Abstract
Peptic ulcer is a widespread disease, with a lifetime frequency of 5–10% among the general population and an annual incidence of 0.1–0.3%. Ovothiol A is naturally produced from sea urchin eggs with special antioxidant activity. Gastric ulcers were induced in rats by a single ethanol dose (5 mL/kg). The rats were divided into control, ulcer, and ulcer with 250 and 500 mg/kg ovothiol A doses. Molecular docking studies were used to examine the interactions between ovothiol A and the H+/K+ ATPase active site residues. Ovothiol A led to a significant decline (p < 0.05) in gastric juice volume, ulcer index, MDA, IL-6, and cytochrome c, while levels of gastric juice pH, GSH, CAT, GST, SOD, and NO increased. Histopathological investigation of stomach sections revealed architecture preservation of the gastric mucosa after ovothiol A administration. The anti-ulcerogenic activity of ovothiol A includes scavenging free radicals, inhibition of inflammation, regulation of apoptosis, and stabilization of fibroblast growth factors to promote gastric ulcers healing.
1. Introduction
Peptic ulcer is a lesion of the digestive system caused by gastric acid that typically develops in the stomach or proximal duodenum [1]. Peptic ulcer disease (PUD) is widespread, with a lifetime frequency of 5–10% among the general population and an annual incidence of 0.1–0.3% [2]. In 2019, there were nearly 8 million prevalent incidences of PUD [3].
PUD is due to a mismatch between defensive factors (mucus secretion, non-enzymatic and enzymatic antioxidants, surface active phospholipids, blood flow, prostaglandins, and cell renewal) and offensive agents such as (gastric acid, pepsin and reactive oxygen species (ROS) [4]. However, the two most important factors that cause an imbalance between the acid and the mucus are H. pylori infection, non-steroidal anti-inflammatory medicines (NSAIDs), and a rise in alcohol and smoking misuse [5,6,7,8,9].
The commonly used proton pump inhibitors (PPIs) and histamine receptor type-2 antagonists have shown side effects and several medication interactions [10]. Therefore, the need to face these challenges has encouraged research reports to explore new active compounds from natural sources [11].
The incredible biodiversity of sea environment creatures contributed to the identification of numerous new chemicals, the biological features, and technological applications of which are being thoroughly researched [12,13]. More attention has recently been paid to marine-isolated ovothiols (1-N-methyl-4-mercaptohistidine) from invertebrates, algae, and protozoa [14]. The three types of ovothiols (A, B, and C) differ in the nitrogen methylation of the aminoacidic side chain (non-, mono- and di-methylated, respectively).
Ovothiol A was first found in some echinoderm’s eggs (for example, the sea urchin Paracentrotus lividus) and in some mollusks and polychaetes biological fluids [15]. The thiol group of all ovothiols on their histidine imidazole ring has exceptional antioxidant characteristics [16,17,18]. Only the anionic thiolate form of thiols functions well as an electron donor, and ovothiol A is almost totally present in this form, giving it its special natural antioxidant capabilities. An in-vitro endothelial dysfunction model revealed hyperglycemic-induced anti-inflammatory and antioxidant effects of ovothiol A’s disulphide form [15,19].
The present study aims to investigate the therapeutic potential of ovothiol isolated from sea urchin eggs against an ethanol-induced peptic ulcer in rats.
2. Results
2.1. Molecular DFT Calculation of Ovothiol A
Figure 1 demonstrates ovothiol A’s optimized structures as the lowest energy configurations. The dipole moment and the natural charges obtained from Natural Bond Orbital Analysis (NBO) are shown on the active sites of oxygen, nitrogen, and sulfur atoms. The MEP loosely or excess electrons) charged electrostatic potential in the molecule. Both bond lengths and angles are shown in Table 1.
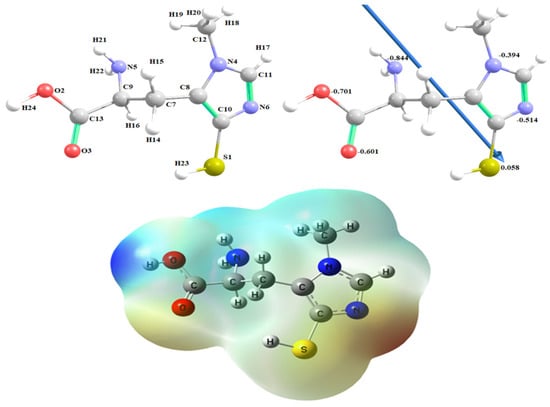
Figure 1.
The optimized structure of ovothiol A, the vector of the dipole moment, the natural charges on atoms and molecular electrostatic potential (MEP) surface by density function B3LYP/6-311G++ (dp).

Table 1.
Selected optimized bond lengths (Å) and bond angles (°) of ovothiol A.
The computed total energy of ovothiol A is −986.465 Hartree, the highest occupied molecular orbital (HOMO) energy is −5.6010 eV, the lowest unoccupied molecular orbital (LUMO) energy is −0.8496 eV and the dipole moment is 6.9314 Debye. The higher total energy negativity indicates compound stability. Also, the energy gap (Eg) = ELUMO − EHOMO = 4.7514 eV, Figure 2 and Table 2. Many reactivity descriptors such as ionization potential (I), electron affinity (A), Electronegativity (χ), chemical potential (μ), hardness (η), softness (S) and electrophilicity index (ω), all derived from the HOMO and LUMO energies, proposed to comprehend many reactivity-related features of chemical processes, Table 2.
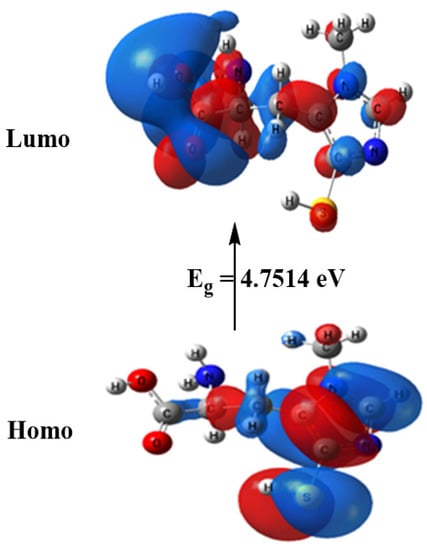
Figure 2.
HOMO and LUMO charge density maps of B3LYP/6-311G++ (dp).

Table 2.
Calculated energies and the properties of ovothiol A.
2.2. Molecular Docking Interaction between Ovothiol A with 1AFC (PDB ID: 1AFC)
The free energy binding of ovothiol A’s to the active sites of the 1AFC receptor was determined in the current investigation to be −4.3 kcal/mol, according to Table 3. The stronger the binding, the more negative the binding energy (Table 3 and Figure 3).

Table 3.
The Docking interaction data calculations of ovothiol A with 1AFC (PDB ID: 1AFC).
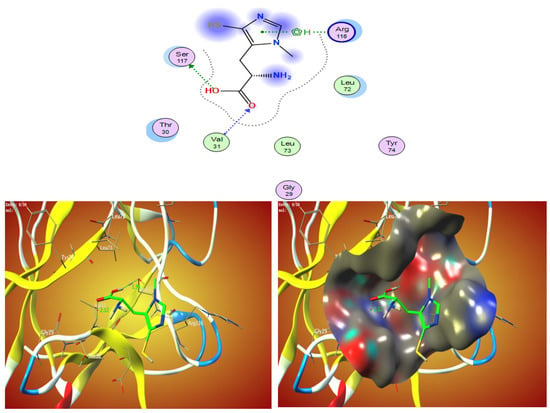
Figure 3.
The 2D and 3D molecular docking simulation studies of the interaction between ovothiol A with 1AFC. Hydrophobic interactions with amino acid residues are shown with dotted curves.
2.3. Ulcer l Markers
The gastric juice volume and ulcer index were significantly elevated (p < 0.05) while the pH was significantly reduced (p < 0.05) in the ulcer group compared to the control group. However, the ovothiol A administration with both concentrations caused a significant decrease (p < 0.05) in gastric juice volume and ulcer index compared to the ulcer group, as shown in Table 4 and Figure 4.

Table 4.
The curative potency of ovothiol A on the gastric ulcer markers in rats.
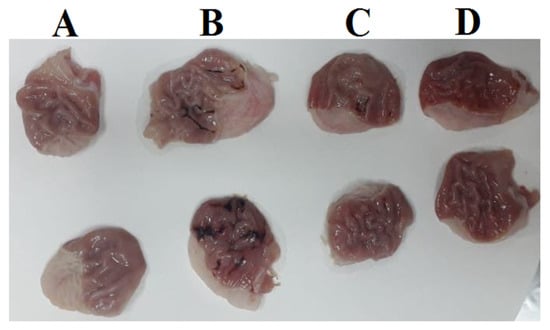
Figure 4.
Stomachs of; control rat (A), ulcer rat (B), ovothiol A (250 mg/kg) rat (C) and ovothiol A (500 mg/kg) rat (D).
2.4. Markers of Oxidative Stress
Gastric MDA level was significantly increased (p < 0.05), while GSH and GST levels were significantly reduced (p < 0.05) in the ulcer group compared to the control group. Moreover, ovothiol A treatment significantly (p < 0.05) decreased MDA levels and elevated (p < 0.05) GSH and GST levels compared to the ulcer group, as shown in Table 5.

Table 5.
The curative potency of ovothiol on oxidative stress markers of ethanol-induced gastric ulcer induced in rats.
2.5. NO, PGE-2, IL6, and Cytochrome C
Table 6 showed significant ethanol-induced elevation (p < 0.05) in the cytochrome c, PGE-2 and IL6 levels compared to the control group. The levels of the aforementioned parameters were restored near the normal level by the administration of ovothiol A with its two doses.

Table 6.
The curative potency of ovothiol on Apoptosis and inflammatory parameters in Ethanol-induced gastric ulcer in rats.
2.6. Histopathological Analysis
The control group revealed a standard histological structure characterized by a long tubular gastric gland (acini) in the mucosal layer that extended deep into the lamina propria and was covered by simple columnar epithelium (Figure 5a). The gastric acini showed the digestive enzyme-secreting zymogenic cells (chief cells) and the gastric acid-secreting parietal cells (oxyntic cells). The submucosal layer showed the blood vessels, nerves and ganglionated plexus within loose connective tissue. The muscular layer was well developed, with serosal covering the outer surface of the stomach (visceral peritoneum).
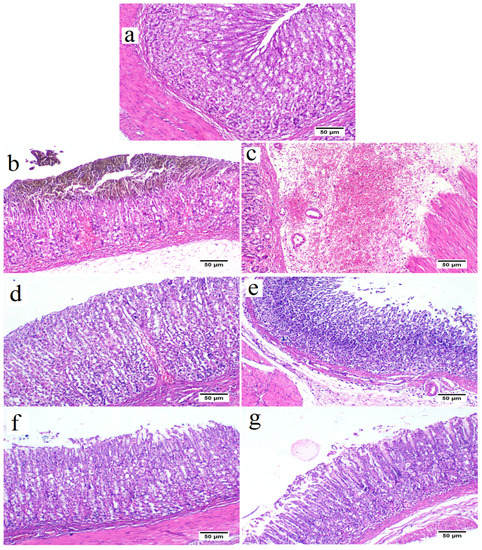
Figure 5.
Stomach from control group (a) showing normal histological structure of stomach tissue that consisted of mucous epithelium covering gastric acini (H&E stain). Stomach from ulcer group showing destruction of the mucosal covering associated with extensive hemorrhage (b), and expansion of the submucosal layer with edema, and diffuse extensive hemorrhage (c). Stomach from ovothiol A (250 mg/kg) treated group showing apparently normal gastric mucosa (d) with mild to moderate submucosal edema and lowered number of mononuclear cells infiltration (e). stomach from ovothiol A (500 mg/kg) treated group showing apparently normal gastric mucosa (f) and few mononuclear cells infiltration with mild submucosal edema (g).
In contrast, the ethanol in the ulcer group triggered a severe gastric injury (Figure 5b,c). The mucosal layer showed severe alteration and loss of the epithelial cells with hemorrhages and congested blood capillaries. The lamina epithelialis and the lamina propria were infiltrated with inflammatory cells. Some examined areas presented severe necrosis of the gastric acini with mononuclear cell infiltration. The submucosal layer was severely expanded with the dispersion of the connective tissue by inflammatory edema and severely dilated blood vessels associated with extensive hemorrhage.
The low dose (250 mg/kg) of ovothiol A reduced the histopathologic scores, attenuating gastric damage, and inflammatory cell infiltration (Figure 5d,e) and decrease gastric hemorrhage with architecture preservation of the gastric mucosa in other examined sections.
Rats received a high dose of (500 mg/kg) ovothiol resulted in the highest protection of the gastric histological structure (Figure 5f,g). Few examined sections showed a reduction of mononuclear inflammatory cells compared with the ulcer group and low dose ovothiol treated group. Most examined sections revealed normal glandular gastric mucosal surface. The total histopathological lesion score showed the peak value recorded by the ulcer group that a significant difference from other experimental groups (Figure 6). The high dose of ovothiol showed a better improvement and scored fewer histopathological lesions compared with a low dose of the same drug.
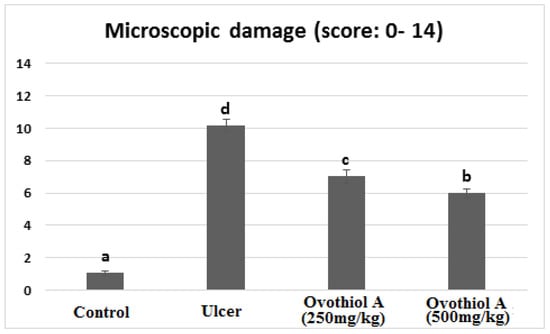
Figure 6.
Histopathological score of stomachs of control, ulcer, ovothiol A (250 mg/kg) and (500 mg/kg). Data are expressed as the mean ± SE. a, b, c and d above the error bar indicate a significant difference.
3. Discussion
Major complications such as hemorrhage, perforation, or stomach outlet obstruction affects roughly 25% of people with peptic ulcer disease [3]. People worldwide are still suffering from peptic ulcer complications and looking for safe and effective treatment. The immense potential of marine natural products has been recently revealed by developments in the discovery, approval, and therapeutic use of marine pharmaceuticals. This work investigated the anti-inflammatory and antioxidant potential of ovothiol A extracted from sea urchins against ethanol-induced gastric ulcers.
In the current study, ethanol administration increased gastric juice secretion and ulcer index and decreased pH in rats. Acid secretion, oedema, haemorrhage, necrosis, and inflammation all contributed to higher gastric juice volume and gastric ulcer index in the ulcer group, as confirmed by the histopathological investigation. The mucous membrane of the digestive tract easily absorbs ethanol. High ethanol concentrations would damage the gastric mucosa and may cause hemorrhagic gastritis within 30 min [20]. Ethanol and its byproduct, acetaldehyde, can damage the gastric mucosa’s epithelial and vascular endothelial cells [21,22,23]. Ovothiol A (250 and 500 mg/kg) showed a therapeutic effect against gastric ulcers indicated by a significantly elevated gastric pH with decreased gastric juice volume and gastric ulcer index.
Under typical physiological conditions, the body has a built-in system to maintain a healthy balance between oxidation and antioxidation [24]. One of the main reasons why gastric ulcers happen is because this system is out of balance [25]. Malondialdehyde (MDA) is the major diagnostic for evaluating lipid peroxidation. In line with prior findings, the administration of ethanol in rats resulted in a considerable elevation in gastric issue’s MDA levels [26,27]. High ethanol intake produces substantial ROS, which react with membrane lipids to produce lipid peroxides [28]. Several investigations have indicated that antioxidant substances that scavenge free radicals prevent ethanol-induced stomach ulcers [26,29]. In our study, the rat treated with ovothiol A (250 and 500 mg/kg) showed a decrease in MDA levels which indicated the antioxidant activity of ovothiol.
According to our results, ulcer groups showed a significant decrease in rats’ antioxidant system (GSH, CAT, GST and SOD), which is considered the initial defense against ethanol-induced free radical damage to gastric mucosal cells [30]. Because of the antioxidant activity of ovothiol A [31] the gastric tissues restored the antioxidant system levels.
Prostaglandin E2 (PGE2) and nitric oxide (NO) are essential mediators for maintaining gastric mucosal defense integrity and healing gastric ulcers [32]. In the current study, ethanol caused a significant decrease in NO and PGE2 levels. NO is a vasodilator factor that keeps the integrity of the gastric epithelial and mucus barriers by regulating blood flow [33]. Most importantly, NO is an important part of angiogenesis and tissue regeneration and could help heal ulcers [34]. Also, several studies found that NO plays a protective role in peptic ulcers and can speed gastric ulcer healing [35,36]. PGE2 is one of the endogenous gastric mucosa protective factors by increasing the blood flow, enhancing mucus and bicarbonate secretion, and making epithelial cells more resistant to stimuli [37]. Having oxidative damage present, which causes prostaglandins to be converted into oxidation products like 8-isoprostaglandin F2alpha, was thought to be responsible for ethanol’s suppressive effect on gastric mucosal PGE2 level [38,39].
However, treatment with ovothiol A (250 and 500 mg/kg) increased NO and PGE2 levels that protect the stomach lining against ethanol-caused ulcers.
Interleukins are important regulators of the mucosal defense barrier [40]. There is evidence that ethanol may stimulate the innate immune system, changing the levels of inflammatory cytokines, including Interleukin 6 (IL-6) [41,42]. The current data demonstrated ethanol-induced elevated levels of IL-6 in the gastric tissue that could activate oxidative stress, drive neutrophils, lymphocytes, and phagocytes in the inflammatory areas and create toxic metabolites and lysosomal enzymes, injuring the gastric mucosa [43]. Our findings showed that ovothiol A treatment with different doses decreased the IL-6 level
Moreover, apoptosis is a key mechanism in multicellular organisms for maintaining homeostasis and responding to environmental stimuli. The release of many substances from mitochondria mediates apoptosis. The interaction of the pro-apoptotic protein’s caspase-3 and -9 regulates the release of cytochrome c that may turn on or off apoptosis [44]. In the current study, ethanol administration increased cytochrome C levels in rats. It was reported that ethanol stimulated the mitochondrial signaling pathway of apoptosis in the gastric mucosa [45]. The release of cytochrome c was linked to ROS production such as H2O2 [46,47]. In our study, ovothiol A treatment (250 and 500 mg/kg) decreased the cytochrome C levels, indicating its effective gastroprotective activity. Furthermore, Zhu et al. [48] reported that fibroblast growth factors (FGFs) binding to their receptors more effectively if they are bound to sulfate. According to the current study, ovothiol A promotes the healing of gastric ulcers by stabilizing FGFs to prevent denaturation in the stomach’s acidic pH.
4. Materials and Methods
4.1. Drugs and Chemicals
All solvents, chemicals and drugs were purchased from Sigma Aldrich Co. (St. Louis, MO, USA).
4.2. Isolation of Ovothiol A from Sea Urchin Eggs
Ovothiol-A was isolated using the Russo et al. technique [49]. Prior to setting the sea urchin on the water’s surface, we injected its eggs with about 1 mL of KCl. The gathered sea urchin eggs were centrifuged at 2000 RPM for 10 min, and the supernatant was discarded. The eggs were crushed and agitated for 12 h in a mixture of 20 mL 1 M HCl and 80 mL ethanol. At 4 °C the homogenate was centrifuged for 15 min at 6000 RPM. A rotatory evaporator was used to evaporate the ethanol (at 40 °C), and the supernatant was then gathered. The lipids were eliminated from the mixture using 50 mL of diethyl ether. Through the use of an alumina column, peroxide is eliminated from the solution. The Dowex 50WX2 (1 cm × 22 cm) column was loaded with the acidic solution. Water, 0.1 M, 0.5 M, and 4 M HCl were used as elusions. The 4M fraction’s ovathiol was converted to ovathiol disulfide after being exposed to air for 4 h at a pH of 8. Before repeating the chromatography on the same Dowex column, the pH of the solution was brought down to 2. A 40 °C oven was used to dry the crystals, which resulted in colorless, glassy solid crystals. 3.5 mg of ovathiol-A is produced from each 10 g eggs. For characterization details of ovathiol-A, please refer to the Supplementary Materials.
4.3. Ovothiol-A Molecular DFT Calculation
Using the Gaussian 09 programme, density functional theory (DFT) simulations were performed to examine the equilibrium geometry of Ovathiol A at the B3LYP/6-311G++ (dp) level of theory.
4.4. Molecular Docking Interaction between Ovothiol A with 1AFC (PDB ID: 1AFC)
Utilizing the MOA2019 programme, molecular docking studies were conducted to determine the potential binding modes for the receptor 1AFC’s most active location. The proton-pump inhibitor drugs target 1AFC, which is gastric H+,K+ ATPase (the gastric acid pump).
4.5. Experimental Animals
Twenty-four male Wister rats Rattus Norvegicus with similar age (± one week) and weight (130–150 (±2 g) were used in the experiments. Steel-wire topped polycarbonate boxes were used for animal housing and were bedded with wood shavings. The animals were provided with a standard laboratory diet, and water ad libitum and kept under fixed housing and handling conditions.
4.6. Ethical Consideration
The experimental techniques and practices of the study were approved by the Faculty of Science Institutional Animal Care and Use Committee (IACUC) at Cairo University, Egypt. Under ethical approval number CUIF3120, all of the experiments were done in line with international rules for the care and use of laboratory animals.
4.7. Induction of Peptic Ulcer
All animals fasted 24 h before administration of ethanol (5 mL/kg of body weight, orally) [50].
4.8. Experimental Design
The rats were divided into four equal groups (n = 6):
- Group I (control group): Rats were administrated orally with dist. water (5 mL/kg), then after one hour, dist. water was administrated again.
- Group II (ulcer group): Rats were administrated orally with ethanol (5 mL/kg), then after one hour, dist. water was administrated again.
- Group III (ovothiol A-500): Rats were administrated orally with ethanol (5 mL/kg), then after one hour, ovothiol A (500 mg/kg) [31] was administrated.
- Group IV (ovothiol A-250): Rats were administrated with ethanol 5 mL/kg orally, then after one hour, ovothiol A (250 mg/kg) was administrated.
4.9. Animal Handling and Specimen Collection
Animals were euthanized using 3% sodium pentobarbital (100 mg/kg) overdose one hour after the last treatment [51]. The stomach was then collected and blotted with filter paper. For the biochemical study, a section of the stomach was frozen at −80 degrees Celsius. Another section of the stomach was fixed before histological analysis by suspending it in 10% formal saline.
4.10. Ulcer Markers
After collecting the gastric juice by cutting open the stomachs, the samples were centrifuged for 10 min at 3000 rpm to remove the solids from the liquid, and the supernatant volume was measured. A pH meter was used to measure the pH level of the gastric juice. Stomachs were inspected for ulcers using magnification (×10). Methods used to evaluate the ulcer index included: Below 1 mm = 1 point, 1–2 mm = 2 points, and >3 mm = 3 points for ulcer length. The ulcer index was calculated by taking the total number of points and dividing it by 10 (lens magnification) [52].
4.11. Stomach Homogenate Preparation
10% w/v of stomach tissue was homogenized in ice-cold 0.1 M Tris-HCl buffers (pH 7.4). The homogenate was centrifuged at 3000 rpm at 4 °C for 15 min, and the supernatant was biochemically analyzed.
4.12. Biochemical Parameters
Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), nitric oxide (NO) reduced glutathione (GSH), and glutathione-S-transferase (GST) were determined following the manufacturer’s instructions. Interleukin 6 (IL-6), prostaglandin E2 (PGE2) and cytochrome c were determined using the relevant enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions.
4.13. Histopathological Analysis
For each rat, a small gastric tissue section was fixed with 10% neutral buffered formalin, embedded in paraffin, cut into 5 μm thickness and stained with hematoxylin and eosin stain. The specimens were examined under Olympus BX43 light microscope, and sections were captured by Olympus DP27 camera connected to Cellsens dimensions software (Olympus). A 0–14 range was used to score the microscopic damage according to [53,54], where epithelial cell loss or the presence of inflammatory cells scored 0–3 and oedema in the upper mucosa or hemorrhagic damage scored 0–4. The total microscopic score was obtained by summating the four histopathological scores.
4.14. Statistical Significance
The statistical analysis was performed using SPSS for Windows (version 15.0). Data were expressed in the form of means ± standard error of the mean (SEM). The one-way analysis of variance (ANOVA) was used to perform within-group comparisons, and the Duncan post hoc test was used to compare the group means. p values < 0.05 were considered statistically significant.
5. Conclusions
The current study revealed that ovothiol A exhibited antiulcerogenic activity by reducing gastric juice volume, ulcer index, MDA, IL-6, and cytochrome c, increasing gastric juice pH, GSH, CAT, GST, SOD, and NO, and improving gastric mucosa architecture. The therapeutic pathways of ovothiol A include scavenging free radicals, inhibition of inflammation, regulation of apoptosis, and promotion of the healing of stomach ulcers by stabilizing fibroblast growth factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21010025/s1, Supplementary Material: Characterization of ovothiol A.
Author Contributions
Conceptualization, M.R.S. and A.S.M.; methodology, A.T.S. and A.S.M.; software, H.I.S.; validation, M.R.S.; formal analysis, A.T.S. and A.S.M.; investigations.; resources, H.I.S. and T.A.; data curation, A.T.S.; writing—original draft preparation, A.T.S.; writing—review and editing, M.R.S. and T.A.; visualization, H.I.S.; supervision, A.S.M. and H.I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The experimental techniques and practices of the study were approved by the Faculty of Science Institutional Animal Care and Use Committee (IACUC) at Cairo University, Egypt. All the experimental procedures followed the international guidelines for laboratory animal care and use (CUIF3120).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PUD | Peptic ulcer disease |
| ROS | Reactive oxygen species |
| NSAIDs | Non-steroidal anti-inflammatory medicines |
| PPIs | Proton pump inhibitors |
| MEP | Molecular electrostatic potential |
| MDA | Malondialdehyde |
| PGE2 | Prostaglandin E2 |
| NO | Nitric oxide |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH | Reduced glutathione |
| GST | Glutathione-S-transferase |
| IL-6 | Interleukin 6 |
| FGFs | Fibroblast growth factors |
| H. pylori | Helicobacter pylori |
References
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Chan, F. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ren, K.; Zhou, Z.; Dang, C.; Zhang, H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: A population-based study. BMC Gastroenterol. 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Ahmad, M.A.; Sumbul, S.; Asif, M. Role of phenolic compounds in peptic ulcer: An overview. J. Pharm. Bioallied Sci. 2011, 3, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A. Time trends of ulcer mortality in Europe. Gastroenterology 2007, 132, 2320–2327. [Google Scholar] [CrossRef]
- Sonnenberg, A. Time trends of ulcer mortality in non-European countries. Am. J. Gastroenterol. 2007, 102, 1101–1107. [Google Scholar] [CrossRef]
- Lanas, A.; Garcia-Rodriguez, L.A.; Polo-Tomas, M.; Ponce, M.; Quintero, E.; PerezAisa, M.A.; Gisbert, J.P.; Bujanda, L.; Castro, M.; Calvet, X.; et al. The changing face of hospitalization due to gastrointestinal bleeding and perforation. Aliment. Pharmacol. Ther. 2011, 33, 385–391. [Google Scholar] [CrossRef]
- Malmi, H.; Kautiainen, H.; Vitra, L.J.; Farkkila, N.; Koakenpato, J.; Farkkila, M.A. Incidence and complications of peptic ulcer disease requiring hospitalization have markedly decreased in Finland. Aliment. Pharmacol. Ther. 2014, 39, 496–506. [Google Scholar] [CrossRef]
- Leow, A.H.; Lim, Y.Y.; Liew, W.C.; Goh, K.L. Time trends in upper gastrointestinal diseases and Helicobacter pylori infection in a multiracial Asian population—A 20-year experience over three time periods. Aliment. Pharmacol. Ther. 2016, 43, 831–837. [Google Scholar] [CrossRef]
- Sherif, M.; Kamel, R.; Kamel, E.; Ahmed, S.K. Role of boswellic acid in the treatment of peptic ulcer disease. Al-Azhar J. Pharm. Sci. 2019, 60, 122–145. [Google Scholar] [CrossRef][Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engstrom-Ost, J. The relevance of marine chemical ecology to plankton and ecosystem function: An emerging field. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.H.; Cerella, C.; Dicato, M.; Diederich, M.A. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules 2013, 18, 3641–3673. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, I.; Castellano; Napolitano, A. Ovothiol: A potent natural antioxidant from marine organisms. In Blue Biotechnology. Production and Use of Marine Molecules. Part 2: Marine Molecules for Disease Treatment/Prevention and for Biological Research (581–608); La Barre, E.S., Bates, S.S., Eds.; Wiley VCH: Weinheim, Germany, 2018. [Google Scholar]
- Castellano, I.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Pipino, C.; Formoso, G.; Pandolfi, A. Anti-inflammatory activity of marine ovothiol A in an in vitro model of endothelial dysfunction induced by hyperglycemia. Oxidative Med. Cell. Longevity 2018, 2018, 2087373. [Google Scholar] [CrossRef]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef]
- Holler, T.P.; Hopkins, P.B. Ovothiols as biological antioxidants. The thiol groups of ovothiol and glutathione are chemically distinct. J. Am. Chem. Soc. 1988, 110, 4837–4838. [Google Scholar] [CrossRef]
- Shapiro, B.M.; Turner, E. Oxidative stress and the role of novel thiol compounds at fertilization. BioFactors 1988, 1, 85–88. [Google Scholar]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Oxidative Molecular Mechanisms Underlying Liver Diseases: From Systems Biology to the Personalized Medicine. Oxidative Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Mei, N.; Ma, C.; Lou, Z.; Lv, W.; He, G. Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol. 2018, 107, 230–235. [Google Scholar] [CrossRef]
- de Araújo, E.; Guerra, G.; Araújo, D.; de Araújo, A.; Fernandes, J.; Júnior, R.D.A.; da Silva, V.; de Carvalho, T.; Ferreira, L.; Zucolotto, S. Gastroprotective and antioxidant activity of Kalanchoe brasiliensis and Kalanchoe pinnata leaf juices against indomethacin and ethanol-induced gastric lesions in rats. Int. J. Mol. Sci. 2018, 19, 1265. [Google Scholar] [CrossRef]
- Xue, Z.; Shi, G.; Fang, Y.; Liu, X.; Zhou, X.; Feng, S.; Zhao, L. Protective effect of polysaccharides from Radix hedysari on gastric ulcers induced by acetic acid in rats. Food Funct. 2019, 10, 3965–3976. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, H.; Li, S.; Han, B.; Liu, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Zhang, Z. Sulforaphane prevents chromiuminduced lung injury in rats via activation of the Akt/GSK-3β/ Fyn pathway. Environ. Pollut. 2020, 259, 113812. [Google Scholar] [CrossRef]
- Ding, W.; Hong, L. Progress research on relationship between oxidative stress and collagen metabolism diseases. J. Jilin Univ. (Med. Ed.) 2013, 39, 1302–1306. [Google Scholar]
- Suo, H.; Zhao, X.; Qian, Y.; Sun, P.; Zhu, K.; Li, J.; Sun, B. Lactobacillus fermentum Suo attenuates HCl/ethanol induced gastric injury in mice through its antioxidant effects. Nutrients 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of mda increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, X.; Gou, L.; Fu, X.; Li, S.; Lan, N.; Yin, X. protective effect of L-citrulline against ethanol-induced gastric ulcer in rats. Environ. Toxicol. Pharmacol. 2012, 34, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, J.; Long, X.; Pan, Y.; Mu, J.; Park, K.Y.; Zhao, X. Lactobacillus plantarum ZS62 alleviates alcohol-induced gastric injury in mice via an anti-oxidative mechanism. Drug Des. Dev. Ther. 2021, 15, 1667–1676. [Google Scholar] [CrossRef]
- Abdulla, M.; Ahmed, K.-A.; Al-Bayaty, F.; Masood, Y. Gastroprotective effect of Phyllanthus niruri leaf extract against ethanol-induced gastric mucosal injury in rats. Afr. J. Pharm. Pharmacol. 2010, 4, 226–230. [Google Scholar]
- Sun, Y.; Ma, N.; Yi, J.; Zhou, L.; Cai, S. Astroprotective effect and mechanisms of Chinese Sumac fruits (Rhus chinensis Mill.) on ethanol-induced gastric ulcers in mice. Food Funct. 2021, 12, 12565–12579. [Google Scholar] [CrossRef]
- Madany, N.M.; Shehata, M.R.; Mohamed, A.S. Ovothiol-a isolated from sea urchin eggs suppress oxidative stress, inflammation, and dyslipidemia resulted in restoration of liver activity in cholestatic rats. Biointerface Res. Appl. Chem. 2022, 12, 8152–8162. [Google Scholar]
- Sánchez-Mendoza, M.E.; López-Lorenzo, Y.; Cruz-Antonio, L.; Matus-Meza, A.-S.; Sánchez-Mendoza, Y.; Arrieta, J. Gastroprotection of Calein D against ethanol-induced gastric lesions in mice: Role of prostaglandins, nitric oxide and sulfhydryls. Molecules 2019, 24, 622. [Google Scholar] [CrossRef]
- Liang, T.-Y.; Deng, R.-M.; Li, X.; Xu, X.; Chen, G. The role of nitric oxide in peptic ulcer: A narrative review. Med. Gas Res. 2021, 11, 42–45. [Google Scholar] [PubMed]
- Moawad, H.; El Awdan, S.A.; Sallam, N.A.; El-Eraky, W.I.; Alkhawlani, M.A. Gastroprotective effect of Cilostazol against ethanol-and pylorus ligation–induced gastric lesions in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Brzozowski, T.; Majka, J.; Pytko-polonczyk, J.; Stachura, J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. Eur. J. Pharmacol. 1993, 239, 215–217. [Google Scholar] [CrossRef]
- El-Abhar, H.S. Coenzyme Q10: A novel gastroprotective effect via modulation of vascular permeability, prostaglandin E2, nitric oxide and redox status in indomethacin-induced gastric ulcer model. Eur. J. Pharmacol. 2010, 649, 314–319. [Google Scholar] [CrossRef]
- Badawy, S.A.; Ogaly, H.A.; Abd-Elsalam, R.M.; Azouz, A.A. Benzyl isothiocyanates modulate inflammation, oxidative stress, and apoptosis via Nrf2/HO-1 and NF-κB signaling pathways on indomethacin-induced gastric injury in rat. Food Funct. 2021, 12, 6001–6013. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, F.; Shen, W.; Fu, A.; Zheng, L.; Yan, Z. Protective effects of DIDS against ethanol-induced gastric mucosal injury in rats. Acta Biochim. Biophys. Sin. 2009, 41, 301–308. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Rizk, S.M.; Shahin, N.N. Gastroprotective effect of Crocin in ethanol-induced gastric injury in rats. Chem.-Biol. Interact. 2015, 229, 26–35. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yin, J.Y.; Zhao, M.M.; Liu, S.Y.; Nie, S.P.; Xie, M.Y. Gastroprotective activity of polysaccharide from Hericium erinaceus against ethanol-induced gastric mucosal lesion and pylorus ligation-induced gastric ulcer, and its antioxidant activities. Carbohydr. Polym. 2018, 186, 100–109. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Scand. J. Gastroenterol. 2009, 27, 897–906. [Google Scholar] [CrossRef]
- Salga, M.S.; Ali, H.M.; Abdulla, M.A.; Abdelwahab, S.I. Gastroprotective activity and mechanism of novel dichlorido-zinc(II)-4-(2-(5-methoxybenzylideneamino)ethyl)piperazin-1-iumphenolate complex on ethanolinduced gastric ulceration. Chem.-Biol. Interact. 2012, 195, 144–153. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.; Nie, S.; Tong, W.; Yin, J. Gastroprotective effect of gamma-aminobutyric acid against ethanol-induced gastric mucosal injury. Chem.-Biol. Interact. 2017, 272, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, Y.M.; Wang, D.C.; Chiu, C.C.; Lin, W.T.; Huang, C.Y.; Hsu, M.C. Cytoprotective effect of American ginseng in a rat ethanol gastric ulcer model. Molecules 2013, 19, 316–326. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Tomisato, W.; Takano, T.; Rokutan, K.; Tsuchiya, T.; Mizushima, T. Gastric irritant-induced apoptosis in guinea pig gastric mucosal cells in primary culture. Biochim. Biophys. Acta 2002, 1589, 168–180. [Google Scholar] [CrossRef]
- Maity, P.; Bindu, S.; Dey, S.; Goyal, M.; Alam, A.; Pal, C.; Mitra, K.; Bandyopadhyay, U. Indomethacin, a non-steroidalanti-inflammatory drug, develops gastropathy by inducingreactive oxygen species-mediated mitochondrial pathology andassociated apoptosis in gastric mucosa: A novel role of mito-chondrial aconitase oxidation. J. Biol. Chem. 2009, 284, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Bindu, S.; Dey, S.; Goyal, M.; Alam, A.; Pal, C.; Reiter, R.; Bandyopadhyay, U. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J. Pineal Res. 2009, 46, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hsu, B.T.; Rees, D.C. Structural studies of the binding of the anti-ulcer drug sucrose octasulfate to acidic fibroblast growth factor. Structure 1993, 1, 27–34. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Castellano, I.; Napolitano, A.; Palumbo, A. Ovothiol isolated from sea urchin oocytes induces autophagy in the Hep-G2 cell line. Mar. Drugs 2014, 12, 4069–4085. [Google Scholar] [CrossRef]
- Sistani Karampour, N.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of Zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Hosney, M.; Bassiony, S.; Hassanein, S.S.; Soliman, A.M.; Fahmy, S.R.; Gaafar, K. Sodium pentobarbital dosages for exsanguination afect biochemical, molecular and histological measurements in rats. Sci. Rep. 2020, 10, 378. [Google Scholar] [CrossRef]
- Schmidt, M.; Polednik, C.; Roller, J.; Hagen, R. Galium verum aqueous extract strongly inhibits the motility of head and neck cancer cell lines and protects mucosal keratinocytes against toxic DNA damage. Oncol. Rep. 2014, 32, 296–1302. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.S.; Maghrabi, I.A. Diosmin protects against ethanol-induced gastric injury in rats: Novel anti-ulcer actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef] [PubMed]
- Loren, L.; Weinstein, W.M. Histology of alcoholic hemorrhagic “gastritis”: A prospective evaluation. Gastroenterology 1988, 94, 1254–1262. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).