Recent Advancement in Anticancer Compounds from Marine Organisms: Approval, Use and Bioinformatic Approaches to Predict New Targets
Abstract
1. Introduction
2. The Evolution of Phenotypic Plasticity in the Marine Environment
3. Approved Anticancer Compounds from Marine Sponges
3.1. Eribulin Mesylate
3.2. Panobinostat
| Compound Name | Chemical Class | Marine Source | Species | Mechanism of Action | References |
|---|---|---|---|---|---|
| Eribulin mesylate | Macrolide | Sponge | Halichondria okadai | Interfering tubulin polymerization | [21,22,24,26,46,47,48] |
| Panobinostat | Hydroxamic acid derivative | Sponge | Pseudoceratina purpurea | HDAC inhibitor | [40,43,45,49,50,51] |
| Aminopeptidase-N inhibitor | [44] | ||||
| DNA Methyltransferase inhibitor | [35] |
4. Approved Anticancer Compounds from Marine Tunicates
4.1. Plitidepsin
4.2. Trabectedin
4.3. Lurbinectedin
| Compound Name | Chemical Class | Marine Source | Species | Mechanism of Action | References |
|---|---|---|---|---|---|
| Lurbinectedin | Alkaloid | Tunicate/bacteria | Ecteinascidia turbinata/ Candidatus Endoecteinascidia frumentensis | Alkylation of DNA | [79] |
| Inhibition of the transcriptional factors binding to DNA | [80,81] | ||||
| Trabectedin | Alkaloid | Tunicate/bacteria | Ecteinascidia turbinata/ Candidatus Endoecteinascidia frumentensis | Block RNA Pol-II | [68,69] |
| Inhibition of the transcriptional factors binding to DNA | [70,71,72] | ||||
| Reduction in TAM | [75,76] | ||||
| Plitidepsin | Peptide | Tunicate | Aplidium albicans | Activation of eEF1A2 | [55,82,83,84] |
5. Approved Anticancer Compounds from Mollusks/Cyanobacteria Association
5.1. Brentuximab Vedotin
5.2. Polatuzumab Vedotin
5.3. Enfortumab Vedotin-Eifv
5.4. Disitamab Vedotin
5.5. Tisotumab Vedotin-tftv
5.6. Belantamab Mafodotin-blmf
| Compound Name | Chemical Class | Marine Source | Species | Mechanism of Action | References |
|---|---|---|---|---|---|
| Brentuximab vedotin | ADC (MMAE + CD30Ab) | Mollusk/ Cyanobacteria | Dolabella auricolaria/ Symploca hynoides, Lyngbya majuscula | Microtubulin targeting agent via CD 30 | [95,96,97] |
| Polatuzumab vedotin | ADC (MMAE + CD-79bAb) | Mollusk/ Cyanobacteria | Dolabella auricolaria/ Symploca hynoides, Lyngbya majuscula | Microtubulin targeting agent via CD79 | [100,101,121] |
| Enfortumab vedotin | ADC (MMAE + Nectin-4 Ab) | Mollusk/ Cyanobacteria | Dolabella auricolaria/ Symploca hynoides, Lyngbya majuscula | Microtubulin targeting agent via Nectin4 | [103,122] |
| Disitamab Vedotin | MMAE + HER-2 Ab | Mollusk/ Cyanobacteria | Dolabella auricolaria/ Symploca hynoides, Lyngbya majuscula | Microtubulin targeting agent via HER-2 | [105,107,108,123] |
| Tisotumab Vedotin | MMAE + TF-011 Ab | Mollusk/ Cyanobacteria | Dolabella auricolaria/ Symploca hynoides, Lyngbya majuscula | Microtubulin targeting agent via TF-011 | [113,114,115,116] |
| Belantamab mafodotin | MMAF + CD38Ab | Mollusk/ Cyanobacteria | Dolabella auricolaria/ Symploca hynoides, Lyngbya majuscula | Microtubulin targeting agent via CD38 | [118,119,120] |
6. Prediction Bioinformatics Tools in Marine-Derived Compounds
6.1. Sponge-Derived Compound Prediction: The Example of Panobinostat
6.2. Tunicate-Derived Compound Prediction: The Example of Plitidepsin
6.3. Mollusks/Cyanobacteria Association-Derived Compound Prediction: The Example of Belantamab Mafodotin
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Wang, Z.; Deisboeck, T.S. Dynamic Targeting in Cancer Treatment. Front. Physiol. 2019, 10, 96. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Bayona, L.M.; de Voogd, N.J.; Choi, Y.H. Metabolomics on the Study of Marine Organisms. Metabolomics 2022, 18, 1–24. [Google Scholar] [CrossRef]
- Lane, A.L.; Moore, B.S. A Sea of Biosynthesis: Marine Natural Products Meet the Molecular Age. Nat. Prod. Rep. 2011, 28, 411–428. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural Products as Reservoirs of Novel Therapeutic Agents. EXCLI J. 2018, 17, 420–451. [Google Scholar] [CrossRef]
- Sigwart, J.D.; Blasiak, R.; Jaspars, M.; Jouffray, J.B.; Tasdemir, D. Unlocking the Potential of Marine Biodiscovery. Nat. Prod. Rep. 2021, 38, 1235–1242. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Coelho, A.V.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and Gene Expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Duncan, E.J.; Gluckman, P.D.; Dearden, P.K. Epigenetics, Plasticity, and Evolution: How Do We Link Epigenetic Change to Phenotype? J. Exp. Zool. B Mol. Dev. Evol. 2014, 322, 208–220. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Sommer, R.J. Phenotypic Plasticity: From Theory and Genetics to Current and Future Challenges. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef]
- World Porifera Database—Species. Available online: https://www.marinespecies.org/porifera/porifera.php?p=stats (accessed on 4 July 2022).
- Amina, M.; Musayeib, N.M. Biological and medicinal importance of sponge. In Biological Resources of Water; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Malve, H. Exploring the Ocean for New Drug Developments: Marine Pharmacology. J. Pharm. Bioallied. Sci. 2016, 8, 83. [Google Scholar] [CrossRef]
- Privat De Garilhe, M.; De Rudder, J. Effect of 2 Arabinose Nucleosides on the multiplication of Herpes Virus and vaccine in cell culture. Comptes Rendus Hebd. Seances Acad. Sci. 1964, 259, 2725–2728. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef]
- Hirata, Y.U.D. Halichondrins-Antitumor Polyether Macrolides from a Marine Sponge. Pure Appl. Chem. 1986, 58, 701–710. [Google Scholar]
- Bai, R.; Paull, K.; Herald, C.; Malspeis, L.; Pettit, G.; Hamel, E. Halichondrin B and Homohalichondrin B, Marine Natural Products Binding in the Vinca Domain of Tubulin. Discovery of Tubulin-Based Mechanism of Action by Analysis of differential cytotoxic data. J. Biol. Chem. 1991, 266, 15882–15889. [Google Scholar] [CrossRef]
- Kaufman, P.A.; Awada, A.; Twelves, C.; Yelle, L.; Perez, E.A.; Velikova, G.; Olivo, M.S.; He, Y.; Dutcus, C.E.; Cortes, J. Phase III Open-Label Randomized Study of Eribulin Mesylate Versus Capecitabine in Patients with Locally Advanced or Metastatic Breast Cancer Previously Treated with an Anthracycline and a Taxane. J. Clin. Oncol. 2015, 33, 594. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus Dacarbazine in Previously Treated Patients with Advanced Liposarcoma or Leiomyosarcoma: A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Barni, S.; Livraghi, L.; Morritti, M.; Vici, P.; Michelotti, A.; Cinieri, S.; Fontanella, C.; Porcu, L.; del Mastro, L.; Puglisi, F. Eribulin in the Treatment of Advanced Breast Cancer: Real-World Scenario from 39 Italian Centers—ESEMPiO Study. Future Oncol. 2019, 15, 3247–3258. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Towle, M.; Cheng, H. Induction of Morphological and Biochemical Apoptosis Following Prolonged Mitotic Blockage by Halichondrin B Macrocyclic Ketone Analog E7389. Cancer Res. 2004, 64, 5760–5764. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Bilela, L.L.; Moulin, C.; Taffin-De-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Dybdal-Hargreaves, N.F.; Risinger, A.L.; Mooberry, S.L. Eribulin Mesylate: Mechanism of Action of a Unique Microtubule-Targeting Agent. Clin. Cancer Res. 2015, 21, 2445–2452. [Google Scholar] [CrossRef]
- Quiñoà, E.; Crews, P. Phenolic Constituents of Psammaplysilla. Tetrahedron Lett. 1987, 28, 3229–3232. [Google Scholar] [CrossRef]
- Kim, D.; Lee, I.S.; Jung, J.H.; Yang, S.i. Psammaplin A, a Natural Bromotyrosine Derivative from a Sponge, Possesses the Antibacterial Activity against Methicillin-Resistant Staphylococcus Aureus and the DNA Gyrase-Inhibitory Activity. Arch. Pharm. Res. 1999, 22, 25–29. [Google Scholar] [CrossRef]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Tani, H.; et al. Psammaplin A Inhibits Hepatitis C Virus NS3 Helicase. J. Nat. Med. 2013, 67, 765–772. [Google Scholar] [CrossRef]
- Saguez, J.; Dubois, F.; Vincent, C.; Laberche, J.C.; Sangwan-Norreel, B.S.; Giordanengo, P. Differential Aphicidal Effects of Chitinase Inhibitors on the Polyphagous Homopteran Myzus Persicae (Sulzer). Pest Manag. Sci. 2006, 62, 1150–1154. [Google Scholar] [CrossRef]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Liu, W.; Xu, F.; Li, Z.; Bai, J.; Hua, H.; Li, D. Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification. Mar. Drugs 2019, 17, 384. [Google Scholar] [CrossRef]
- Tabudravu, J.N.; Eijsink, V.G.H.; Gooday, G.W.; Jaspars, M.; Komander, D.; Legg, M.; Synstad, B.; van Aalten, D.M.F. Psammaplin A, a Chitinase Inhibitor Isolated from the Fijian Marine Sponge Aplysinella Rhax. Bioorg. Med. Chem. 2002, 10, 1123–1128. [Google Scholar] [CrossRef]
- Kim, D.; Lee, I.S.; Jung, J.H.; Lee, C.O.; Choi, S.U. Psammaplin A, a Natural Phenolic Compound, Has Inhibitory Effect on Human Topoisomerase II and Is Cytotoxic to Cancer Cells. Anticancer Res. 1999, 19, 4085–4090. [Google Scholar] [PubMed]
- Jiménez, C.; Crews, P. Novel Marine Sponge Derived Amino Acids 13. Additional Psammaplin Derivatives from Psammaplysilla Purpurea. Tetrahedron 1991, 47, 2097–2102. [Google Scholar] [CrossRef]
- Piña, I.C.; Gautschi, J.T.; Wang, G.Y.S.; Sanders, M.L.; Schmitz, F.J.; France, D.; Cornell-Kennon, S.; Sambucetti, L.C.; Remiszewski, S.W.; Perez, L.B.; et al. Psammaplins from the Sponge Pseudoceratina purpurea: Inhibition of Both Histone Deacetylase and DNA Methyltransferase. J. Org. Chem. 2003, 68, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Dixon, G.; Liao, Y.; Bay, L.K.; Matz, M.v. Role of Gene Body Methylation in Acclimatization and Adaptation in a Basal Metazoan. Proc. Natl. Acad. Sci. USA 2018, 115, 13342–13346. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Li, J.; Du, L.; Mahdi, F.; Le, T.P.; Chen, W.L.; Swanson, S.M.; Watabe, K.; Nagle, D.G. Biochemical and Anti-Triple Negative Metastatic Breast Tumor Cell Properties of Psammaplins. Mar. Drugs 2018, 16, 442. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Jung, J.H.; Na, Y.J.; Kim, H.S. A Natural Histone Deacetylase Inhibitor, Psammaplin A, Induces Cell Cycle Arrest and Apoptosis in Human Endometrial Cancer Cells. Gynecol. Oncol. 2008, 108, 27–33. [Google Scholar] [CrossRef]
- Anne, M.; Sammartino, D.; Barginear, M.F.; Budman, D. Profile of Panobinostat and Its Potential for Treatment in Solid Tumors: An Update. Onco Targets Ther. 2013, 6, 1613–1624. [Google Scholar] [CrossRef]
- Wahaib, K.; Beggs, A.E.; Campbell, H.; Kodali, L.; Ford, P.D. Panobinostat: A Histone Deacetylase Inhibitor for the Treatment of Relapsed or Refractory Multiple Myeloma. Am. J. Health Syst. Pharm. 2016, 73, 441–450. [Google Scholar] [CrossRef]
- Qian, D.Z.; Kato, Y.; Shabbeer, S.; Wei, Y.; Verheul, H.M.W.; Salumbides, B.; Sanni, T.; Atadja, P.; Pili, R. Targeting Tumor Angiogenesis with Histone Deacetylase Inhibitors: The Hydroxamic Acid Derivative LBH589. Clin. Cancer Res. 2006, 12, 634–642. [Google Scholar] [CrossRef]
- Bali, P.; Pranpat, M.; Bradner, J.; Balasis, M.; Fiskus, W.; Guo, F.; Rocha, K.; Kumaraswamy, S.; Boyapalle, S.; Atadja, P.; et al. Inhibition of Histone Deacetylase 6 Acetylates and Disrupts the Chaperone Function of Heat Shock Protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005, 280, 26729–26734. [Google Scholar] [CrossRef]
- Singh, A.; Patel, V.K.; Jain, D.K.; Patel, P.; Rajak, H. Panobinostat as Pan-Deacetylase Inhibitor for the Treatment of Pancreatic Cancer: Recent Progress and Future Prospects. Oncol. Ther. 2016, 4, 73. [Google Scholar] [CrossRef]
- Shim, J.S.; Lee, H.S.; Shin, J.; Kwon, H.J. Psammaplin A, a Marine Natural Product, Inhibits Aminopeptidase N and Suppresses Angiogenesis in Vitro. Cancer Lett. 2004, 203, 163–169. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.M.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Overall Survival of Patients with Relapsed Multiple Myeloma Treated with Panobinostat or Placebo plus Bortezomib and Dexamethasone (the PANORAMA 1 Trial): A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Haematol. 2016, 3, e506–e515. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Kaklamani, V.; Kalinsky, K. Perspectives on the Mechanism of Action and Clinical Application of Eribulin for Metastatic Breast Cancer. Future Oncol. 2019, 15, 1641–1653. [Google Scholar] [CrossRef]
- Tate, C.R.; Rhodes, L.V.; Segar, H.C.; Driver, J.L.; Pounder, F.N.; Burow, M.E.; Collins-Burow, B.M. Targeting Triple-Negative Breast Cancer Cells with the Histone Deacetylase Inhibitor Panobinostat. Breast Cancer Res. 2012, 14, R79. [Google Scholar] [CrossRef]
- Dias, J.; Aguiar, S.; Pereira, D.; André, A.; Gano, L.; Correia, J.; Carrapiço, B.; Rütgen, B.; Malhó, R.; Peleteiro, C.; et al. The Histone Deacetylase Inhibitor Panobinostat Is a Potent Antitumor Agent in Canine Diffuse Large B-Cell Lymphoma. Oncotarget 2018, 9, 28586. [Google Scholar] [CrossRef]
- Shenkar, N.; Swalla, B.J. Global Diversity of Ascidiacea. PLoS ONE 2011, 6, e20657. [Google Scholar] [CrossRef]
- Dou, X.; Dong, B. Origins and Bioactivities of Natural Compounds Derived from Marine Ascidians and Their Symbionts. Mar. Drugs 2019, 17, 670. [Google Scholar] [CrossRef]
- Rinehart, K.; Lithgow-Bertelloni, A.M. Novel Antiviral and Cytotoxic Agent, dehydrodidemnin B. PCT Int. Pat. Appl. 1991, 15, 248086q. [Google Scholar]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.; Mateos, M.; Martín, A. Plitidepsin: Design, Development, and Potential Place in Therapy. Drug Des. Dev. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef]
- Urdiales, J.; Morata, P.; de Castro, I.; Sánchez-Jiménez, F. Antiproliferative Effect of Dehydrodidemnin B (DDB), a Depsipeptide Isolated from Mediterranean Tunicates. Cancer Lett. 1996, 102, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Losada, A.; Muñoz-Alonso, M.; García, C.; Sánchez-Murcia, P.; Martínez-Leal, J.; Domínguez, J.; Lillo, M.; Gago, F.; Galmarini, C. Translation Elongation Factor EEF1A2 Is a Novel Anticancer Target for the Marine Natural Product Plitidepsin. Sci. Rep. 2016, 6, 35100. [Google Scholar] [CrossRef] [PubMed]
- Leisch, M.; Egle, A.; Greil, R. Plitidepsin: A Potential New Treatment for Relapsed/Refractory Multiple Myeloma. Future Oncol. 2018, 15, 109–120. [Google Scholar] [CrossRef]
- Capalbo, A.; Lauritano, C. Multiple Myeloma: Possible Cure from the Sea. Cancers 2022, 14, 2965. [Google Scholar] [CrossRef] [PubMed]
- Spicka, I.; Ocio, E.; Oakervee, H.; Greil, R.; Banh, R.; Huang, S.; D’Rozario, J.; Dimopoulos, M.; Martínez, S.; Extremera, S.; et al. Randomized Phase III Study (ADMYRE) of Plitidepsin in Combination with Dexamethasone vs. Dexamethasone Alone in Patients with Relapsed/Refractory Multiple Myeloma. Ann. Hematol. 2019, 98, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Aplidin. Available online: https://www.ema.europa.eu/en/aplidin (accessed on 23 October 2022).
- Varona, J.; Landete, P.; Lopez-Martin, J.; Estrada, V.; Paredes, R.; Guisado-Vasco, P.; de Orueta, L.; Torralba, M.; Fortun, J.; Vates, R.; et al. Preclinical and Randomized Phase I Studies of Plitidepsin in Adults Hospitalized with COVID-19. Life Sci Alliance 2022, 5, e202101200. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, M.; Papoutsi, E.; Giannakas, T.; Katsaounou, P. Plitidepsin: Mechanisms and Clinical Profile of a Promising Antiviral Agent against COVID-19. J. Pers. Med. 2021, 11, 668. [Google Scholar] [CrossRef]
- Guisado-Vasco, P.; Carralón-González, M.; Aguareles-Gorines, J.; Martí-Ballesteros, E.; Sánchez-Manzano, M.; Carnevali-Ruiz, D.; García-Coca, M.; Barrena-Puertas, R.; de Viedma, R.; Luque-Pinilla, J.; et al. Plitidepsin as a Successful Rescue Treatment for Prolonged Viral SARS-CoV-2 Replication in a Patient with Previous Anti-CD20 Monoclonal Antibody-Mediated B Cell Depletion and Chronic Lymphocytic Leukemia. J. Hematol. Oncol. 2022, 15, 4. [Google Scholar] [CrossRef]
- Carter, N.; Keam, S. Trabectedin. Drugs 2007, 67, 2257–2276. [Google Scholar] [CrossRef]
- Rinehart, K.L. Antitumor Compounds from Tunicates. Med. Res. Rev. 2000, 20, 1–27. [Google Scholar] [CrossRef]
- Larsen, A.K.; Galmarini, C.M.; D’Incalci, M. Unique Features of Trabectedin Mechanism of Action. Cancer Chemother. Pharmacol. 2016, 77, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Aune, G.J.; Takagi, K.; Sordet, O.; Guirouilh-Barbat, J.; Antony, S.; Bohr, V.; Pommier, Y. Von Hippel-Lindau Coupled and Transcription-Coupled Nucleotide Excision Repair Dependent Degradation of RNA Polymerase II in Response to Trabectedin. Clin. Cancer Res. 2008, 14, 6449. [Google Scholar] [CrossRef] [PubMed]
- Feuerhahn, S.; Giraudon, C.; Martínez-Díez, M.; Bueren-Calabuig, J.A.; Galmarini, C.M.; Gago, F.; Egly, J.M. XPF-Dependent DNA Breaks and RNA Polymerase II Arrest Induced by Antitumor DNA Interstrand Crosslinking-Mimetic Alkaloids. Chem. Biol. 2011, 18, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Minuzzo, M.; Marchini, S.; Broggini, M.; Faircloth, G.; D’Incalci, M.; Mantovani, R. Interference of Transcriptional Activation by the Antineoplastic Drug Ecteinascidin-743. Proc. Natl. Acad. Sci. USA 2000, 97, 6780–6784. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gorfajn, B.; Faircloth, G.; Scotto, K.W. Ecteinascidin 743, a Transcription-Targeted Chemotherapeutic That Inhibits MDR1 Activation. Proc. Natl. Acad. Sci. USA 2000, 97, 6775–6779. [Google Scholar] [CrossRef]
- Bonfanti, M.; la Valle, E.; Fernandez Sousa Faro, J.M.; Faircloth, G.; Caretti, G.; Mantovani, R.; D’Incalci, M. Effect of Ecteinascidin-743 on the Interaction between DNA Binding Proteins and DNA. Anticancer Drug Des. 1999, 14, 179–186. [Google Scholar]
- Di Giandomenico, S.; Frapolli, R.; Bello, E.; Uboldi, S.; Licandro, S.A.; Marchini, S.; Beltrame, L.; Brich, S.; Mauro, V.; Tamborini, E.; et al. Mode of Action of Trabectedin in Myxoid Liposarcomas. Oncogene 2014, 33, 5201–5210. [Google Scholar] [CrossRef]
- Demetri, G.; von Mehren, M.; Jones, R.; Hensley, M.; Schuetze, S.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of Macrophage Targeting in the Antitumor Activity of Trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef]
- D’Incalci, M.; Badri, N.; Galmarini, C.M.; Allavena, P. Trabectedin, a Drug Acting on Both Cancer Cells and the Tumour Microenvironment. Br. J. Cancer 2014, 111, 646. [Google Scholar] [CrossRef]
- Allavena, P.; Signorelli, M.; Chieppa, M.; Erba, E.; Bianchi, G.; Marchesi, F.; Olimpio, C.; Bonardi, C.; Garbi, A.; Lissoni, A.; et al. Anti-Inflammatory Properties of the Novel Antitumor Agent Yondelis (Trabectedin): Inhibition of Macrophage Differentiation and Cytokine Production. Cancer Res. 2005, 65, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as Second-Line Treatment for Patients with Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jaigirdar, A.; Mulkey, F.; Cheng, J.; Hamed, S.; Li, Y.; Liu, J.; Zhao, H.; Goheer, A.; Helms, W.; et al. FDA Approval Summary: Lurbinectedin for the Treatment of Metastatic Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Harlow, M.; Maloney, N.; Roland, J.; Guillen Navarro, M.; Easton, M.; Kitchen-Goosen, S.; Boguslawski, E.; Madaj, Z.; Johnson, B.; Bowman, M.; et al. Lurbinectedin Inactivates the Ewing Sarcoma Oncoprotein EWS-FLI1 by Redistributing It within the Nucleus. Cancer Res. 2016, 76, 6657–6668. [Google Scholar] [CrossRef]
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M. Targeting Transcription Factors for Cancer Treatment. Molecules 2018, 23, 1479. [Google Scholar] [CrossRef]
- Muñoz-Alonso, M.; González-Santiago, L.; Zarich, N.; Martínez, T.; Alvarez, E.; Rojas, J.M.; Muñoz, A. Plitidepsin Has a Dual Effect Inhibiting Cell Cycle and Inducing Apoptosis via Rac1/c-Jun NH2-Terminal Kinase Activation in Human Melanoma Cells. J. Pharmacol. Exp. Ther. 2008, 324, 1093–1101. [Google Scholar] [CrossRef]
- Erba, E.; Serafini, M.; Gaipa, G.; Tognon, G.; Marchini, S.; Celli, N.; Rotilio, O.; Broggini, M.; Jimeno, J.; Faircloth, G.; et al. Effect of Aplidin in Acute Lymphoblastic Leukaemia Cells. Br. J. Cancer 2003, 89, 763–773. [Google Scholar] [CrossRef]
- Cuadrado, A.; González, L.; Suárez, Y.; Martínez, T.; Muñoz, A. JNK Activation Is Critical for AplidinTM-Induced Apoptosis. Oncogene 2004, 23, 4673–4680. [Google Scholar] [CrossRef]
- Guiry, M. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Molluscabase. Available online: https://www.molluscabase.org/ (accessed on 5 July 2022).
- Hrouzek, P. Secondary metabolites produced by cyanobacteria in symbiotic associations. In Algal and Cyanobacteria Symbioses; World Scientific: Singapore, 2017; pp. 611–626. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyj, L.; Tomer, K.B.; Bontems, R.J. The Isolation and Structure of a Remarkable Marine Animal Antineoplastic Constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Pettit, G.; Kamano, Y.; Fujii, Y.; Herald, C.; Inoue, M.; Brown, P.; Gust, D.; Kitahara, K.; Schmidt, J.; Doubek, D.; et al. Marine Animal Biosynthetic Constituents for Cancer Chemotherapy. J. Nat. Prod. 1981, 44, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, G.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Nagle, D.G.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H.; Valeriote, F.A. Symplostatin 1: A Dolastatin 10 Analogue from the Marine Cyanobacterium Symploca Hydnoides. J. Nat. Prod. 1998, 61, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Robles-Bañuelos, B.; Durán-Riveroll, L.; Rangel-López, E.; Pérez-López, H.; González-Maya, L. Marine Cyanobacteria as Sources of Lead Anticancer Compounds: A Review of Families of Metabolites with Cytotoxic, Antiproliferative, and Antineoplastic Effects. Molecules 2022, 27, 4814. [Google Scholar] [CrossRef] [PubMed]
- Maderna, A.; Leverett, C. Recent Advances in the Development of New Auristatins: Structural Modifications and Application in Antibody Drug Conjugates. Mol. Pharm. 2015, 12, 1798–1812. [Google Scholar] [CrossRef]
- Maderna, A.; Doroski, M.; Subramanyam, C.; Porte, A.; Leverett, C.; Vetelino, B.; Chen, Z.; Risley, H.; Parris, K.; Pandit, J.; et al. Discovery of Cytotoxic Dolastatin 10 Analogues with N-Terminal Modifications. J. Med. Chem. 2014, 57, 10527–10543. [Google Scholar] [CrossRef]
- Akaiwa, M.; Dugal-Tessier, J.; Mendelsohn, B. Antibody-Drug Conjugate Payloads; Study of Auristatin Derivatives. Chem. Pharm. Bull. 2020, 68, 201–211. [Google Scholar] [CrossRef]
- Yu, B.; Liu, D. Antibody-Drug Conjugates in Clinical Trials for Lymphoid Malignancies and Multiple Myeloma. J. Hematol. Oncol. 2019, 12, 94. [Google Scholar] [CrossRef]
- Van de Donk, N.W.C.J.; Dhimolea, E. Brentuximab Vedotin. MAbs 2012, 4, 458. [Google Scholar] [CrossRef]
- Eisenberg, R. Immune compromise associated with biologics. In Stiehm’s Immune Deficiencies; Academic Press: Cambridge, MA, USA, 2014; pp. 889–906. [Google Scholar] [CrossRef]
- Jiang, J.; Li, S.; Shan, X.; Wang, L.; Ma, J.; Huang, M.; Dong, L.; Chen, F. Preclinical Safety Profile of Disitamab Vedotin: A Novel Anti-HER2 Antibody Conjugated with MMAE. Toxicol. Lett. 2020, 324, 30–37. [Google Scholar] [CrossRef]
- Young, R.; Shaffer, A.; Phelan, J.; Staudt, L. B-Cell Receptor Signaling in Diffuse Large B-Cell Lymphoma. Semin. Hematol. 2015, 52, 77–85. [Google Scholar] [CrossRef]
- Choi, Y.; Diefenbach, C. Polatuzumab Vedotin: A New Target for B Cell Malignancies. Curr. Hematol. Malig. Rep. 2020, 15, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Tilly, H.; Morschhauser, F.; Bartlett, N.L.; Mehta, A.; Salles, G.; Haioun, C.; Munoz, J.; Chen, A.I.; Kolibaba, K.; Lu, D.; et al. Polatuzumab Vedotin in Combination with Immunochemotherapy in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma: An Open-Label, Non-Randomised, Phase 1b–2 Study. Lancet Oncol. 2019, 20, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.; Sonpavde, G.; Loriot, Y.; Durán, I.; Lee, J.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab Vedotin: A Novel Antibody-Drug Conjugates for Cancer Therapy. Drug Deliv. 2022, 29, 1335. [Google Scholar] [CrossRef]
- Deeks, E.D. Disitamab Vedotin: First Approval. Drugs 2021, 81, 1929–1935. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. A Study of RC48-ADC in Subjects with Advanced Breast Cancer—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT03052634 (accessed on 22 June 2022).
- Patelli, G.; Zeppellini, A.; Spina, F.; Righetti, E.; Stabile, S.; Amatu, A.; Tosi, F.; Ghezzi, S.; Siena, S.; Sartore-Bianchi, A. The Evolving Panorama of HER2-Targeted Treatments in Metastatic Urothelial Cancer: A Systematic Review and Future Perspectives. Cancer Treat. Rev. 2022, 104, 102351. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.; Yao, X.; Luo, H.; Shi, B.; Liu, J.; He, Z.; Yu, G.; et al. Open-Label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 43–51. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, C.; Gou, J.; Yi, T.; Qian, Y.; Du, X.; Zhao, X. Expression of Tissue Factor in Human Cervical Carcinoma Tissue. Exp. Ther. Med. 2018, 16, 4075–4081. [Google Scholar] [CrossRef]
- Cocco, E.; Varughese, J.; Buza, N.; Bellone, S.; Lin, K.Y.; Bellone, M.; Todeschini, P.; Silasi, D.A.; Azodi, M.; Schwartz, P.E.; et al. Tissue Factor Expression in Ovarian Cancer: Implications for Immunotherapy with HI-Con1, a Factor VII-IgGF(c) Chimeric Protein Targeting Tissue Factor. Clin. Exp. Metastasis 2011, 28, 689–700. [Google Scholar] [CrossRef]
- Patry, G.; Hovington, H.; Larue, H.; Harel, F.; Fradet, Y.; Lacombe, L. Tissue Factor Expression Correlates with Disease-Specific Survival in Patients with Node-Negative Muscle-Invasive Bladder Cancer. Int. J. Cancer 2008, 122, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Concin, N.; Hong, D.; Thistlethwaite, F.; Machiels, J.; Arkenau, H.; Plummer, R.; Jones, R.; Nielsen, D.; Windfeld, K.; et al. Tisotumab Vedotin in Patients with Advanced or Metastatic Solid Tumours (InnovaTV 201): A First-in-Human, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, Y.W.; Osanto, S.; Reitsma, P.H.; Versteeg, H.H. The Relationship between Tissue Factor and Cancer Progression: Insights from Bench and Bedside. Blood 2012, 119, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, R.S.; Taubman, M.B.; Mackman, N. Role of Tissue Factor in Cancer. J. Clin. Oncol. 2009, 27, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Seidel, U.; Schlegel, P.; Lang, P. Natural Killer Cell Mediated Antibody-Dependent Cellular Cytotoxicity in Tumor Immunotherapy with Therapeutic Antibodies. Front. Immunol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (InnovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- FDA. FDA Grants Accelerated Approval to Tisotumab Vedotin-Tftv for Recurrent or Metastatic Cervical Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer (accessed on 23 June 2022).
- Offidani, M.; Corvatta, L.; Morè, S.; Olivieri, A. Belantamab Mafodotin for the Treatment of Multiple Myeloma: An Overview of the Clinical Efficacy and Safety. Drug Des. Devel. Ther. 2021, 15, 2401–2415. [Google Scholar] [CrossRef]
- Condorelli, A.; Garibaldi, B.; Gagliano, C.; Romano, A.; del Fabro, V.; Parrinello, N.L.; Longo, A.; Cosentino, S.; di Raimondo, F.; Conticello, C. Belantamab Mafodotin and Relapsed/Refractory Multiple Myeloma: This Is Not Game Over. Acta Haematol. 2021, abstract. [Google Scholar] [CrossRef]
- Markham, A. Belantamab Mafodotin: First Approval. Drugs 2020, 80, 1607–1613. [Google Scholar] [CrossRef]
- Li, D.; Lee, D.; Dere, R.C.; Zheng, B.; Yu, S.F.; Fuh, F.K.; Kozak, K.R.; Chung, S.; Bumbaca Yadav, D.; Nazzal, D.; et al. Evaluation and Use of an Anti-cynomolgus Monkey CD79b Surrogate Antibody–Drug Conjugate to Enable Clinical Development of Polatuzumab Vedotin. Br. J. Pharmacol. 2019, 176, 3805. [Google Scholar] [CrossRef]
- Lagunin, A.; Dubovskaja, V.; Rudik, A.; Pogodin, P.; Druzhilovskiy, D.; Gloriozova, T.; Filimonov, D.; Sastry, N.; Poroikov, V. CLC-Pred: A Freely Available Web-Service for in Silico Prediction of Human Cell Line Cytotoxicity for Drug-like Compounds. PLoS ONE 2018, 13, e0191838. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of Activity Spectra for Biologically Active Substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Lagunin, A.; Ivanov, S.; Rudik, A.; Filimonov, D.; Poroikov, V. DIGEP-Pred: Web Service for in Silico Prediction of Drug-Induced Gene Expression Profiles Based on Structural Formula. Bioinformatics 2013, 29, 2062–2063. [Google Scholar] [CrossRef]
- Ge, S.; Jung, D.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, M.; Katz, C.; Kass, S.; Smith, D.; Hunter, K.; Warshal, D.; Aikins, J.; Ostrovsky, O. Epigenetic Therapy Augments Classic Chemotherapy in Suppressing the Growth of 3D High-Grade Serous Ovarian Cancer Spheroids over an Extended Period of Time. Biomolecules 2021, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cheong, H.; Kim, S.; Yoon, J.; Kim, H.; Kim, K.; Kim, S.; Kim, H.; Bae, S.; Kim, C. Low-Dose Combinations of LBH589 and TRAIL Can Overcome TRAIL-Resistance in Colon Cancer Cell Lines. Anticancer. Res. 2011, 31, 3385–3394. [Google Scholar] [PubMed]
- Huang, H.y.; Wang, Y.; Wang, W.d.; Wei, X.l.; Gale, R.P.; Li, J.y.; Zhang, Q.y.; Shu, L.l.; Li, L.; Li, J.; et al. A Prognostic Survival Model Based on Metabolism-Related Gene Expression in Plasma Cell Myeloma. Leukemia 2021, 35, 3212–3222. [Google Scholar] [CrossRef]
- Fedeli, E.; Lancelot, A.; Dominguez, J.; Serrano, J.; Calvo, P.; Sierra, T. Self-Assembling Hybrid Linear-Dendritic Block Copolymers: The Design of Nano-Carriers for Lipophilic Antitumoral Drugs. Nanomaterials 2019, 9, 161. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Rozen-Gagnon, K.; Miles, L.A.; Schuster, F.; Razooky, B.; Jacobson, E.; Wu, X.; Yi, S.; Rudin, C.M.; et al. TMEM41B Is a Pan-Flavivirus Host Factor. Cell 2021, 184, 133–148.e20. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Gu, Z.; Wang, H.; Xia, J.; Wu, X.; Zhan, X.; Levasseur, D.; Zhou, Y.; Janz, S.; et al. ALDH1 Activity Identifies Tumor-Initiating Cells and Links to Chromosomal Instability Signatures in Multiple Myeloma. Leukemia 2014, 28, 1155–1158. [Google Scholar] [CrossRef]
- Conte, M.; Fontana, E.; Nebbioso, A.; Altucci, L. Marine-Derived Secondary Metabolites as Promising Epigenetic Bio-Compounds for Anticancer Therapy. Mar. Drugs 2020, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine Power on Cancer: Drugs, Lead Compounds, and Mechanisms. Mar. Drugs 2021, 19, 488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Wang, X.; Smith, L.S.; He, B.; Liu, S.; Zhu, H.J. Tissue- and Cell-Expression of Druggable Host Proteins Provide Insights into Repurposing Drugs for COVID-19. Clin. Transl. Sci. 2022, 15, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Plitidepsin: A Repurposed Drug for the Treatment of COVID-19. Antimicrob. Agents Chemother. 2021, 65, e00200-21. [Google Scholar] [CrossRef]


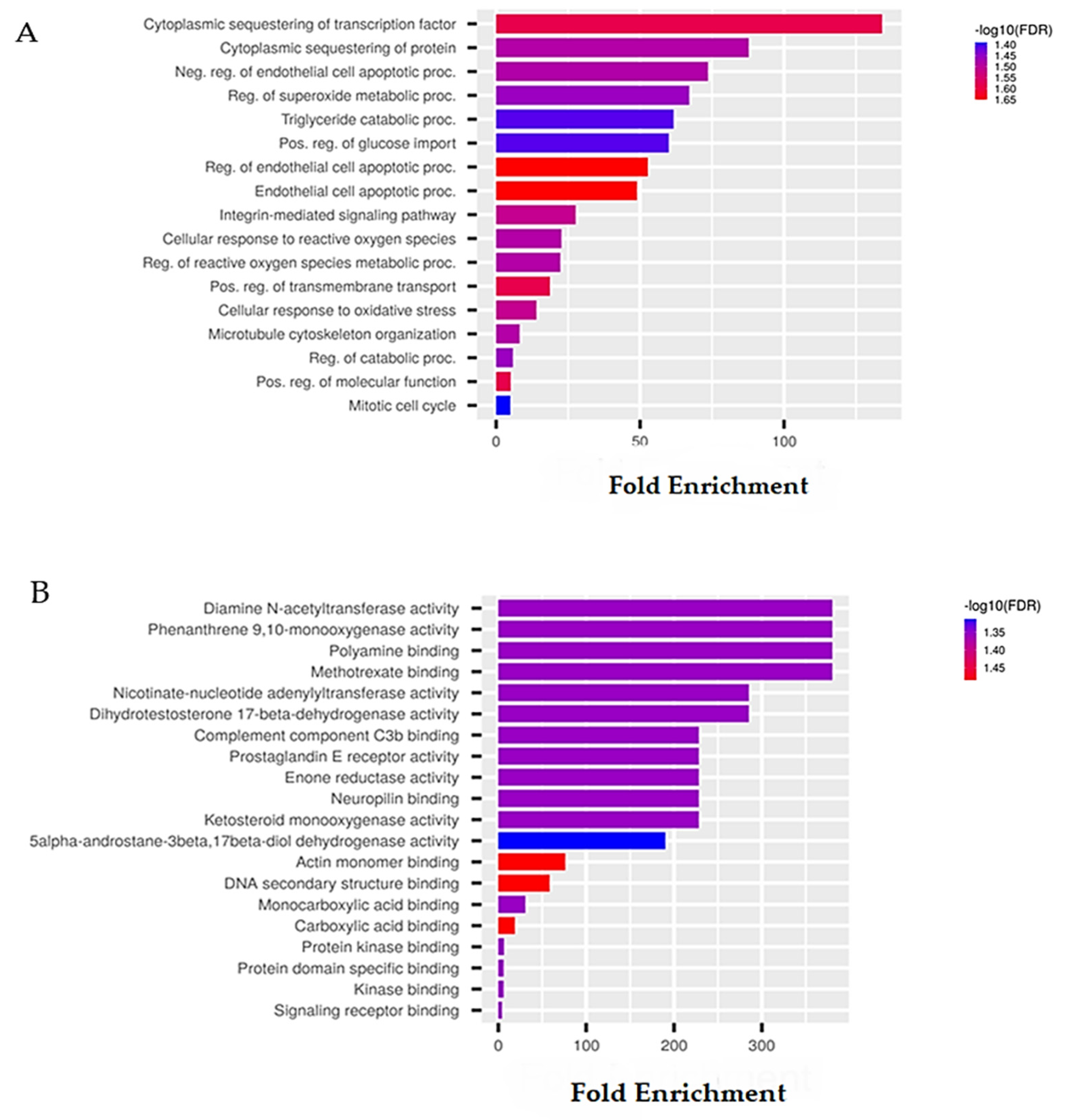
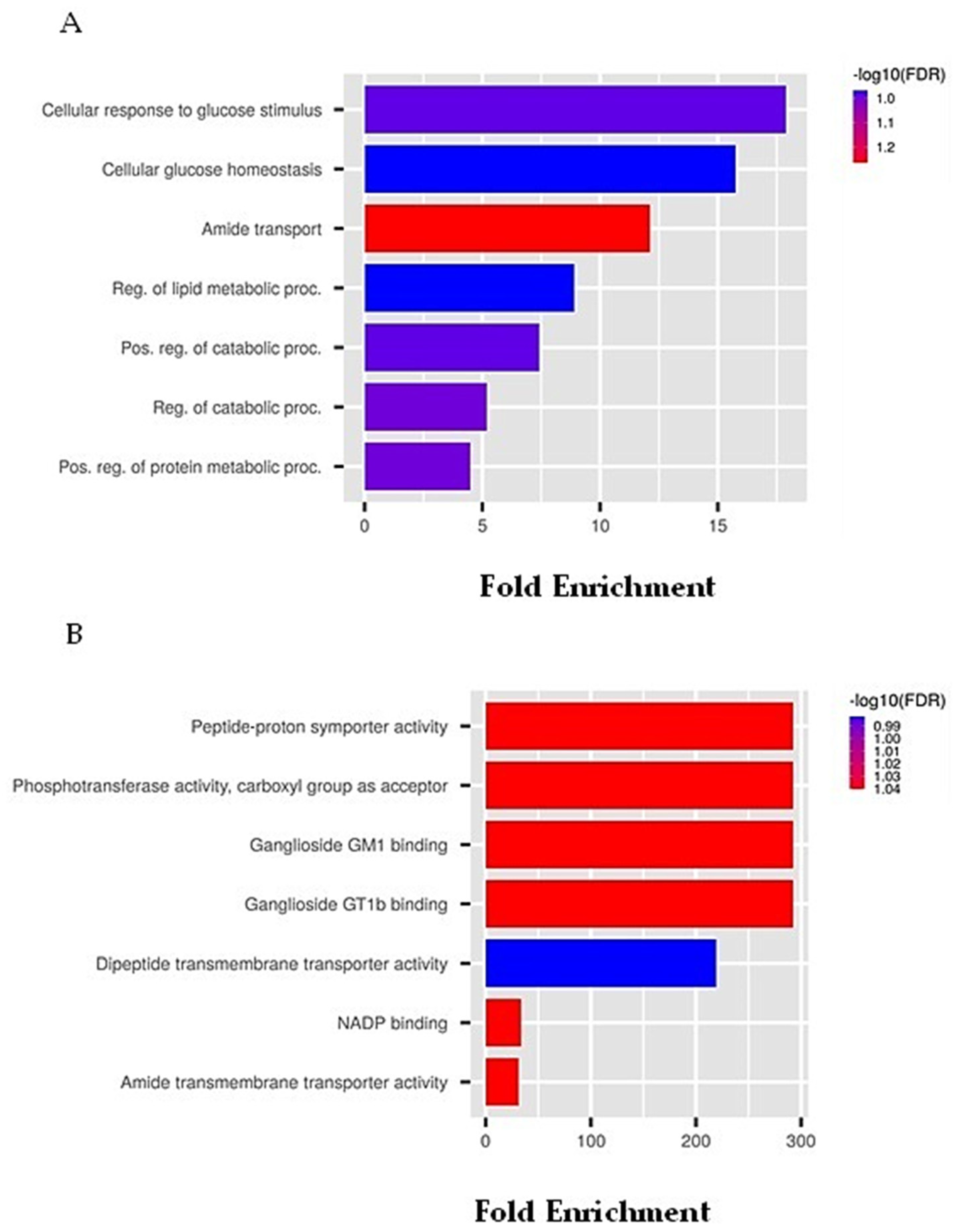

| Activity | Pa | Pi |
|---|---|---|
| HDAC 1 inhibitor | 0.843 | 0.001 |
| HDAC class I inhibitor | 0.842 | 0.001 |
| HDAC inhibitor | 0.792 | 0.002 |
| HDAC 2 inhibitor | 0.760 | 0.001 |
| HDAC 4 inhibitor | 0.706 | 0.001 |
| HDAC IIa inhibitor | 0.700 | 0.001 |
| HDAC 8 inhibitor | 0.530 | 0.001 |
| Chemosensitizer | 0.523 | 0.016 |
| Calcium channel (voltage-sensitive) activator | 0.521 | 0.064 |
| Possible Adverse and Toxic Effects | Pa | Pi |
|---|---|---|
| Ulcer, gastric | 0.362 | 0.057 |
| Occult bleeding Ulcer, peptic | 0.421 0.346 | 0.131 0.088 |
| Cell Line Full Name and Code | Tissue | Pa | Pi |
|---|---|---|---|
| Colon Carcinoma HCT-116 | Colon | 0.784 | 0.0070 |
| Non-small cell lung carcinoma NCI-H1299 | Lung | 0.543 | 0.004 |
| Down-Regulated Genes | Pa | Pi | Up-Regulated Genes | Pa | Pi |
|---|---|---|---|---|---|
| CTPS1 INCENP KEAP1 AKR1C3 EIF4G2 RCC2 ABL1 PHF11 TOB1 DHFR FABP4 MAPK4 NPM | 0.875 0.863 0.795 0.802 0.700 0.697 0.676 0.683 0.715 0.701 0.664 0.563 0.568 | 0.001 0.006 0.008 0.022 0.003 0.002 0.002 0.013 0.050 0.048 0.049 0.114 0.137 | TACC1 ITGAM H1F0 HPGD FGF21 TMSB4X OCLN SAT1 | 0.833 0.819 0.759 0.748 0.686 0.659 0.573 0.592 | 0.004 0.021 0.005 0.032 0.01 0.034 0.039 0.129 |
| Activity | Pa | Pi |
|---|---|---|
| Immunosuppressant | 0.788 | 0.006 |
| Antibiotic glycopeptide-like | 0.738 | 0.003 |
| General pump inhibitor | 0.649 | 0.014 |
| Antineoplastic | 0.657 | 0.034 |
| CYP2H substrate | 0.650 | 0.050 |
| Antifungal | 0.585 | 0.020 |
| Antineoplastic (colorectal cancer) | 0.548 | 0.010 |
| Antineoplastic (colon cancer) | 0.541 | 0.010 |
| Xenobiotic-transporting ATPase inhibitor | 0.524 | 0.009 |
| Antibacterial | 0.453 | 0.021 |
| Possible Adverse and Toxic Effects | Pa | Pi |
|---|---|---|
| Dyskinesia Sleep disturbance Dyspnea Ataxia | 0.960 0.898 0.892 0.776 | 0.004 0.012 0.006 0.013 |
| Cell Line Full Name and Code | Tissue | Pa | Pi |
|---|---|---|---|
| Lung carcinoma A549 Colon adenocarcinoma HT-29 Breast adenocarcinoma MDA-MB-231 Lung carcinoma DMS-114 | Lung Colon Breast Lung | 0.808 0.801 0.554 0.501 | 0.011 0.005 0.020 0.038 |
| Down-Regulated Genes | Pa | Pi | Up-Regulated Genes | Pa | Pi |
|---|---|---|---|---|---|
| NSF ALDH18A1 H6PD SLC15A1 MYBL1 BACE1 SLC14A1 TOB1 VTN BARD1 FKBP5 CTPS1 HSPB11 TAGLN | 0.907 0.879 0.782 0.765 0.739 0.697 0.675 0.682 0.605 0.636 0.599 0.566 0.501 0.516 | 0.012 0.015 0.019 0.036 0.040 0.016 0.053 0.063 0.036 0.120 0.086 0.090 0.053 0.088 | TMEM41B C10ORF118 FAM49A PLXNA2 HMGCR PSAP TXNDC9 PLK3 FGF21 C8ORF4 WIPI1 GPRC5A NUCB2 | 0.823 0.826 0.756 0.751 0.637 0.635 0.542 0.595 0.517 0.552 0.530 0.507 0.523 | 0.009 0.012 0.041 0.047 0.035 0.055 0.025 0.092 0.034 0.083 0.106 0.087 0.105 |
| Activity | Pa | Pi |
|---|---|---|
| Immunostimulant | 0.823 | 0.008 |
| Proteasome ATPase inhibitor | 0.774 | 0.007 |
| Antineoplastic (non-Hodgkin’s lymphoma) | 0.729 | 0.003 |
| Muramoyltetrapeptide carboxypeptidase inhibitor | 0.636 | 0.021 |
| Antineoplastic (solid tumors) | 0.537 | 0.010 |
| Peptide agonist | 0.537 | 0.039 |
| Neuropeptide Y4 antagonist | 0.461 | 0.027 |
| Antineoplastic (pancreatic cancer) | 0.437 | 0.010 |
| CYP2H substrate | 0.502 | 0.122 |
| Fibroblast growth factor agonist | 0.419 | 0.056 |
| Cell Line Full Name and Code | Tissue | Pa | Pi |
|---|---|---|---|
| Breast adenocarcinoma MDA-MB-231 Breast carcinoma MCF7 | Breast Breast | 0.501 0.513 | 0.028 0.049 |
| Down-Regulated Genes | Pa | Pi | Up-Regulated Genes | Pa | Pi |
|---|---|---|---|---|---|
| ALDH18A1 SHC1 NSF H6PD AKR1C3 | 0.698 0.550 0.546 0.524 0.511 | 0.098 0.040 0.117 0.127 0.211 | C10ORF118 TMEM41B | 0.631 0.606 | 0.063 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santaniello, G.; Nebbioso, A.; Altucci, L.; Conte, M. Recent Advancement in Anticancer Compounds from Marine Organisms: Approval, Use and Bioinformatic Approaches to Predict New Targets. Mar. Drugs 2023, 21, 24. https://doi.org/10.3390/md21010024
Santaniello G, Nebbioso A, Altucci L, Conte M. Recent Advancement in Anticancer Compounds from Marine Organisms: Approval, Use and Bioinformatic Approaches to Predict New Targets. Marine Drugs. 2023; 21(1):24. https://doi.org/10.3390/md21010024
Chicago/Turabian StyleSantaniello, Giovanna, Angela Nebbioso, Lucia Altucci, and Mariarosaria Conte. 2023. "Recent Advancement in Anticancer Compounds from Marine Organisms: Approval, Use and Bioinformatic Approaches to Predict New Targets" Marine Drugs 21, no. 1: 24. https://doi.org/10.3390/md21010024
APA StyleSantaniello, G., Nebbioso, A., Altucci, L., & Conte, M. (2023). Recent Advancement in Anticancer Compounds from Marine Organisms: Approval, Use and Bioinformatic Approaches to Predict New Targets. Marine Drugs, 21(1), 24. https://doi.org/10.3390/md21010024








