Development of Bi- and Tri-Layer Nanofibrous Membranes Based on the Sulfated Polysaccharide Carrageenan for Periodontal Tissue Regeneration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of the GTR Nanofibrous Membranes
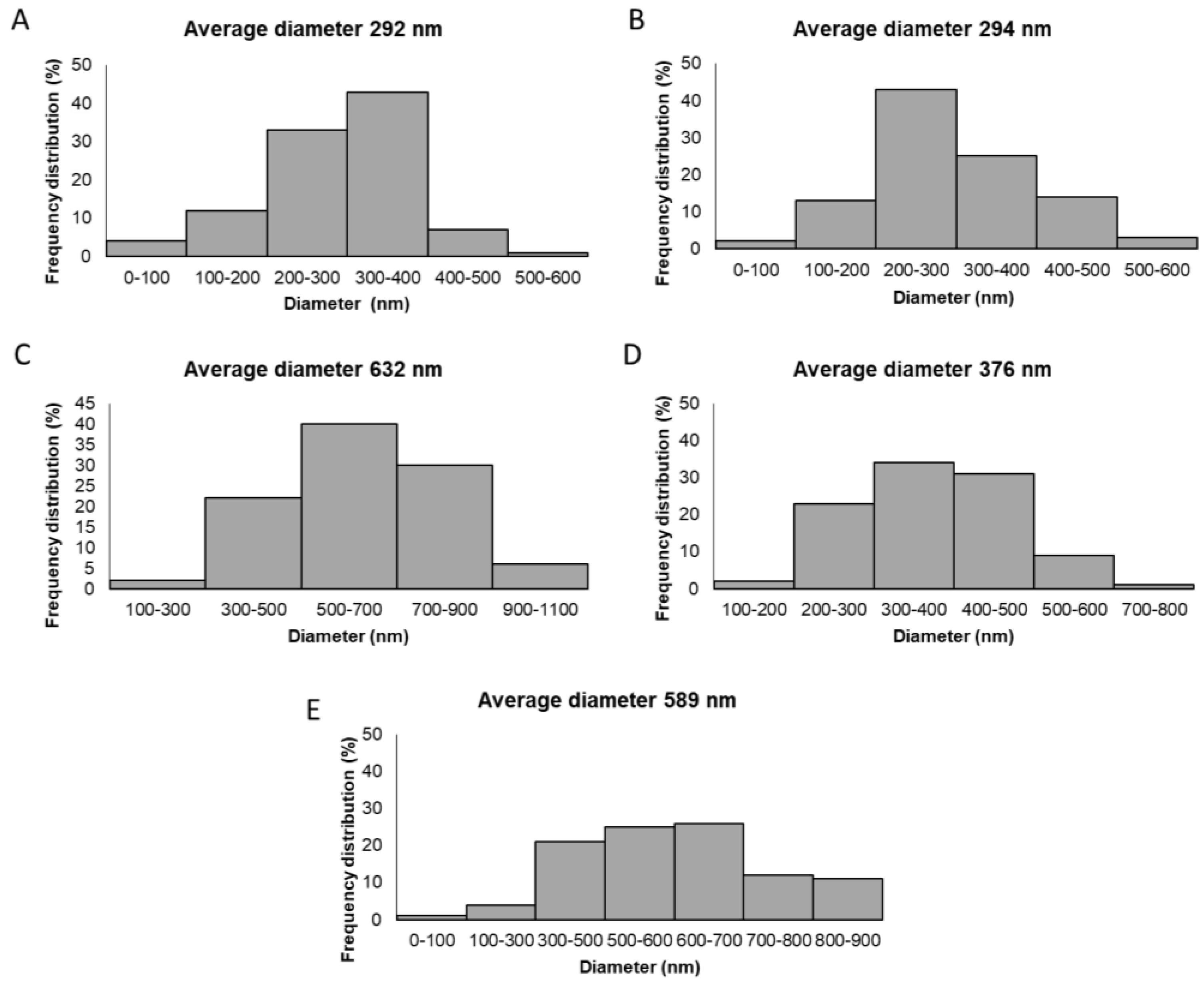
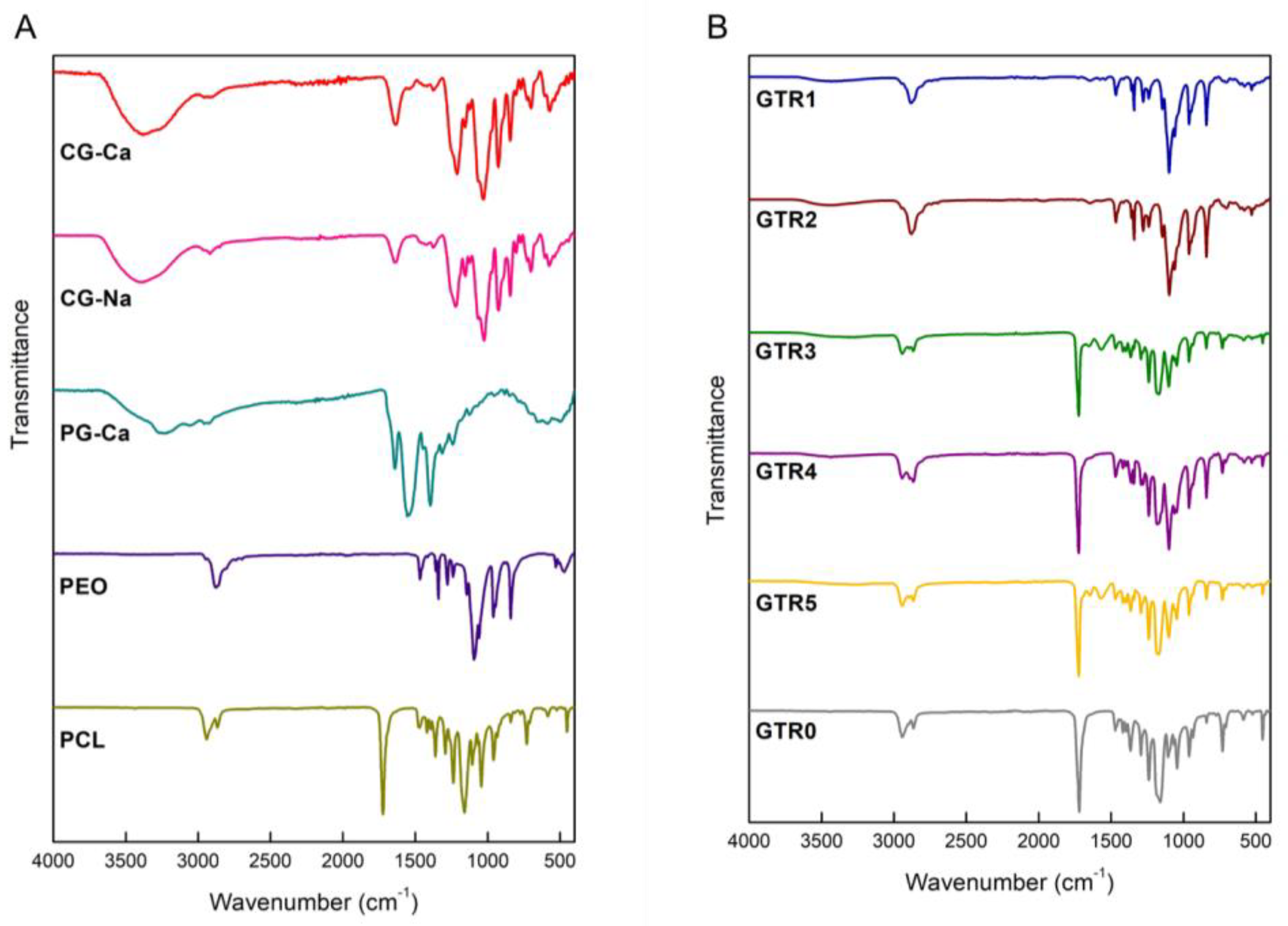
2.2. Characterization of the Fabricated GTR Membranes
2.3. Determination of the Degradation Rate of the Fabricated GTR Membranes
2.4. Release of Ca+2 from the Fabricated GTR Membranes
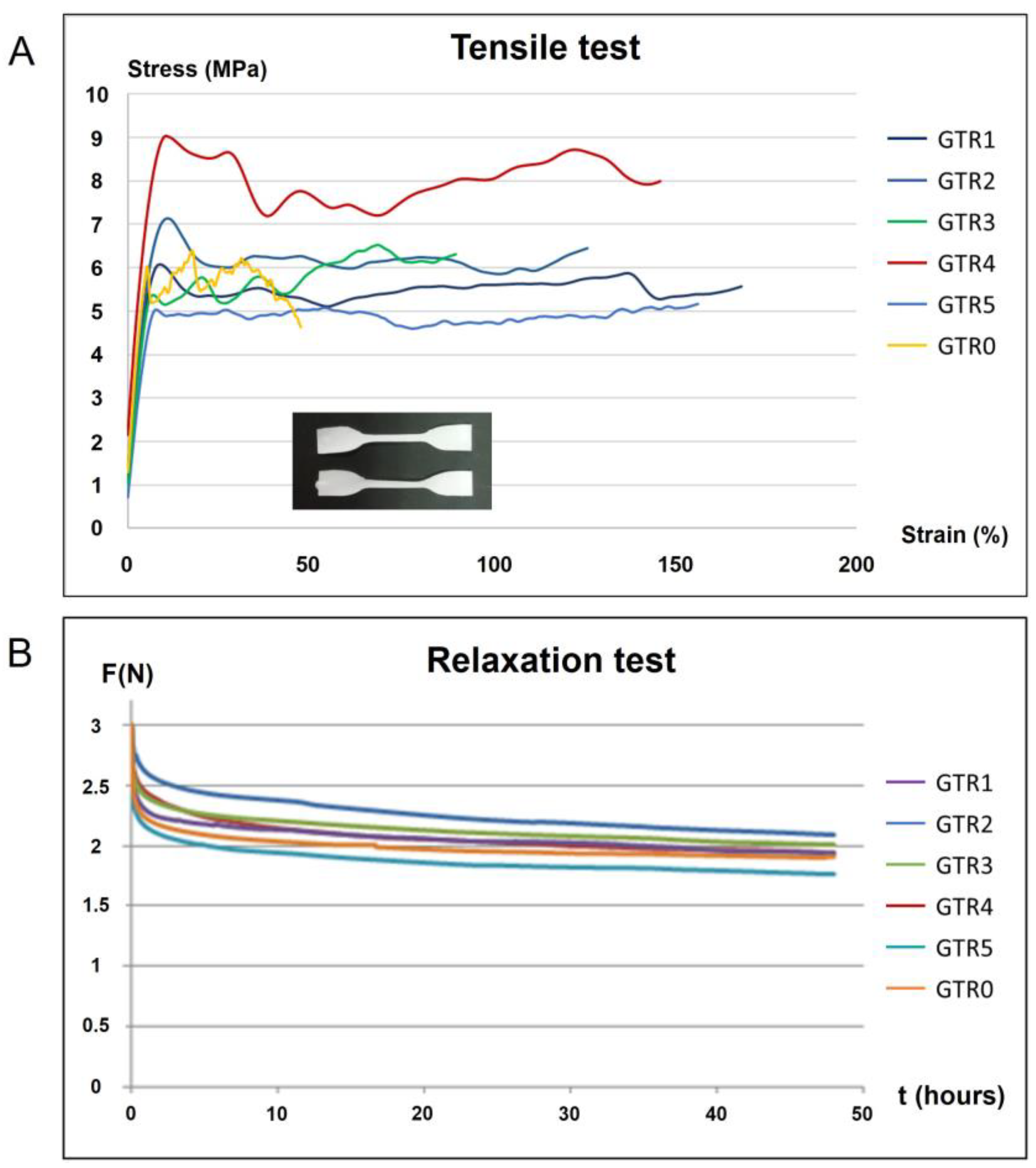
2.5. Determination of the Mechanical Properties of the Fabricated GTR Membranes
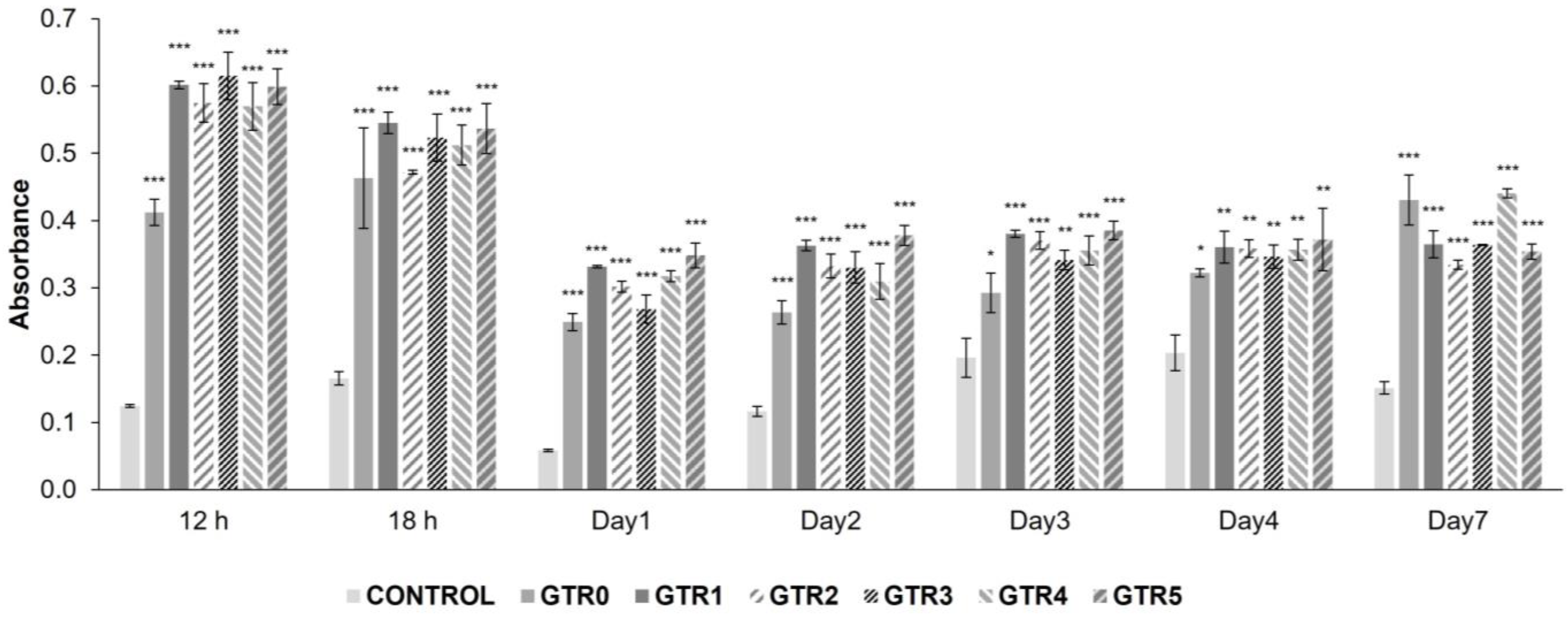
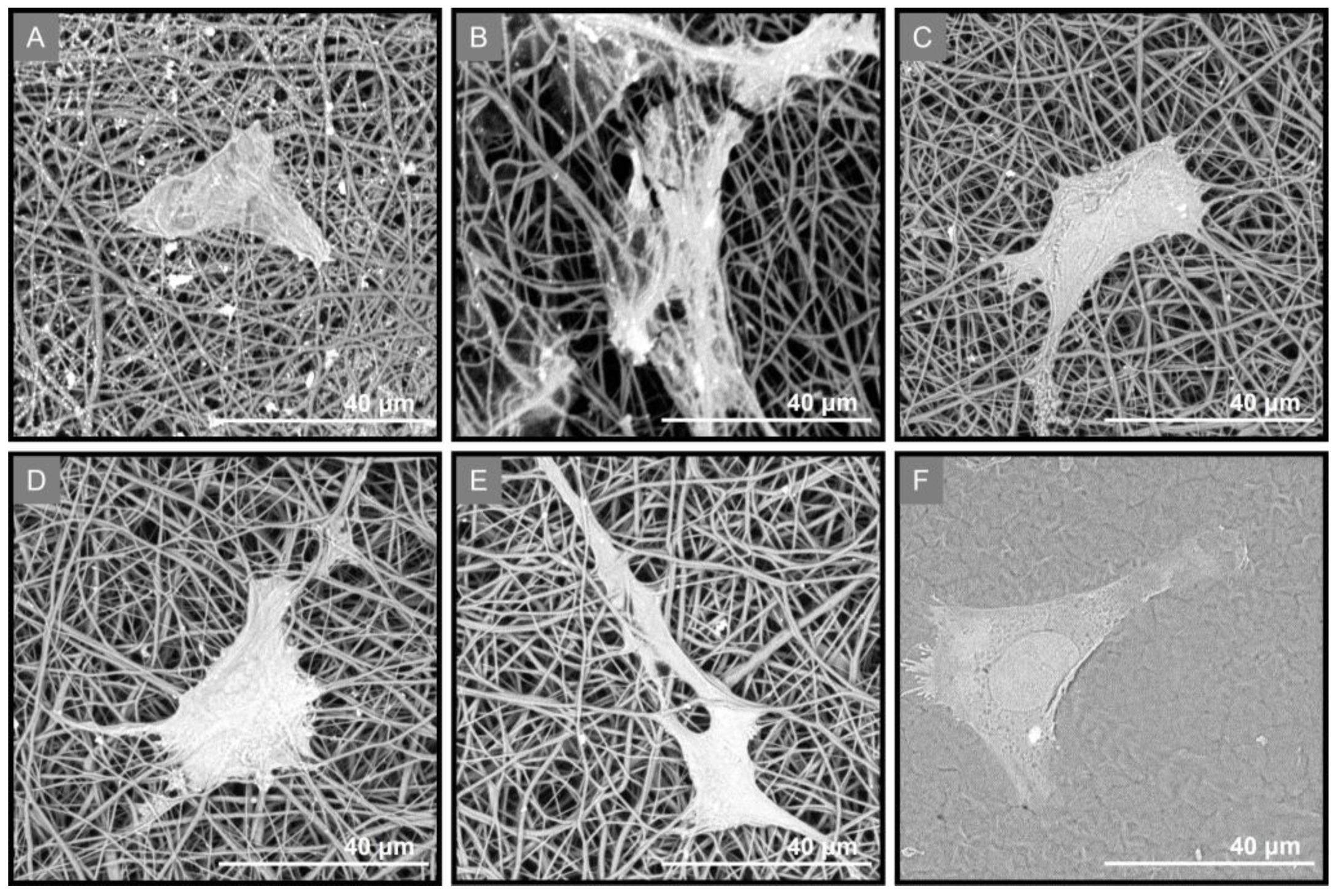
2.6. Evaluation of the Growth and Attachment of PDL Cells Seeded on the GTR Membranes
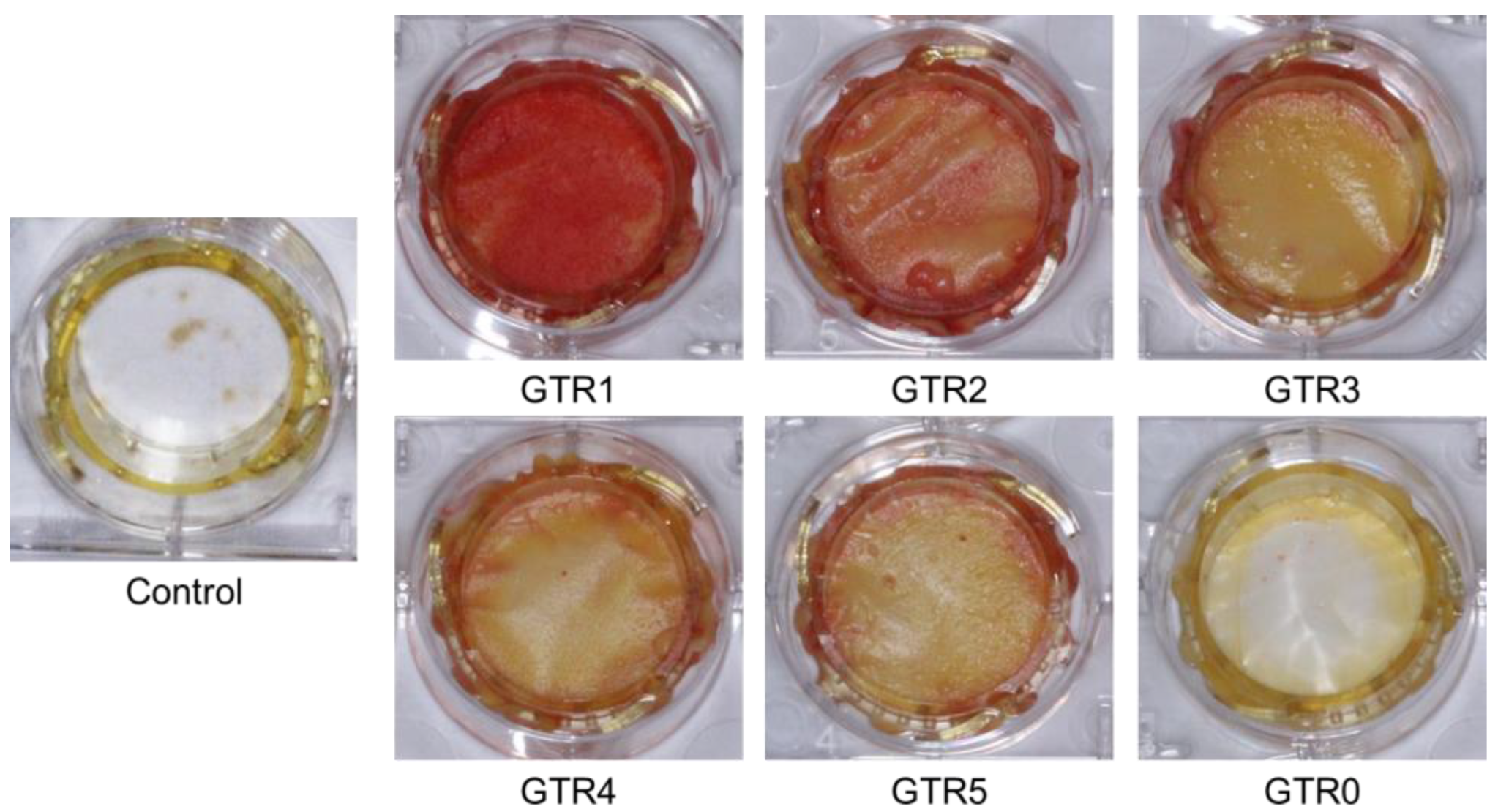
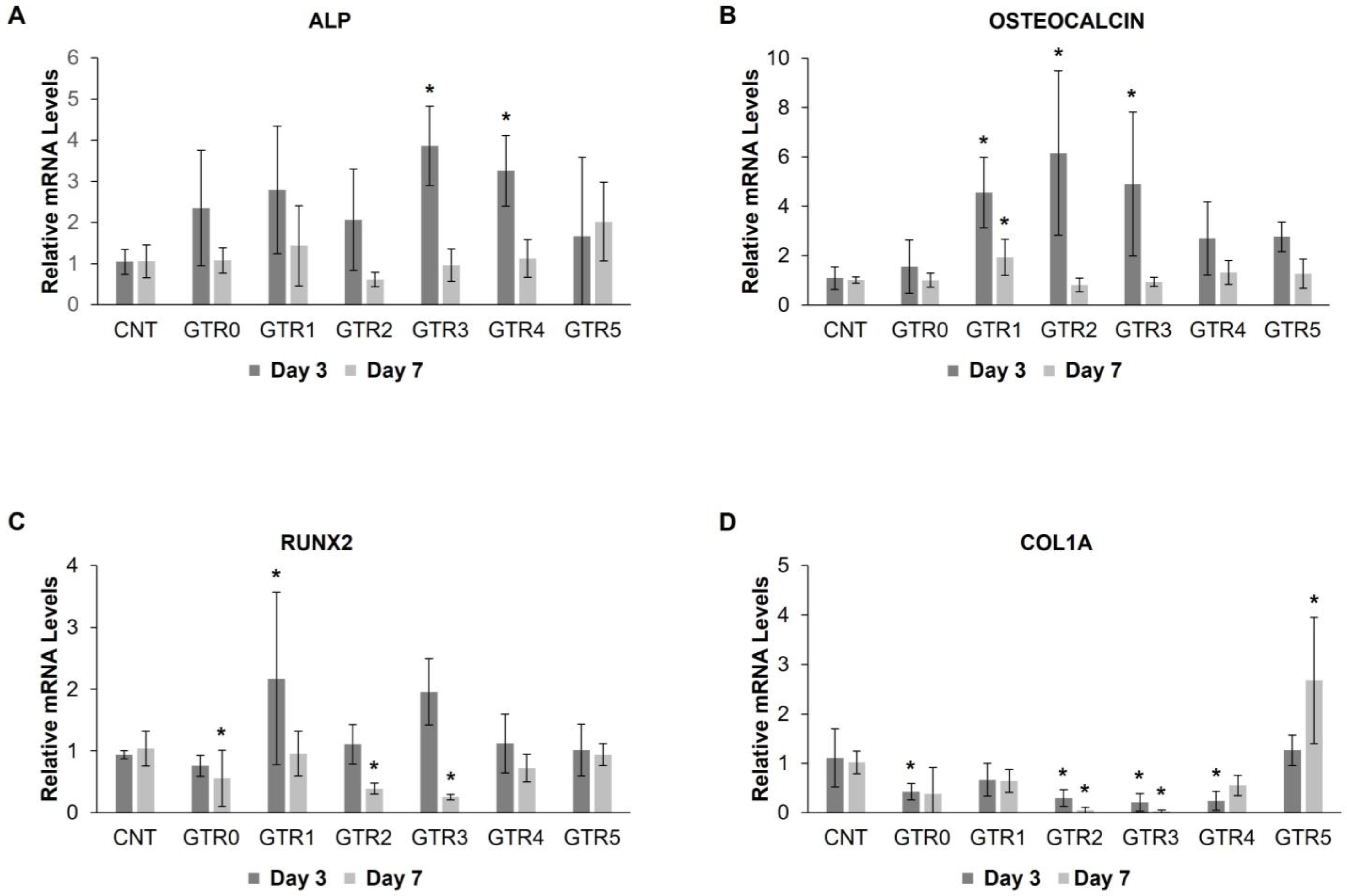
2.7. Evaluation of the Effect of the GTR Membranes on the Osteo-Induction of PDL Cells
3. Materials and Methods
3.1. Materials
3.2. Extraction and Characterization of Calcium Salt of Carrageenan (CG-Ca) and Sodium Salt of Carrageenan (CG-Na)
3.3. Preparation of Calcium Poly(L-glutamate) (PG-Ca)
3.4. Fabrication of the GTR Membranes
3.4.1. Preparation of the Cast Outer Layer
3.4.2. Preparation of the Spinning Solutions and Electrospinning
3.4.3. Preparation of the Tri-Layer GTR1 Membrane
3.4.4. Preparation of the Tri-Layer GTR2 Membrane
3.4.5. Preparation of the Bi-Layer GTR3 Membrane
3.4.6. Preparation of the Bi-Layer GTR4 Membrane
3.4.7. Preparation of the Bi-Layer GTR5 Membrane
3.5. Scanning Electron Microscopy (SEM)
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Thermogravimetric Analysis (TGA)
3.8. Degradation Rate Study
3.9. Ca+2 Release Study
3.10. Mechanical Properties of the GTR Membranes
3.11. Biocompatibility of the GTR Membranes
3.11.1. Cell Culture
3.11.2. Treatment of the GTR Membranes
3.11.3. Cytocompatibility of the GTR Membranes and Proliferation of PDL Cells
3.12. Osteo-Differentiation of PDL Cells Seeded on the GTR Membranes
3.13. qRT-PCR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, D.; Caton, J.; Benoit, D.S.W. Periodontal wound healing and regeneration: Insights for engineering new therapeutic approaches. Front. Dent. Med. 2022, 3, 815810. [Google Scholar] [CrossRef]
- Uskoković, V.; Pejčić, A.; Koliqi, R.; Anđelković, Z. Polymeric nanotechnologies for the treatment of periodontitis: A chronological review. Int. J. Pharm. 2022, 625, 122065. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Ezzati, A.; Mehrandish, S.; Asare-Addo, K.; Nokhodchi, A. An overview of guided tissue regeneration (GTR) systems designed and developed as drug carriers for management of periodontitis. J. Drug Deliv. Sci. Technol. 2022, 71, 103341. [Google Scholar] [CrossRef]
- Gerritsen, A.E.; Allen, P.F.; Witter, D.J.; Bronkhorst, E.M.; Creugers, N.H.J. Tooth loss and oral health-related quality of life: A systematic review and meta-analysis. Health Qual. Life Outcomes 2010, 8, 126. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A. Asymmetric resorbable-based dental barrier membrane for periodontal guided tissue regeneration and guided bone regeneration: A review. J. Biomed. Mater. Res. 2022, 110, 2157–2182. [Google Scholar] [CrossRef]
- Chapple, I.L.C. Time to take periodontitis seriously. B.M.J. 2014, 348, 2645. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration-A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Sam, G.; Pillai, B.R. Evolution of Barrier Membranes in Periodontal Regeneration-“Are the third Generation Membranes really here? ” J. Clin. Diagn. Res. 2014, 8, ZE14–ZE17. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef]
- Carter, S.S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Huthmacher, D.W.; Malda, J. Additive biomanufacturing: An advanced approach for periodontal tissue regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Pankajakshan, D.; Albuquerque, M.T.P.; Bottino, M.C. 16–Electrospun nanofibers for regenerative dentistry. In Electrospun Materials for Tissue Engineering and Biomedical Applications, 1st ed.; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 357–384. [Google Scholar] [CrossRef]
- Zupančič, Š.; Casula, L.; Rijavec, T.; Lapanze, A.; Luštrik, M.; Fadda, A.M.; Kocbek, P.; Kristl, M. Sustained release of antimicrobials from double-layer nanofiber mats for local treatment of periodontal disease, evaluated using a new micro flow through apparatus. J. Control. Release 2019, 316, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.-G. Electrospun nanofibers for periodontal treatment: A recent progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef]
- Santos, M.S.; Carvalho, M.S.; Silva, J.C. Recent advances on electrospun nanofibers for periodontal regeneration. Nanomaterials 2023, 13, 1307. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ren, S.; Li, L.; Zhou, Y.; Peng, W.; Xu, Y. Biodegradable engineered fiber scaffolds fabricated by electrospinning for periodontal tissue regeneration. J. Biomater. Appl. 2021, 36, 55–75. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-derived polymeric materials and biomimetics: An overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of marine biomaterials and their applications in biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B. Marine-Derived Biomaterials for Tissue Engineering Applications, 1st ed.; Springer: Singapore, 2019; pp. 1–550. [Google Scholar]
- Iliou, K.; Kikionis, S.; Ioannou, E.; Roussis, V. Marine biopolymers as bioactive functional ingredients of electrospun nanofibrous scaffolds for biomedical applications. Mar. Drugs 2022, 20, 314. [Google Scholar] [CrossRef]
- Zaitseva, O.O.; Sergushkina, M.I.; Khudyakov, A.N.; Polezhaeva, T.V.; Solomina, O.N. Seaweed sulfated polysaccharides and their medicinal properties. Algal Res. 2022, 68, 102885. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, L.; Wang, C.; Cheng, M.; Liu, M.; Wang, L.; Pan, P.; Chen, J. Renewable marine polysaccharides for microenvironment-responsive wound healing. Int. J. Biol. Macromol. 2023, 225, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, I.; Dheer, D.; Nagpal, M.; Kumar, P.; Venkatesh, D.N.; Puri, V.; Singh, I. A propitious role of marine sourced polysaccharides: Drug delivery and biomedical applications. Carbohydr. Polym. 2023, 308, 120448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Li, J.; Chen, Z.; Fan, M.; Lin, F.; Xie, Y. Electrospun polysaccharides for periodontal tissue engineering: A review of recent advances and future perspectives. Ann. Biomed. Eng. 2022, 50, 769–793. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva Jr, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis–A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-carrageenan/chitosan/gelatin scaffold for the osteogenic differentiation of adipose-derived MSCs in vitro. J. Biomed. Mater. Res. Part B 2015, 103B, 1498–1510. [Google Scholar] [CrossRef]
- Goonoo, N.; Khanbabaee, B.; Steuber, M.; Bhaw-Luximon, A.; Jonas, U.; Pietsch, U.; Jhurry, D.; Schönherr, H. κ-Carrageenan enhances the biomineralization and osteogenic differentiation of electrospun PHB and PHBV fibers. Biomacromolecules 2017, 18, 1563–1573. [Google Scholar] [CrossRef]
- Cao, W.; Jin, J.; Wu, G.; Bravenboer, N.; Helder, M.N.; Pathak, J.L.; Zandieh-Doulabi, B.; Hogervorst, J.M.A.; Matsukawa, S.; Geonzon, L.C.; et al. K-Carrageenan stimulates pre-osteoblast proliferation and osteogenic differentiation: A potential factor for the promotion of bone regeneration? Molecules 2021, 26, 6131. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Jonas, U.; Jhurry, D.; Schönherr, H. Enhanced differentiation of human preosteoblasts on electrospun blend fiber mats of polydioxanone and anionic sulfated polysaccharides. ACS Biomater. Sci. Eng. 2017, 3, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Roshanfar, F.; Hesaraki, S.; Dolatshahi-Pirouz, A. Electrospun Silk Fibroin/kappa-carrageenan hybrid nanofibers with enhanced osteogenic properties for bone regeneration Applications. Biology 2022, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, K.; Yan, S.; Wu, J.; Yin, J. A tough and self-healing poly(l-glutamic acid)-based composite hydrogel for tissue engineering. J. Mater. Chem. B 2018, 6, 6865–6876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, S.; Gong, L.; Wang, J.; Chen, X.; Cui, L.; Yin, J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(L-glutamic acid) and chitosan. Acta Biomater. 2013, 9, 7276–7288. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef]
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable in situ self-cross-linking hydrogels based on poly(l-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef]
- Parati, M.; Clarke, L.; Anderson, P.; Hill, R.; Khalil, I.; Tchuenbou-Magaia, F.; Stanley, M.S.; McGee, D.; Mendrek, B.; Kowalczuk, M.; et al. Microbial poly-γ-glutamic acid (γ-PGA) as an effective tooth enamel protectant. Polymers 2022, 14, 2937. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Dehnavi, S.S.; Mehdikhani, M.; Rafienia, M.; Bonakdar, S. Preparation and in vitro evaluation of polycaprolactone/PEG/bioactive glass nanopowders nanocomposite membranes for GTR/GBR applications. Mater. Sci. Eng. C 2018, 90, 236–247. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel bilayer polycaprolactone membrane for guided bone regeneration: Combining electrospinning and emulsion templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef]
- Osathanon, T.; Chanjavanakul, P.; Kongdecha, P.; Clayhan, P.; Huynh, N.C.-N. Polycaprolactone-Based Biomaterials for Guided Tissue Regeneration Membrane; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Castro, A.G.B.; Diba, M.; Kersten, M.; Jansen, J.A.; van den Beucken, J.J.J.P.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for guided bone regeneration. Mater. Sci. Eng. C 2018, 85, 154–161. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Shaltooki, M.; Dini, G.; Mehdikhani, M. Fabrication of chitosan-coated porous polycaprolactone/strontium substituted bioactive glass nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110138. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gao, X.; Shen, Z.; Shi, X.; Lin, Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv. 2019, 9, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C 2020, 109, 110618. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Shahrousvand, M.; Hsu, Y.-T.; Su, W.-T. Polycaprolactone/polyethylene glycol blended with Dipsacus asper wall extract nanofibers promote osteogenic differentiation of periodontal ligament stem cells. Polymers 2021, 13, 2245. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Aggelidou, E.; Tziveleka, L.-A.; Demiri, E.; Bakopoulou, A.; Zinelis, S.; Kritis, A.; Roussis, V. The marine polysaccharide ulvan confers potent osteoinductive capacity to PCL-based scaffolds for bone tissue engineering applications. Int. J. Mol. Sci. 2021, 22, 3086. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, S.; Demirtaş, T.T.; Yüksel, E.; Karakeçili, A.; Doğan, A.; Gümüşderelioğlu, M. Multi-layered functional membranes for periodontal regeneration: Preparation and characterization. Mat. Lett. 2016, 178, 256–259. [Google Scholar] [CrossRef]
- Nivedhitha Sundaram, M.; Sowmya, S.; Deepthi, S.; Bumgardener, J.D.; Jayakumar, R. Bilayered construct for simultaneous regeneration of alveolar bone and periodontal ligament. J. Biomed. Mater. Res. Part B 2016, 104B, 761–770. [Google Scholar] [CrossRef]
- Abedi, N.; Rajabi, N.; Kharaziha, M.; Nejatidanesh, F.; Tayebi, L. Layered scaffolds in periodontal regeneration. J. Oral. Biol. Craniofac. Res. 2022, 12, 782–797. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, L.; Ngai, T. Multiphasic membranes/scaffolds for periodontal guided tissue regeneration. Macromol. Mater. Eng. 2023, 2300081. [Google Scholar] [CrossRef]
- Kotroni, E.; Simirioti, E.; Kikionis, S.; Sfiniadakis, I.; Siamidi, A.; Karalis, V.; Vitsos, A.; Vlachou, M.; Ioannou, E.; Roussis, V.; et al. In vivo evaluation of the anti-inflammatory activity of electrospun micro/nanofibrous patches loaded with Pinus halepensis bark extract on hairless mice skin. Materials 2019, 12, 2596. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Sapalidis, A.; Kikionis, S.; Aggelidou, E.; Demiri, E.; Kritis, A.; Ioannou, E.; Roussis, V. Hybrid Sponge-like scaffolds based on ulvan and gelatin: Design, characterization and evaluation of their potential use in bone tissue engineering. Materials 2020, 13, 1763. [Google Scholar] [CrossRef]
- Kyritsi, A.; Kikionis, S.; Tagka, A.; Koliarakis, N.; Evangelatou, A.; Papagiannis, P.; Stratigos, A.; Karalis, V.; Dallas, P.; Vitsos, A.; et al. Management of acute radiodermatitis in non-melanoma skin cancer patients using electrospun nanofibrous patches loaded with Pinus halepensis bark extract. Cancers 2021, 13, 2596. [Google Scholar] [CrossRef]
- Kikionis, S.; Koromvoki, M.; Tagka, A.; Polichronaki, E.; Stratigos, A.; Panagiotopoulos, A.; Kyritsi, A.; Karalis, V.; Vitsos, A.; Rallis, M.; et al. Ulvan-based nanofibrous patches enhance wound healing of skin trauma resulting from cryosurgical treatment of keloids. Mar. Drugs 2022, 20, 551. [Google Scholar] [CrossRef]
- Terezaki, A.; Kikionis, S.; Ioannou, E.; Sfiniadakis, I.; Tziveleka, L.-A.; Vitsos, A.; Roussis, V.; Rallis, M. Ulvan/gelatin-based nanofibrous patches as a promising treatment for burn wounds. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Kikionis, S.; Karkatzoulis, L.; Bethanis, K.; Roussis, V.; Ioannou, E. Valorization of fish waste: Isolation and characterization of acid- and pepsin-soluble collagen from the scales of mediterranean fish and fabrication of collagen-based nanofibrous scaffolds. Mar. Drugs 2022, 20, 664. [Google Scholar] [CrossRef]
- Qamar, Z.; Haji Abdul Rahim, Z.B.; Neon, G.S.; Chew, H.P.; Zeeshan, T. Effectiveness of poly-γ-glutamic acid in maintaining enamel integrity. Arch. Oral Biol. 2019, 106, 104482. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Tran, H.L.; Mai, P.T.; Duong, V.B.; Nguyen, L.-T.T.; Nguyen, H.T. Poly(L-glutamic acid) via catalytical hydrogenation for the fabrication of carbon nanotube nanocomposites. Mater. Res. 2021, 24, e20200321. [Google Scholar] [CrossRef]
- Ionescu, O.M.; Mignon, A.; Iacob, A.T.; Simionescu, N.; Confederat, L.G.; Tuchilus, C.; Profire, L. New Hyaluronic acid/polyethylene oxide-based electrospun nanofibers: Design, characterization and in vitro biological evaluation. Polymers 2021, 13, 1291. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Hezma, A.M.; El-Khodary, A.; Elzayat, A.M. Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Milella, E.; Ramires, P.A.; Brescia, E.; La Sala, G.; Di Paola, L.; Bruno, V. Physicochemical, mechanical, and biological properties of commercial membranes for GTR. J. Biomed. Mater. Res. 2001, 58, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Jose, M.V.; Thomas, V.; Dean, D.R.; Janowski, G.M. Freeze-dried acellular dermal matrix graft: Effects of rehydration on physical, chemical, and mechanical properties. Dent. Mater. 2009, 25, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Coic, M.; Placet, V.; Jacquet, E.; Meyer, C. Mechanical properties of collagen membranes used in guided bone regeneration: A comparative study of three models. Rev. Stomatol. Chir. Maxillofac. 2010, 111, 286–290. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Zhang, N.; Geng, X.; Meng, C.; Wang, X.; Yang, Y. Osteogenic capacity and cytotherapeutic potential of periodontal ligament cells for periodontal regeneration in vitro and in vivo. PeerJ 2019, 7, e6589. [Google Scholar] [CrossRef]
- Pereira, L.; Mesquita, J.F. Population studies and carrageenan properties of Chondracanthus teedei var. lusitanicus (Gigartinaceae, Rhodophyta). J. Appl. Phycol. 2004, 16, 369–383. [Google Scholar] [CrossRef]
- Correa-Diaz, F.; Aguilar-Rosas, R.; Aguilar-Rosas, L.E. Infrared analysis of eleven carrageenophytes from Baja California, Mexico. Hydrobiologia 1990, 204, 609–614. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Mavrogiorgis, D.; Bilalis, P.; Karatzas, A.; Skoulas, D.; Fotinogiannopoulou, A.; Iatrou, H. Controlled polymerization of histidine and synthesis of well-defined stimuli responsive polymers. Elucidation of the structure–aggregation relationship of this highly multifunctional material. Polym. Chem. 2014, 5, 6256–6278. [Google Scholar] [CrossRef]
- Han, J.; Ding, J.; Wang, Z.; Yan, S.; Zhuang, X.; Chen, X.; Yin, J. The synthesis, deprotection and properties of poly(γ-benzyl-L-glutamate). Sci. China Chem. 2013, 56, 729–738. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Membrane | Outer Cast Layer | Middle Nanofibrous Layer | Inner Nanofibrous Layer |
|---|---|---|---|
| GTR1 | PCL | PG-Ca/PEO 1, PCL | CG-Ca/PEO 2 |
| GTR2 | PCL | PG-Ca/PEO 1, PCL | CG-Na/PEO 2 |
| GTR3 | PCL | - | CG-Ca/PG-Ca/PEO 1, PCL |
| GTR4 | PCL | - | CG-Ca/PEO 2, PCL |
| GTR5 | PCL | - | PG-Ca/PEO 1, PCL |
| GTR0 | PCL | - | - |
| GTR Membranes | E (MPa) | UTS (MPa) | Strain (%) | RAS (%) |
|---|---|---|---|---|
| GTR1 | 117.6 (13.6) ABC | 7.6 (0.4) C | 78 [72, 120] ABC | 32.2 (2.2) A |
| GTR2 | 118.4 (15.7) AB | 9.6 (1.6) A | 138 [136, 320] A | 34.1 (5.7) A |
| GTR3 | 90.5 (8.2) AB | 5.6 (0.4) Β | 100 [89, 118] ABC | 37.0 (3.6) A |
| GTR4 | 88.0 (20.9) C | 5.9 (1.2) ΒC | 150 [96, 159] AB | 33.4 (4.8) A |
| GTR5 | 128.3 (26.8) B | 6.5 (1.1) ΒC | 70 [59, 86] BC | 37.7 (2.3) A |
| GTR0 | 97.3 (12.3) AC | 5.7 (1.1) B | 45 [36, 53] C | 33.3 (6.3) A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikionis, S.; Iliou, K.; Karra, A.G.; Polychronis, G.; Choinopoulos, I.; Iatrou, H.; Eliades, G.; Kitraki, E.; Tseti, I.; Zinelis, S.; et al. Development of Bi- and Tri-Layer Nanofibrous Membranes Based on the Sulfated Polysaccharide Carrageenan for Periodontal Tissue Regeneration. Mar. Drugs 2023, 21, 565. https://doi.org/10.3390/md21110565
Kikionis S, Iliou K, Karra AG, Polychronis G, Choinopoulos I, Iatrou H, Eliades G, Kitraki E, Tseti I, Zinelis S, et al. Development of Bi- and Tri-Layer Nanofibrous Membranes Based on the Sulfated Polysaccharide Carrageenan for Periodontal Tissue Regeneration. Marine Drugs. 2023; 21(11):565. https://doi.org/10.3390/md21110565
Chicago/Turabian StyleKikionis, Stefanos, Konstantina Iliou, Aikaterini G. Karra, Georgios Polychronis, Ioannis Choinopoulos, Hermis Iatrou, George Eliades, Efthymia Kitraki, Ioulia Tseti, Spiros Zinelis, and et al. 2023. "Development of Bi- and Tri-Layer Nanofibrous Membranes Based on the Sulfated Polysaccharide Carrageenan for Periodontal Tissue Regeneration" Marine Drugs 21, no. 11: 565. https://doi.org/10.3390/md21110565
APA StyleKikionis, S., Iliou, K., Karra, A. G., Polychronis, G., Choinopoulos, I., Iatrou, H., Eliades, G., Kitraki, E., Tseti, I., Zinelis, S., Ioannou, E., & Roussis, V. (2023). Development of Bi- and Tri-Layer Nanofibrous Membranes Based on the Sulfated Polysaccharide Carrageenan for Periodontal Tissue Regeneration. Marine Drugs, 21(11), 565. https://doi.org/10.3390/md21110565













