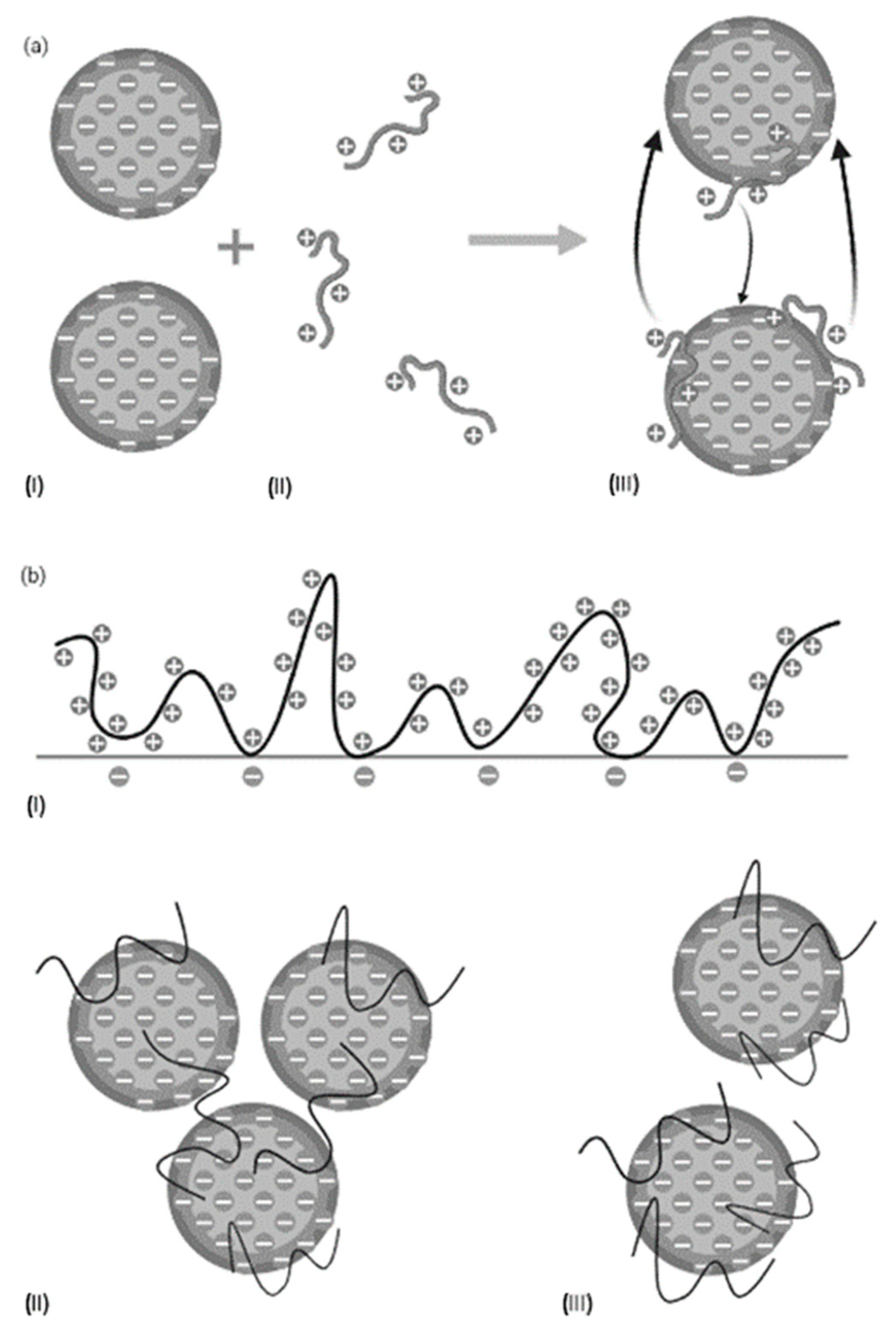
2.2. Flocculation Treatments
2.2.1. Protein Sedimentation
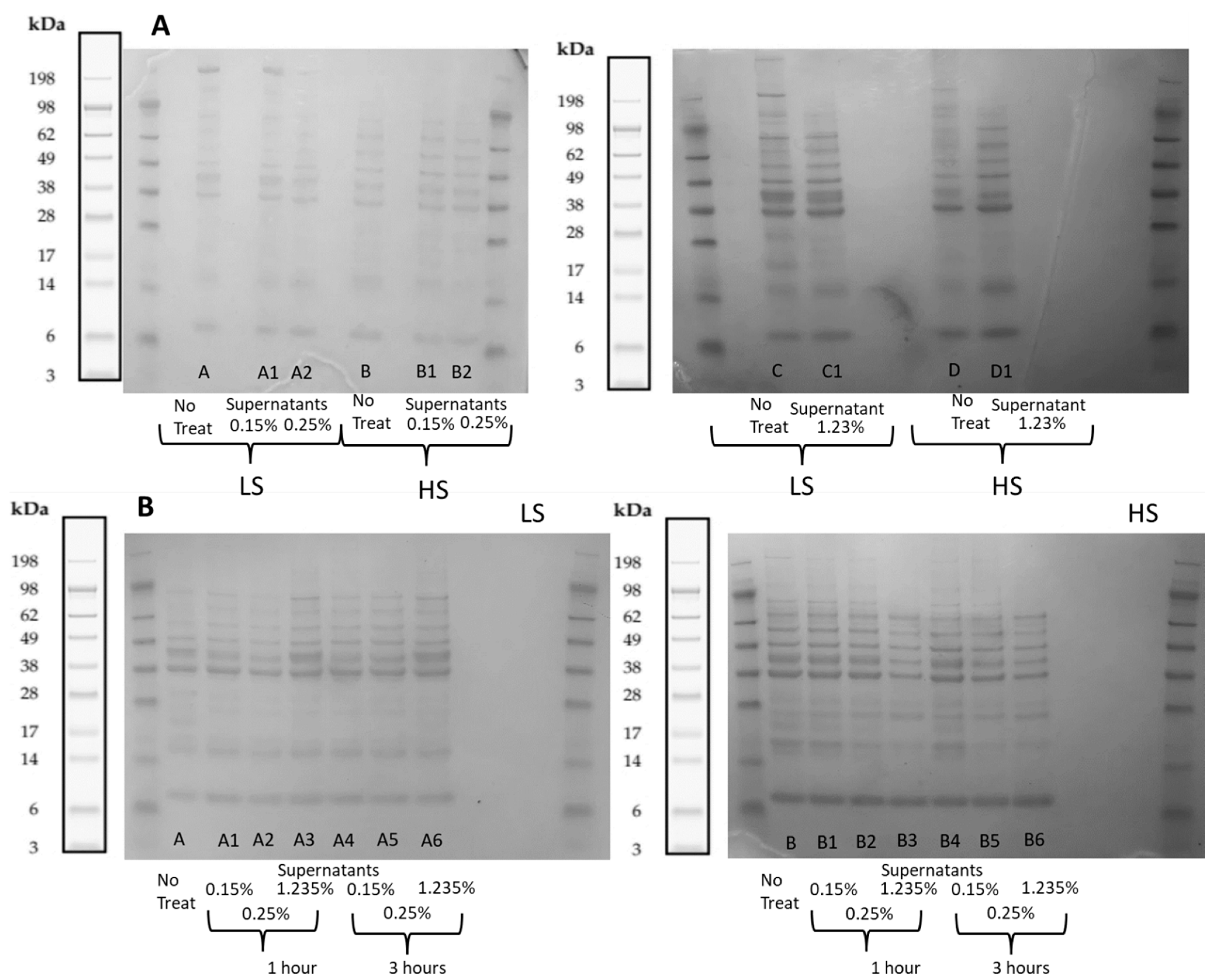
2.2.2. Polypeptide Profiling Using Sodium Dodecyl Sulfate—Polyacrylamide Gel Electrophoresis (SDS-PAGE)
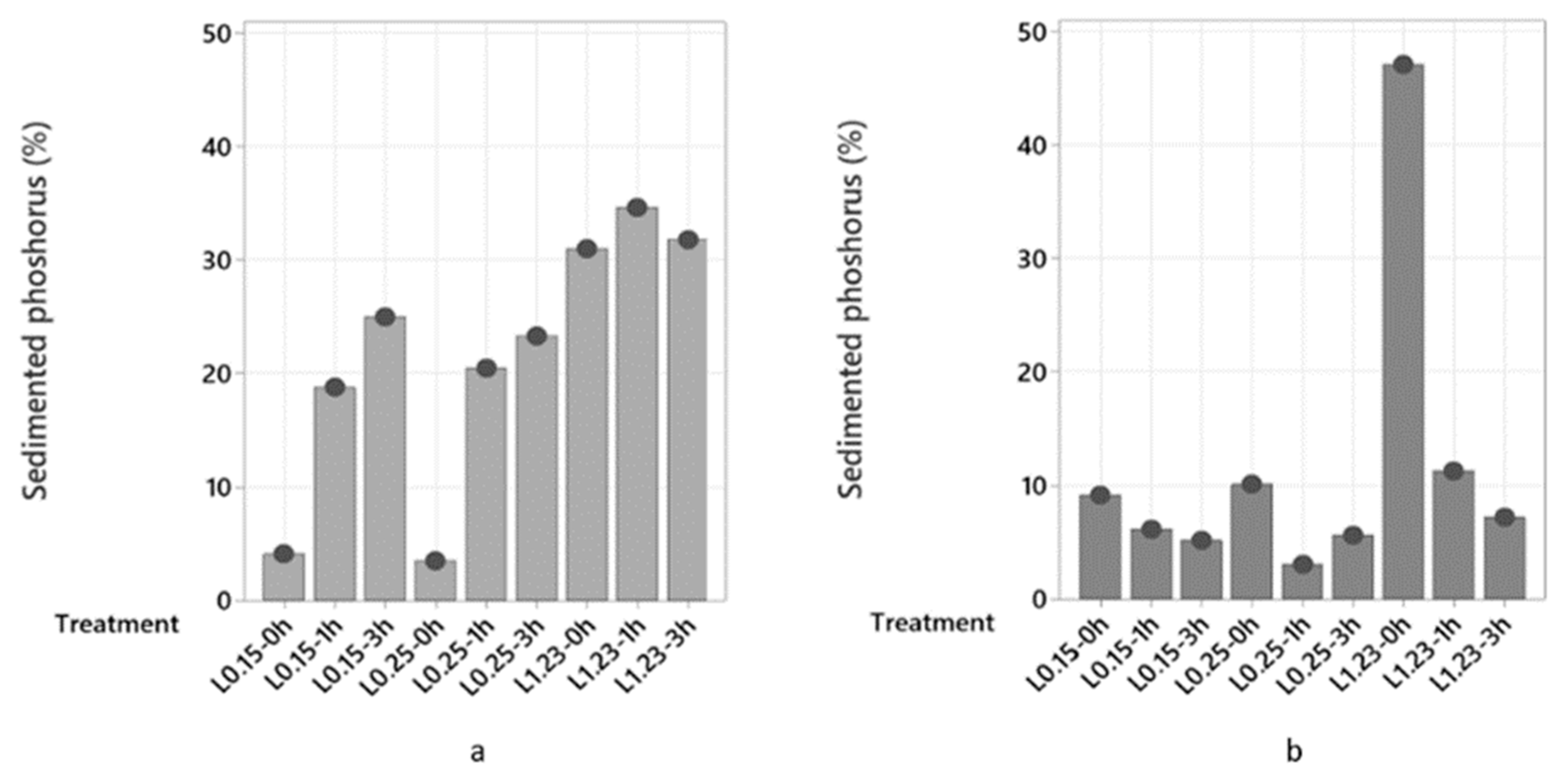
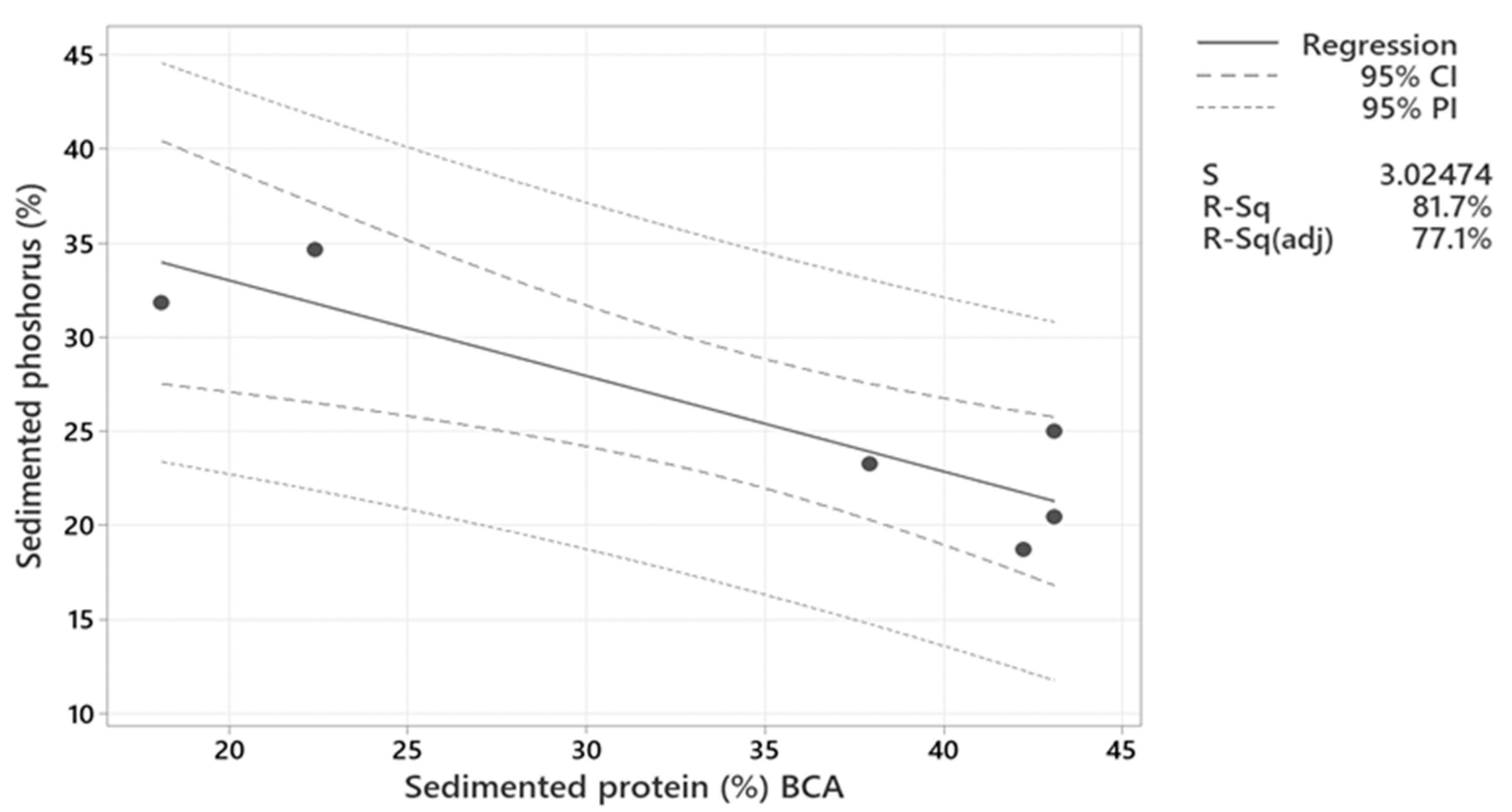
2.2.3. Phosphorus and Other Minerals Sedimentation
2.3. Membrane Technology Treatments
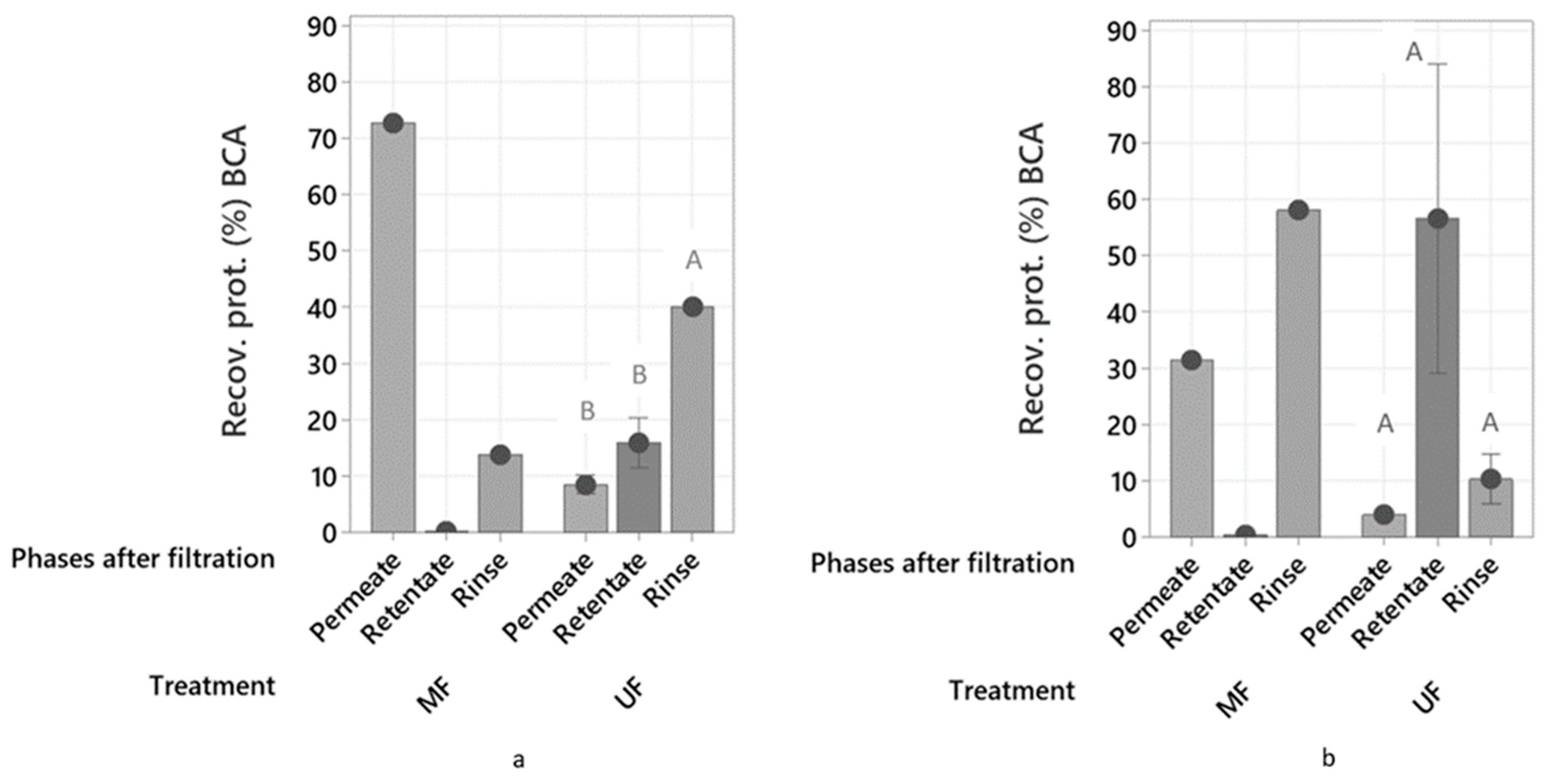
2.3.1. Protein Recovery
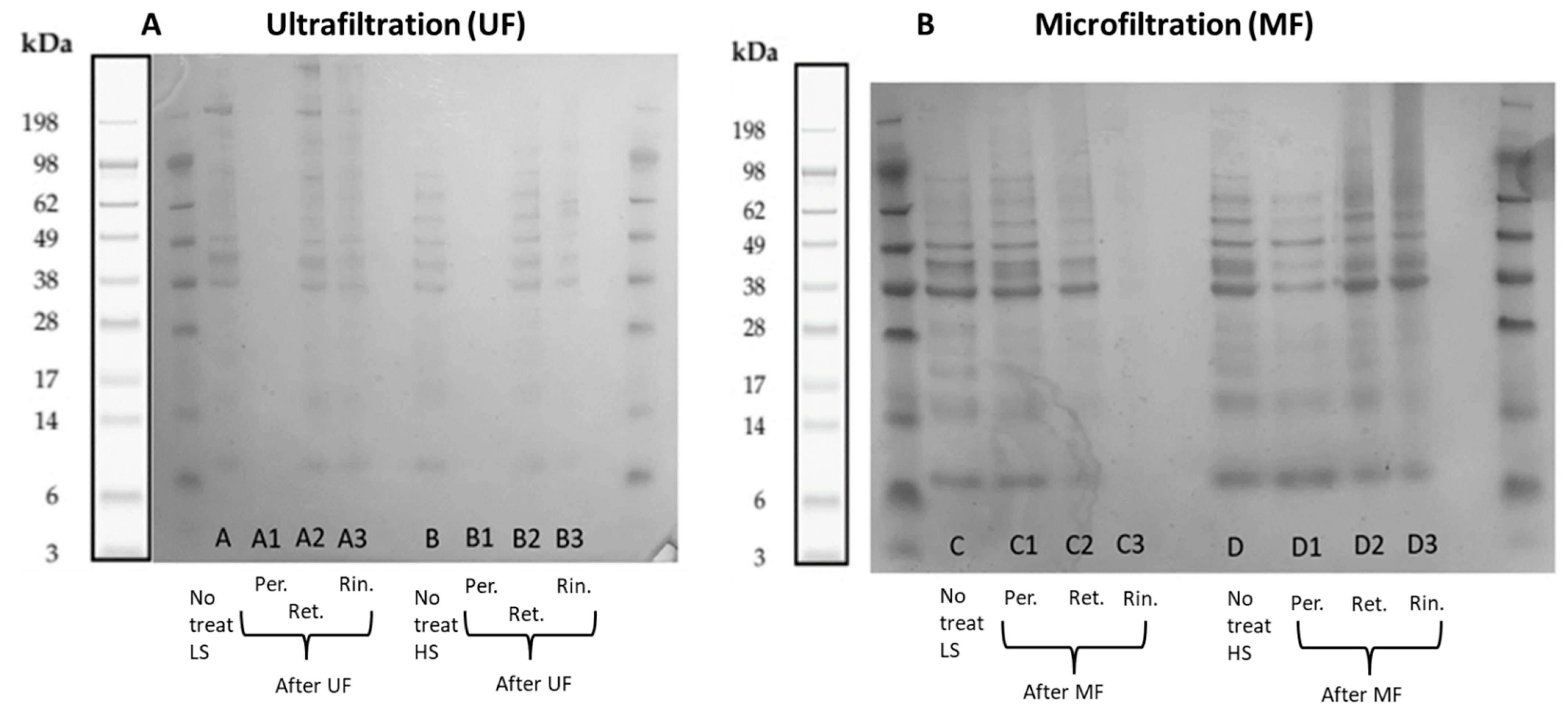
2.3.2. Polypeptide Profiling Using SDS-PAGE
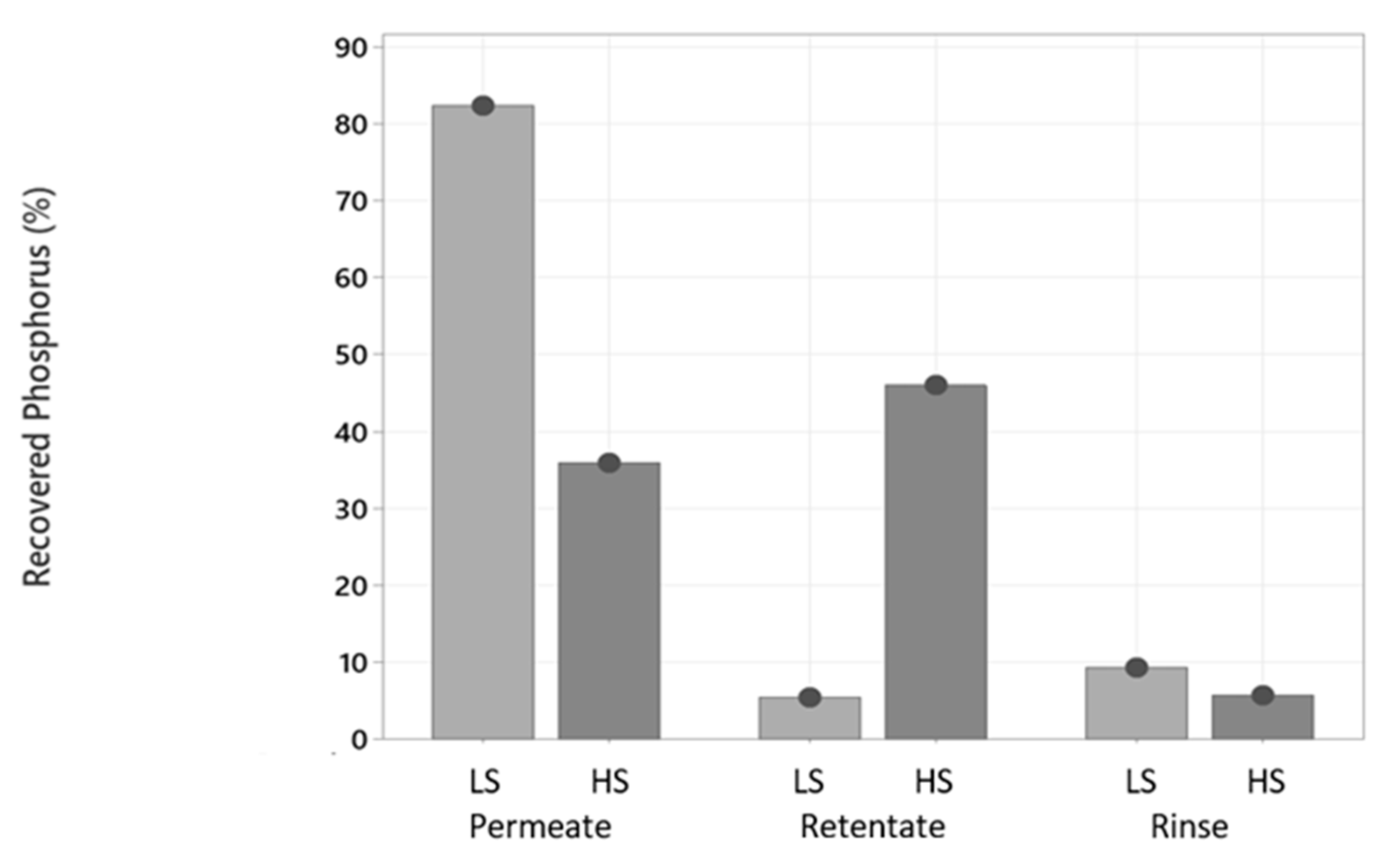
2.3.3. Phosphorus and Other Minerals Determination
2.4. Combination of Technologies
3. Materials and Methods
3.1. Chemicals
3.2. Side-Stream Waters
3.3. Separation of Protein and Phosphorus
3.3.1. Flocculation without Maturation Period
3.3.2. Flocculation with Maturation Period
3.3.3. Membrane Technology
3.4. Protein Determination Measured via BCA
3.5. Element Determination
3.6. Polypeptide Profiling Using SDS-PAGE
3.7. Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venugopal, V.; Sasidharan, A. Seafood industry effluents: Environmental hazards, treatment and resource recovery. J. Environ. Chem. Eng. 2021, 9, 104758. [Google Scholar] [CrossRef]
- Gringer, N.; Hosseini, S.V.; Svendsen, T.; Undeland, I.; Christensen, M.L.; Baron, C.P. Recovery of biomolecules from marinated herring (Clupea harengus) brine using ultrafiltration through ceramic membranes. LWT Food Sci. Technol. 2015, 63, 423–429. [Google Scholar] [CrossRef]
- Cassini, A.S.; Tessaro, I.C.; Marczak, L.D.F.; Pertile, C. Ultrafiltration of wastewater from isolated soy protein production: A comparison of three UF membranes. J. Clean. Prod. 2010, 18, 260–265. [Google Scholar] [CrossRef]
- Sniatala, B.; Kurniawan, T.A.; Sobotka, D.; Makinia, J.; Othman, M.H.D. Macro-nutrients recovery from liquid waste as a sustainable resource for production of recovered mineral fertilizer: Uncovering alternative options to sustain global food security cost-effectively. Sci. Total Environ. 2023, 856, 159283. [Google Scholar] [CrossRef]
- Forghani, B.; Mihnea, M.; Svendsen, T.C.; Undeland, I. Protein and long-chain n-3 polyunsaturated fatty acids recovered from herring brines upon flocculation and flotation—A case study. ACS Sustain. Chem. Eng. 2023, 11, 6523–6534. [Google Scholar] [CrossRef]
- Forghani, B.; Sørensen, A.-D.M.; Johannesson, J.; Svendsen, T.C.; Undeland, I. Flocculation and flotation to recover protein-enriched biomasses from shrimp boiling and peeling process waters—A case study. ACS Sustain. Chem. Eng. 2020, 8, 9660–9668. [Google Scholar] [CrossRef]
- Pereira, M.J.; Grosjean, O.; Pintado, M.; Brazinha, C.; Crespo, J. Clean technologies for production of valuable fractions from sardine cooking wastewaters: An integrated process of flocculation and reverse osmosis. Clean Technol. 2022, 4, 276–295. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Koch, F.A. Phosphorus recovery from wastewater through struvite formation in fluidized bed reactors: A sustainable approach. Water Sci. Technol. 2008, 57, 175. [Google Scholar] [CrossRef]
- Qurie, M.; Abbadi, J.; Scrano, L.; Mecca, G.; Bufo, S.; Khamis, M.; Karaman, R. Inland treatment of the brine generated from reverse osmosis advanced membrane wastewater treatment plant using epuvalisation system. Int. J. Mol. Sci. 2013, 14, 13808–13825. [Google Scholar] [CrossRef]
- Wagner, J. Membrane Filtration Handbook—Practical Tips and Hints, 2nd ed.; Osmonics, Inc.: Minnetonka, MN, USA, 2001. [Google Scholar]
- Renault, F.; Sancey, B.; Charles, J.; Morin-Crini, N.; Badot, P.-M.; Winterton, P.; Crini, G. Chitosan flocculation of cardboard-mill secondary biological wastewater. Chem. Eng. J. 2009, 155, 775–783. [Google Scholar] [CrossRef]
- Chong, M. Direct flocculation process for wastewater treatment. In Advances in Water Treatment and Pollution Prevention; Sharma, S.K., Sanghi, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 201–230. [Google Scholar]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Sharma, B.R.; Dhuldhoya, N.C.; Merchant, U.C. Flocculants—An ecofriendly approach. J. Polym. Environ. 2006, 14, 195–202. [Google Scholar] [CrossRef]
- Caskey, J.A.; Primus, R.J. The effect of anionic polyacrylamide molecular conformation and configuration on flocculation effectiveness. Environ. Prog. 1986, 5, 98–103. [Google Scholar] [CrossRef]
- Blanco, A.; Fuente, E.; Negro, C.; Tijero, J. Flocculation monitoring: Focused beam reflectance measurement as a measurement tool. Can. J. Chem. Eng. 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Energy-efficient AnMBRs technology for treatment of wastewaters: A review. Energies 2022, 15, 4981. [Google Scholar] [CrossRef]
- Ditgens, B. Membrane and filtration technologies and the separation and recovery of food processing waste. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; Chapter 11; pp. 282–304. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; Baker, R.W., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Bolzonella, D.; Cecchi, F.; Pavan, P. Treatment of food processing wastewater. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; Chapter 21; pp. 282–304. [Google Scholar]
- Gésan-Guiziou, G. Liquid Milk Processing; Wiley Blackwell: Hoboken, NJ, USA, 2012; Chapter 6; pp. 128–142. [Google Scholar]
- Chmiel, H.; Takors, R.; Weuster-Botz, D. Chapter 9—Aufarbeitung (downstream processing). In Bioprozesstechnik; Springer Spektrum: Oxford, UK, 2021; pp. 299–402. [Google Scholar]
- AlSawaftah, N.; Abuwalfa, W.; Darwish, N.; Husseini, G.A. A review on membrane biofouling: Prediction, characterization, and mitigation. Membranes 2022, 12, 1271. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Murado, M.A.; GonZález, M.P. Recovery of astaxanthin from shrimp cooking wastewaters: Optimization of astaxanthin extraction by response surface methodology and kinetic studies. Food Bioprocess Technol. 2015, 8, 371–381. [Google Scholar] [CrossRef]
- Remmen, K.; Müller, B.; Köser, J.; Wessling, M.; Wintgens, T. Assessment of layer-by-layer modified nanofiltration membrane stability in phosphoric acid. Membranes 2020, 10, 61. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Eliassen, J.L.; Vahl, O. Seasonal variations in the gonad size and the protein and water content of cod, Gadus morhua (L.), muscle from Northern Norway. J. Fish Biol. 1982, 20, 527–533. [Google Scholar] [CrossRef]
- Brown, T.; LeMay, E.; Bursten, B.; Escalona y García, H.J.; Escalona, M.C.R.; Serrano, M.d.C. Quimica: La Ciencia Central, 7th ed.; México, D.F., Ed.; Prentice Hall: Hoboken, NJ, USA, 1998. [Google Scholar]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Surimi wash water treatment for protein recovery: Effect of chitosan–alginate complex concentration and treatment time on protein adsorption. Bioresour. Technol. 2005, 96, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H. Ultrafiltration for Bioprocessing; Woodhead Publishing: Oxford, UK, 2005; pp. 103–130. [Google Scholar]
- Mameri, N.; Abdessemed, D.; Belhocine, D.; Lounici, H.; Gavach, C.; Sandeaux, J.; Sandeaux, R. Treatment of Fishery Washing Water by Ultrafiltration. J. Chem. Technol. Biotechnol. 1996, 67, 169–175. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Abdi, N.; Drouiche, N.; Lounici, H.; Pauss, A.; Goosen, M.F.A.; Mameri, N. Protein recovery by ultrafiltration during isolation of chitin from shrimp shells Parapenaeus longirostris. Food Hydrocoll. 2013, 32, 28–34. [Google Scholar] [CrossRef]
- Osman, A.; Gringer, N.; Svendsen, T.; Yuan, L.F.; Hosseini, S.V.; Baron, C.P.; Undeland, I. Quantification of biomolecules in herring (Clupea harengus) industry processing waters and their recovery using electroflocculation and ultrafiltration. Food Bioprod. Process. 2015, 96, 198–210. [Google Scholar] [CrossRef]
- Jarrault, C.; Dornier, M.; Labatut, M.-L.; Giampaoli, P.; Lameloise, M.-L. Coupling nanofiltration and osmotic evaporation for the recovery of a natural flavouring concentrate from shrimp cooking juice. Innov. Food Sci. Emerg. Technol. 2017, 43, 182–190. [Google Scholar] [CrossRef]
- Forghani, B.; Bordes, R.; Ström, A.; Undeland, I. Recovery of protein-rich biomass from shrimp (Pandalus borealis) boiling water: A colloidal study. Food Chem. 2020, 302, 125299. [Google Scholar] [CrossRef]
- Kreissig, K.J.; Hansen, L.T.; Jensen, P.E.; Wegeberg, S.; Geertz-Hansen, O.; Sloth, J.J. Characterisation and chemometric evaluation of 17 elements in ten seaweed species from Greenland. PLoS ONE 2021, 16, e0243672. [Google Scholar] [CrossRef]
| Sample | Protein [%] | Salt [%] |
|---|---|---|
| LS | 0.91 | 6.95–13.44 |
| HS | 0.85–1.06 | 22.75–25.63 |
| Sample | Date of Collection | Protein [%] |
|---|---|---|
| LS | 9 March 2022 | 0.24 ± 0.01 |
| 28 March 2022 | 0.41 ± 0.02 | |
| 25 April 2022 | 0.10 ± 0.02 | |
| HS | 21 February 2022 | 0.28 ± 0.02 |
| 28 March 2022 | 0.35 ± 0.01 | |
| 25 April 2022 | 0.39 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coque, J.; Jacobsen, C.; Forghani, B.; Meyer, A.; Jakobsen, G.; Sloth, J.J.; Sørensen, A.-D.M. Recovery of Nutrients from Cod Processing Waters. Mar. Drugs 2023, 21, 558. https://doi.org/10.3390/md21110558
Coque J, Jacobsen C, Forghani B, Meyer A, Jakobsen G, Sloth JJ, Sørensen A-DM. Recovery of Nutrients from Cod Processing Waters. Marine Drugs. 2023; 21(11):558. https://doi.org/10.3390/md21110558
Chicago/Turabian StyleCoque, Jorge, Charlotte Jacobsen, Bita Forghani, Anders Meyer, Greta Jakobsen, Jens J. Sloth, and Ann-Dorit Moltke Sørensen. 2023. "Recovery of Nutrients from Cod Processing Waters" Marine Drugs 21, no. 11: 558. https://doi.org/10.3390/md21110558
APA StyleCoque, J., Jacobsen, C., Forghani, B., Meyer, A., Jakobsen, G., Sloth, J. J., & Sørensen, A.-D. M. (2023). Recovery of Nutrients from Cod Processing Waters. Marine Drugs, 21(11), 558. https://doi.org/10.3390/md21110558








