Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of Physicochemical Properties of Polysaccharides
2.1.1. FTIR and UV Spectroscopy
2.1.2. Surface Charge and Sulphate Groups
2.1.3. Molecular Weight
2.1.4. Monosaccharides Composition
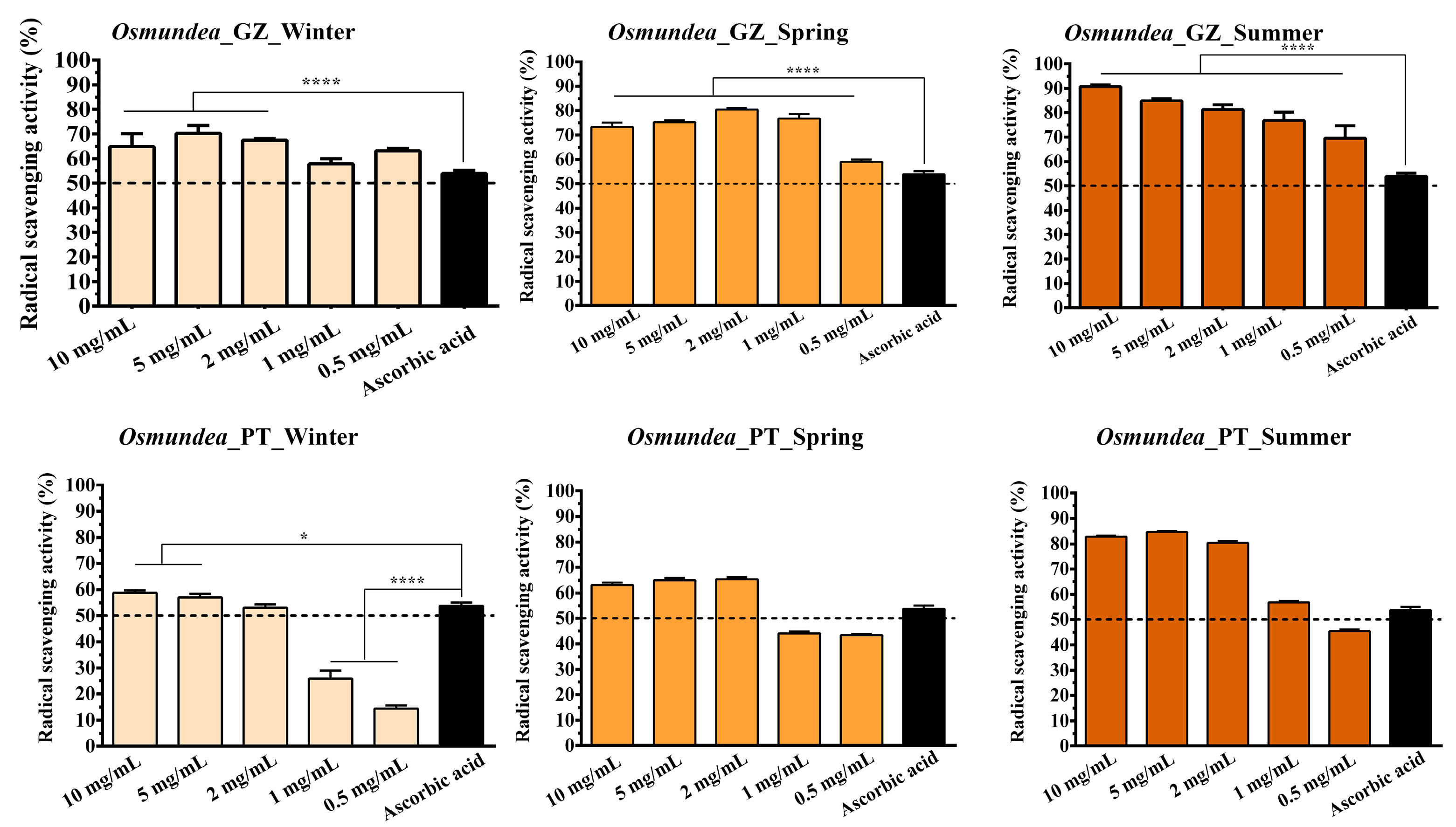
2.2. Determination of the Antioxidant Activity of Polysaccharides
2.3. Characterisation of Biological Properties of Polysaccharides in Contact with Fibroblasts
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extraction of Polysaccharides from Macroalga Codium sp. and Osmundea sp.
3.2.2. Characterisation of the Physicochemical Properties of Extracted Polysaccharides
FTIR, UV Analysis, Surface Charge and Sulphate Groups Determination
Molecular Weight of Polysaccharides
Neutral Sugars Composition
3.2.3. Determination of the Antioxidant Activity of the Polysaccharides
3.2.4. Evaluation of the Biological Activity of the Polysaccharides
3.2.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated polysaccharides from marine algae as a basis of modern biotechnologies for creating wound dressings: Current achievements and future prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Effect of season on the composition of bioactive polysaccharides from the brown seaweed saccharina longicruris. Phytochemistry 2009, 70, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from ulva spp. Along the swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Varadavenkatesan, T.; Singh, R.S.; Giri, B.S.; Selvaraj, R.; Vinayagam, R. Evaluation of seasonal variation and the optimization of reducing sugar extraction from ulva prolifera biomass using thermochemical method. Environ. Sci. Pollut. Res. 2021, 28, 58857–58871. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Grozdanić, N.; Zdunić, G.; Šavikin, K.; Duričić, I.; Kosanić, M.; Mačić, V.; Matić, I.Z.; Stanojković, T.P. Seasonal variation in biopharmaceutical activity and fatty acid content of endemic fucus virsoides algae from adriatic sea. Acta Pol. Pharm.-Drug Res. 2019, 76, 833–844. [Google Scholar] [CrossRef]
- Spicer, S.E.; Adams, J.M.M.; Thomas, D.S.; Gallagher, J.A.; Winters, A.L. Novel rapid method for the characterisation of polymeric sugars from macroalgae. J. Appl. Phycol. 2017, 29, 1507–1513. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. Ftir-atr spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from buarcos bay in central west coast of portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; Van de Velde, F.; Ribeiro-Claro, P.J. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (ftir-atr and ft-raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G. Sulfated polysaccharide extracted from the green algae codium bernabei: Physicochemical characterization and antioxidant, anticoagulant and antitumor activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef]
- Arunkumar, K.; Sreena, K.S.; Moosa, M.; Mohan, G.; Raja, R. Cytotoxic characterization of optically negative codium fragile polysaccharide against hela and mcf cell lines. Bioact. Carbohydr. Diet. Fibre 2023, 29, 100341. [Google Scholar] [CrossRef]
- Trabelsi, L.; M’sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Guo, R.; Li, X.; Chen, X.; Kou, Y.; Hu, H.; Liu, X.; Li, D.; Liu, Y.; Ai, L.; Song, Z. An ultrasonic-extracted arabinoglucan from tamarindus indica l. Pulp: A study on molecular and structural characterizations. Int. J. Biol. Macromol. 2020, 164, 3687–3697. [Google Scholar] [CrossRef]
- Yada, R.Y. (Ed.) Proteins in Food Processing; Woodhead Publishing Ltd: Sawston, UK, 2004; ISBN 9781855737235. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Yamamoto, Y.; Nagasaki, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Long-circulating poly (ethylene glycol)–poly (d, l-lactide) block copolymer micelles with modulated surface charge. J. Control. Release 2001, 77, 27–38. [Google Scholar] [CrossRef]
- Ptak, S.H.; Hjuler, A.L.; Ditlevsen, S.I.; Fretté, X.; Errico, M.; Christensen, K.V. The effect of seasonality and geographic location on sulphated polysaccharides from brown algae. Aquac. Res. 2021, 52, 6235–6243. [Google Scholar] [CrossRef]
- Imbs, T.; Shevchenko, N.; Sukhoverkhov, S.; Semenova, T.; Skriptsova, A.; Zvyagintseva, T. Seasonal variations of the composition and structural characteristics of polysaccharides from the brown alga costaria costata. Chem. Nat. Compd. 2009, 45, 786–791. [Google Scholar] [CrossRef]
- Skriptsova, A. Seasonal variations in the fucoidan content of brown algae from peter the great bay, sea of Japan. Russ. J. Mar. Biol. 2016, 42, 351–356. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly changes in the content and monosaccharide composition of fucoidan from undaria pinnatifida (laminariales, phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- de Azevedo, T.C.G.; Bezerra, M.E.B.; Santos, M.d.G.d.L.; Souza, L.A.; Marques, C.T.; Benevides, N.M.B.; Leite, E.L. Heparinoids algal and their anticoagulant, hemorrhagic activities and platelet aggregation. Biomed. Pharmacother. 2009, 63, 477–483. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.d.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Eder, S.; Zueblin, P.; Diener, M.; Peydayesh, M.; Boulos, S.; Mezzenga, R.; Nyström, L. Effect of polysaccharide conformation on ultrafiltration separation performance. Carbohydr. Polym. 2021, 260, 117830. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Bases, E.; El Shafay, S.M.; El-shenody, R. Influence of seasonal variations on extract yield and antioxidant activities of some seaweed species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 1–9. [Google Scholar] [CrossRef]
- Lahaye, M.; Yaphe, W. Effects of seasons on the chemical structure and gel strength of gracilaria pseudoverrucosa agar (gracilariaceae, rhodophyta). Carbohydr. Polym. 1988, 8, 285–301. [Google Scholar] [CrossRef]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 322, 32–39. [Google Scholar] [CrossRef]
- Yan, S.; Pan, C.; Yang, X.; Chen, S.; Qi, B.; Huang, H. Degradation of codium cylindricum polysaccharides by h2o2-vc-ultrasonic and h2o2-fe2+-ultrasonic treatment: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 182, 129–135. [Google Scholar] [CrossRef]
- Li, P.; Yan, Z.; Chen, Y.; He, P.; Yang, W. Analysis of monosaccharide composition of water-soluble polysaccharides from codium fragile by ultra-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2021, 44, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Pereira, L. Concise review of osmundea pinnatifida (hudson) stackhouse. J. Appl. Phycol. 2020, 32, 2761–2771. [Google Scholar] [CrossRef]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.A.; Costa, J.P.d.; Silva, A.; Duarte, A.C. Sargassum muticum and osmundea pinnatifida enzymatic extracts: Chemical, structural, and cytotoxic characterization. Mar. Drugs 2019, 17, 209. [Google Scholar] [CrossRef]
- Biancacci, C.; Abell, R.; McDougall, G.; Day, J.; Stanley, M. Annual compositional variation in wild osmundea pinnatifida (hudson) stackhouse from the west coast of Scotland. J. Appl. Phycol. 2022, 34, 1661–1675. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W. Fucoidan from new zealand undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- de Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from ulva pertusa (chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Ma, X.-T.; Sun, X.-Y.; Yu, K.; Gui, B.-S.; Gui, Q.; Ouyang, J.-M. Effect of content of sulfate groups in seaweed polysaccharides on antioxidant activity and repair effect of subcellular organelles in injured hk-2 cells. Oxidative Med. Cell. Longev. 2017, 2017, 2542950. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Zhang, J.; Wang, X.; Zhao, B.; Yao, J.; Wang, Y. Sulfated modification, characterization and structure–antioxidant relationships of artemisia sphaerocephala polysaccharides. Carbohydr. Polym. 2010, 81, 897–905. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, Y.-J.; Jeon, Y.-J.; Ryu, B. Bioactivities of the edible brown seaweed, undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Wang, L.; Jeon, Y.-J.; Lee, W.W. Anti-inflammatory potential of alginic acid from sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.D.; Randall Hughes, A.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Faveri, C.; Farias, J.; Scherner, F.; Oliveira, E.D.; Horta, P. Temporal changes in the seaweed flora in southern brazil and its potential causes. Panam. J. Aquat. Sci. 2010, 5, 350–357. [Google Scholar]
- Ansari, A.A.; Ghanem, S.M. Seasonal variation in the growth responses of some chlorophytic algal flora of the red sea. Egypt. J. Aquat. Res. 2017, 43, 129–134. [Google Scholar] [CrossRef]
- He, J.-Z.; Ru, Q.-M.; Dong, D.-D.; Sun, P.-L. Chemical characteristics and antioxidant properties of crude water soluble polysaccharides from four common edible mushrooms. Molecules 2012, 17, 4373–4387. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, E.-A.; Kim, Y.-S.; Yu, S.-K.; Choi, C.; Lee, J.-S.; Kim, Y.-T.; Nah, J.-W.; Jeon, Y.-J. Protective effects of polysaccharides from psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol. 2016, 91, 804–811. [Google Scholar] [CrossRef]
- Vavilala, S.L.; D’Souza, J.S. Algal polysaccharides and their biological applications. In Marine Algae extracts: Processes, Products, and Applications; Wiley: Weinheim, Germany, 2015; pp. 411–451. [Google Scholar]
- Muhamad, I.I.; Zulkifli, N.; Lazim, N.A.M. Bioactive algal-derived polysaccharides: Multi-functionalization, therapeutic potential and biomedical applications. Curr. Pharm. Des. 2019, 25, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Alipanahi, Z.; Mozafari, M. Emerging biomedical applications of algal polysaccharides. Curr. Pharm. Des. 2019, 25, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Veeraperumal, S.; Qiu, H.-M.; Zeng, S.-S.; Yao, W.-Z.; Wang, B.-P.; Liu, Y.; Cheong, K.-L. Polysaccharides from gracilaria lemaneiformis promote the hacat keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Chen, Z.; Shi, J.; Qu, Y.; Zhang, J. Effect of polysaccharides from bletilla striata on the healing of dermal wounds in mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 9212314. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Bhadja, P.; Tan, C.-Y.; Ouyang, J.-M.; Yu, K. Repair effect of seaweed polysaccharides with different contents of sulfate group and molecular weights on damaged hk-2 cells. Polymers 2016, 8, 188. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Miguel, S.P.; Araujo, A.R.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan gum–konjac glucomannan blend hydrogel for wound healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]









| Fraction | Yield (%) | Retention Time (min) | MW (kDa) |
|---|---|---|---|
| Osmundea sp.—Spring_PT | 85.0 | 13.05; 14.67; 15.17; 15.52; 16.63; 17.28 | 152 |
| Osmundea sp.—Spring_GZ | 85.2 | 13.13; 14.93; 15.40; 16.02; 16.75; 22.10; 22.65 | 149 |
| Osmundea sp.—Summer_PT | 83.1 | 13.27; 14.98; 15.42; 16.02; 16.75; 22.15; 23.05 | 124 |
| Osmundea sp.—Summer_GZ | 85.5 | 13.05; 14.53; 15.03; 15.5; 16.62 | 186 |
| Osmundea sp.—Winter_PT | 82.9 | 13.23; 14.97; 15.38; 15.93; 16.72 | 139 |
| Osmundea sp.—Winter_GZ | 95.1 | 13.15; 14.83; 15.33; 15.93; 16.70 | 138 |
| Codium sp.—Spring_PT | 82.3 | 13.02; 14.85; 15.0; 10.08; 16.73; 21.0; 21.37; 22.0 | 94 |
| Codium sp.—Spring_GZ | 62.4 | 13.08; 14.73; 15.28; 15.95; 16.66; 20.43 | 169 |
| Codium sp.—Summer_PT | 95.0 | 12.98; 14.43; 14.97; 15.5; 16.57 | 147 |
| Codium sp.—Summer_GZ | 89.1 | 13.07; 14.80; 15.30; 16.0; 16.75; 17.72; 22.95 | 140 |
| Codium sp.—Winter_PT | 93.3 | 13.05; 14.65; 15.20; 15.88; 16.68; 20.98 | 178 |
| Codium sp.—Winter_GZ | 95.0 | 13.05; 14.65; 15.25; 15.90; 16.68; 22.95 | 171 |
| Osmundea | Codium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Winter | Spring | Summer | Winter | |||||||
| GZ | PT | GZ | PT | GZ | PT | GZ | PT | GZ | PT | GZ | PT | |
| Glucose * | 13.6 | 5.8 | 4.9 | 7.9 | 11.4 | 7.5 | 30.6 | 37.7 | 35.7 | 37.3 | 43.2 | 41.7 |
| Xylose | 9.0 | 9.0 | 10.2 | 6.3 | 8.4 | 8.7 | 4.8 | 3.4 | 4.7 | 2.9 | 2.5 | 5.8 |
| Mannose | 1.4 | 1.6 | 1.0 | 0.6 | 0.9 | 1.0 | 10.9 | 26.8 | 12.9 | 0.4 | 4.8 | 10.7 |
| Galactose | 36.9 | 42.6 | 39.8 | 56.0 | 34.8 | 38.4 | 28.0 | 12.3 | 25.4 | 37.6 | 18.0 | 17.2 |
| Arabinose | 6.1 | 8.0 | 9.4 | 6.1 | 5.9 | 5.8 | 9.7 | 5.8 | 6.2 | 9.1 | 9.9 | 8.5 |
| Formic acid | 3.70 | 6.8 | 4.1 | 3.3 | 4.1 | 6.2 | 2.7 | 3.0 | 4.4 | n.d. | 12.4 | 0 |
| Gluc. acid | n.d. | 4.9 | n.d. | n.d. | 6.6 | 7.3 | 5.0 | 3.6 | 3.5 | 9.5 | n.d. | 6.2 |
| HMF | 9.4 | 9.8 | 9.6 | 8.7 | 8.5 | 7.7 | 1.9 | 1.7 | 2.9 | 3.1 | 1.4 | 1.3 |
| NI | 19.9 | 10.1 | 21.0 | 11.1 | 19.4 | 17.4 | 6.4 | 5.8 | 4.3 | 0.0 | 7.8 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, S.P.; D’Angelo, C.; Ribeiro, M.P.; Simões, R.; Coutinho, P. Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties. Mar. Drugs 2023, 21, 589. https://doi.org/10.3390/md21110589
Miguel SP, D’Angelo C, Ribeiro MP, Simões R, Coutinho P. Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties. Marine Drugs. 2023; 21(11):589. https://doi.org/10.3390/md21110589
Chicago/Turabian StyleMiguel, Sónia P., Caíque D’Angelo, Maximiano P. Ribeiro, Rogério Simões, and Paula Coutinho. 2023. "Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties" Marine Drugs 21, no. 11: 589. https://doi.org/10.3390/md21110589
APA StyleMiguel, S. P., D’Angelo, C., Ribeiro, M. P., Simões, R., & Coutinho, P. (2023). Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties. Marine Drugs, 21(11), 589. https://doi.org/10.3390/md21110589







