Preparation and Characterization of Tilapia Collagen-Thermoplastic Polyurethane Composite Nanofiber Membranes
Abstract
1. Introduction
2. Results and Discussion
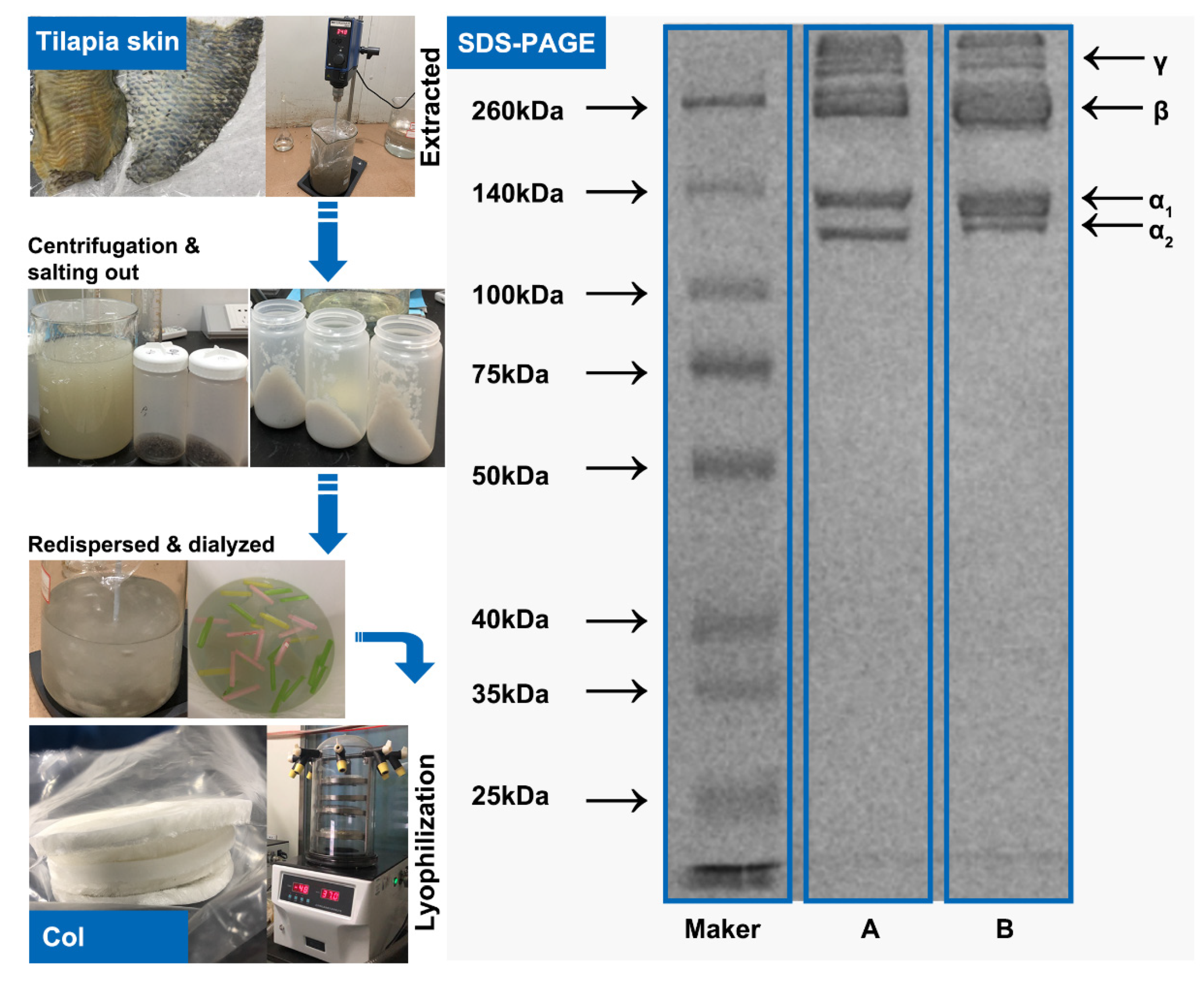
2.1. Collagen Structure Identification
2.2. Structural Analysis of Col-TPU Composite Nanofiber Membranes
2.2.1. Scanning Electron Microscope (SEM) Analysis
2.2.2. FTIR
Main Characteristic Peak
Secondary Structure
2.2.3. XRD
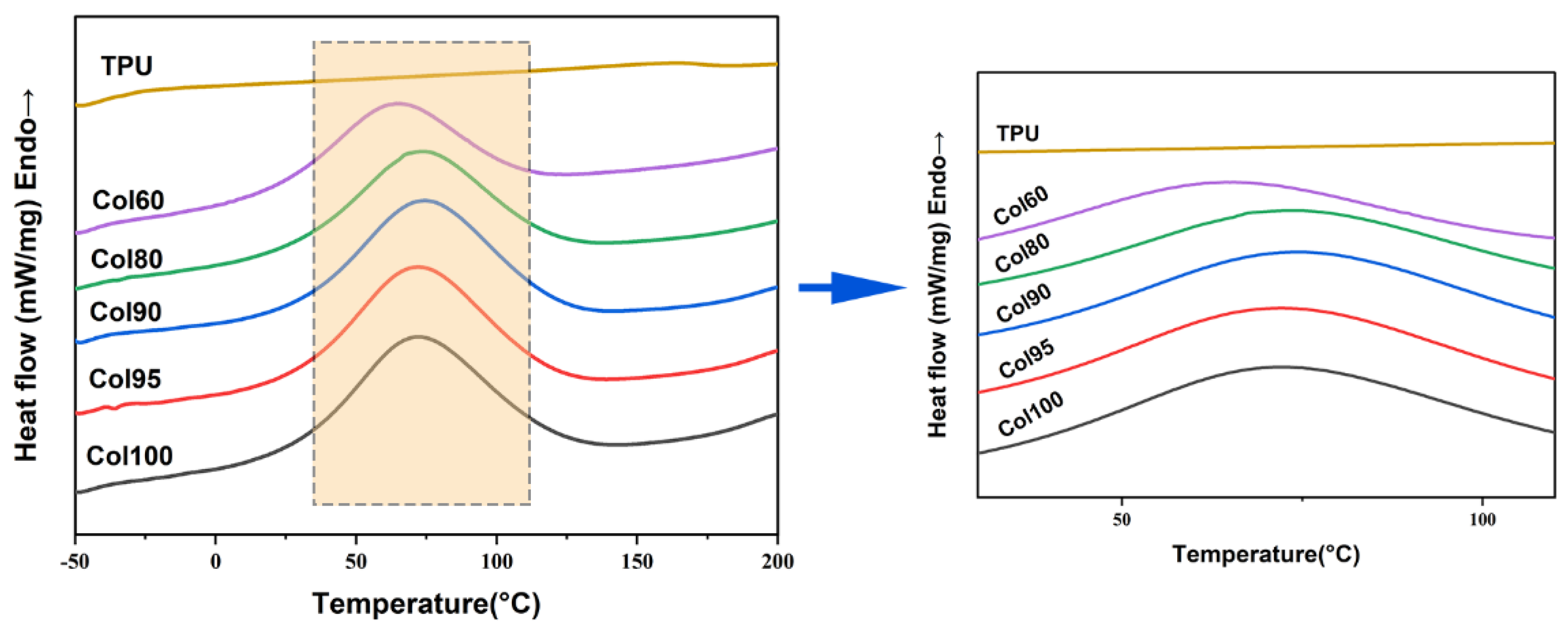
2.3. Differential Scanning Calorimetry (DSC)
2.4. TGA
2.5. Water Contact Angle (WCA)
2.6. Mechanical Properties
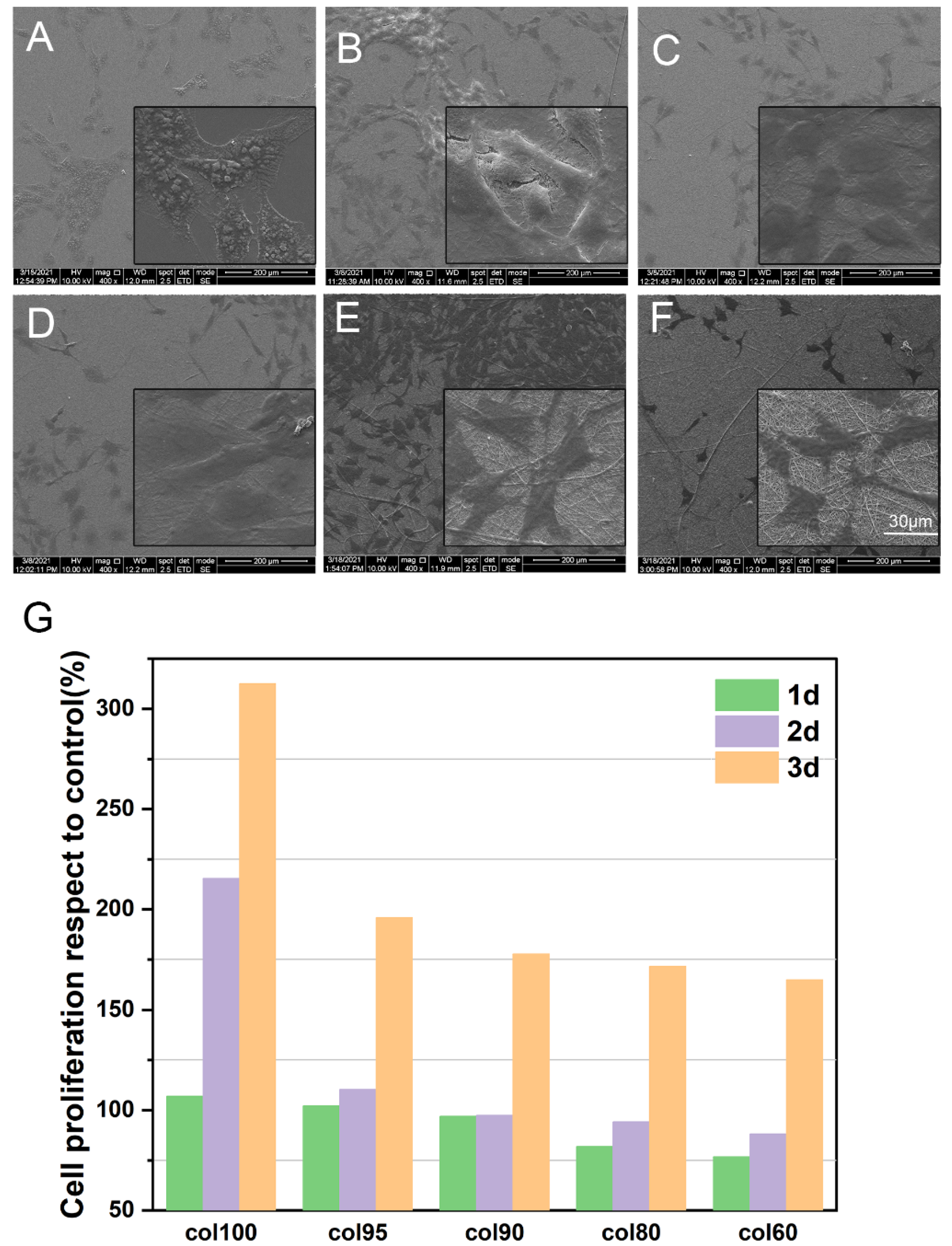
2.7. Cytocompatibility
2.7.1. Cell Proliferation and Cytotoxicity
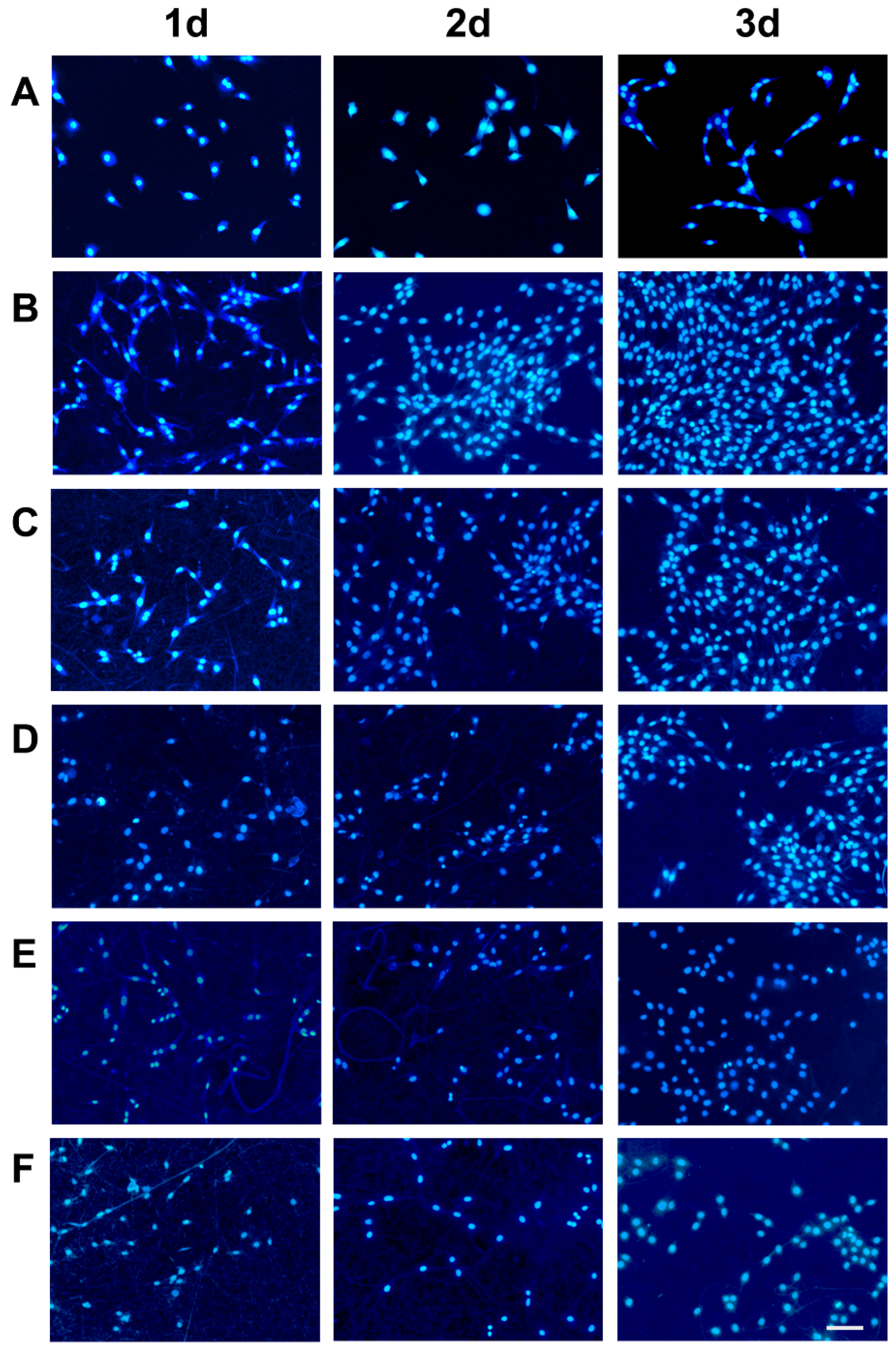
2.7.2. Cell Morphology
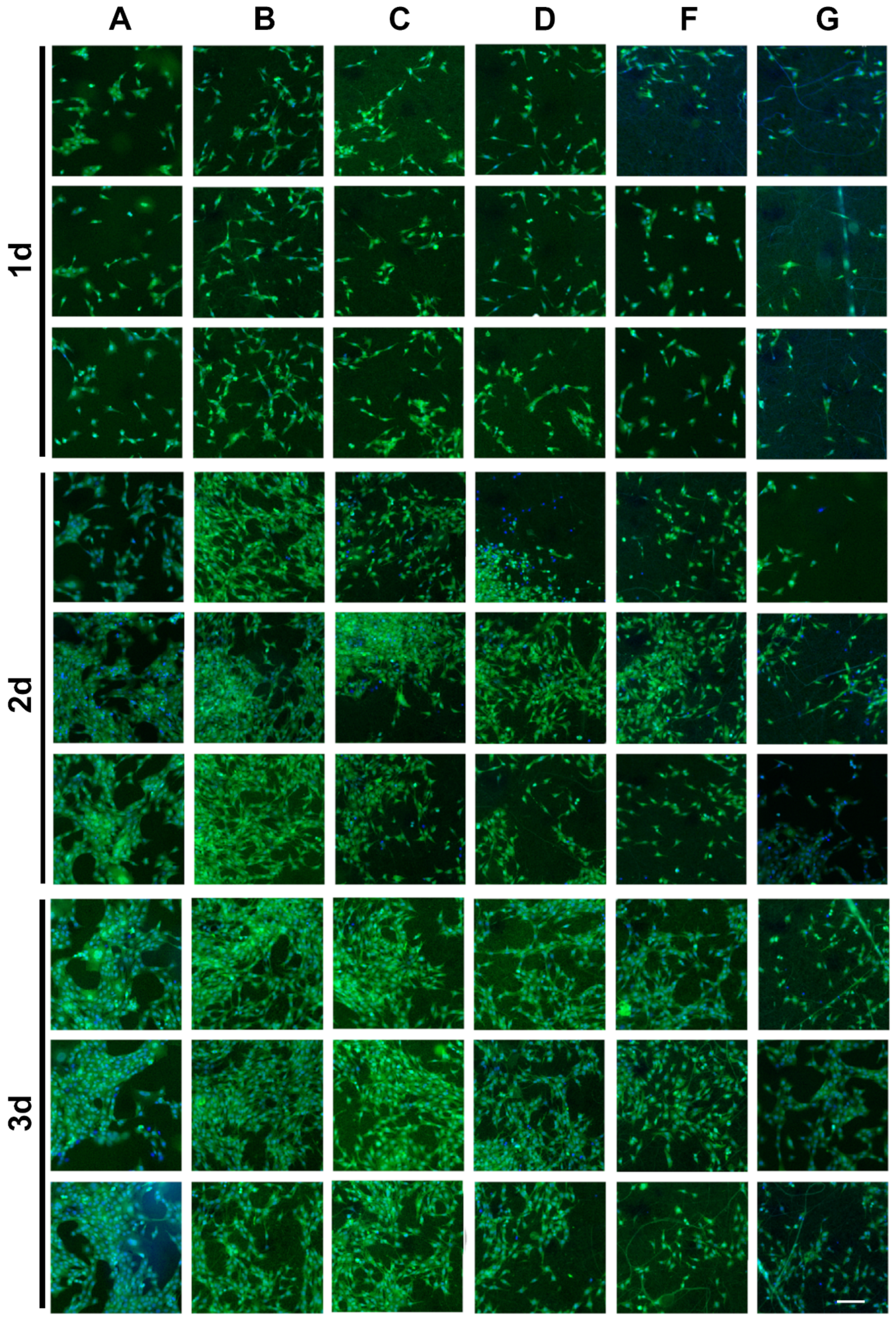
2.7.3. Cell Adhesion
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of Collagen
3.3. Collagen-Based Composite Electrospun Fiber Membranes
3.4. Structural Analysis of Col-TPU Nanofiber Membranes
3.4.1. SEM
3.4.2. FTIR
3.4.3. XRD
3.5. DSC
3.6. Thermal Stability
3.7. WCA
3.8. Mechanical Properties
3.9. Cytocompatibility
3.9.1. Cell Proliferation
3.9.2. Cell Morphology
3.9.3. Cell Adhesion
3.10. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hart, D.A.; Nakamura, N.; Shrive, N.G. Perspective: Challenges presented for regeneration of heterogeneous musculoskeletal tissues that normally develop in unique biomechanical environments. Front. Bioeng. Biotechnol. 2021, 9, 760273. [Google Scholar] [CrossRef] [PubMed]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Synthetic-based blended electrospun scaffolds in tissue engineering applications. J. Mater. Sci. 2022, 57, 4020–4079. [Google Scholar] [CrossRef]
- Dorthe, E.W.; Williams, A.B.; Grogan, S.P.; D’Lima, D.D. Pneumatospinning biomimetic scaffolds for meniscus tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 810705. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regener. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; MacKnight, C. Clinical implications of bovine spongiform encephalopathy. Clin. Infect. Dis. 2019, 32, 1726–1731. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Zou, Y.F.; Jiang, S.; Xu, J.M.; Jiang, S.H.; Hu, Q.H. Chromatographic separation and physicochemical properties of collagen species in the skin of deep-sea redfish (Sebastes mentella). Food Hydrocoll. 2011, 25, 1134–1138. [Google Scholar] [CrossRef]
- Chen, J.D.; Wang, G.Y.; Li, Y.S. Preparation and characterization of thermally stable collagens from the scales of lizardfish (Synodus macrops). Mar. Drugs 2021, 19, 597. [Google Scholar] [CrossRef]
- Yunoki, S.; Hatayama, H.; Ohyabu, Y.; Kobayashi, K. Fibril matrices created with collagen from the marine fish barramundi for use in conventional three-dimensional cell culture. Int. J. Biol. Macromol. 2022, 203, 361–368. [Google Scholar] [CrossRef]
- Huang, R.Q.; Chen, S.W.; Ma, G.Z.; Yang, B.W.; Guo, R.J.; Li, Q.X.; Zhong, J.Y. Research on the extraction of collagen from scales of tilapia. Adv. Mater. Res. 2011, 295–297, 796–799. [Google Scholar] [CrossRef]
- Liu, C.W.; Hsieh, C.Y.; Chen, J.Y. Investigations on the wound healing potential of tilapia piscidin (TP)2-5 and TP2-6. Mar. Drugs 2022, 20, 205. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.S.; Wang, H.N.; Li, J.; Liu, H.H.; Yin, Y.H.; Zhang, N.L.; Qin, S. Comprehensive assessment of nile tilapia skin (Oreochromis niloticus) collagen hydrogels for wound dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zheng, D.X.; Yang, G.Y.; Li, W.; Yang, H.; Jiang, Q.; Chen, Y.J.; Zhang, Y.X.; Xie, X. Tilapia skin peptides ameliorate diabetic nephropathy in STZ-induced diabetic rats and HG-induced GMCs by Improving mitochondrial dysfunction. Mar. Drugs 2020, 18, 363. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Zou, Q.; Huang, J.H.; Zuo, Y.; Li, Y.B.; Wang, S.P.; Cheng, L.; Man, Y.; Yang, F.; et al. Fish collagen and hydroxyapatite reinforced poly(lactide-co-glycolide) fibrous membrane for guided bone regeneration. Biomacromolecules 2019, 20, 2058–2067. [Google Scholar] [CrossRef]
- He, X.L.; Wang, L.; Lv, K.N.; Li, W.J.; Qin, S.; Tang, Z.H. Polyethylene oxide assisted fish collagen-poly-epsilon-caprolactone nanofiber membranes by electrospinning. Biomacromolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- Zhou, T.; Sui, B.Y.; Mo, X.M.; Sun, J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: Fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3495–3507. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Wang, X.Y.; Huang, H. Thermoplastic polyurethane–urea elastomers with superior mechanical and thermal properties prepared from alicyclic diisocyanate and diamine. J. Appl. Polym. Sci. 2020, 137, 49575. [Google Scholar] [CrossRef]
- Tatai, L.; Moore, T.G.; Adhikari, R.; Malherbe, F.; Jayasekara, R.; Griffiths, I.; Gunatillake, P.A. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef]
- Enayati, M.; Eilenberg, M.; Grasl, C.; Riedl, P.; Kaun, C.; Messner, B.; Walter, I.; Liska, R.; Schima, H.; Wojta, J.; et al. Biocompatibility assessment of a new biodegradable vascular graft via in vitro co-culture approaches and in vivo model. Ann. Biomed. Eng. 2016, 44, 3319–3334. [Google Scholar] [CrossRef]
- Xu, C.C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2022, 15, 250–271. [Google Scholar] [CrossRef]
- Lee, J.K.; Kang, S.I.; Kim, Y.J.; Kim, M.J.; Heu, M.S.; Choi, B.D.; Kim, J.S. Comparison of collagen characteristics of sea- and freshwater-rainbow trout skin. Food Sci. Biotechnol. 2016, 25, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Anton, E.D.; Iosageanu, A.; Berger, D.; Matei, C.; Mitran, R.A.; Negreanu-Pirjol, T.; Craciunescu, O.; Moldovan, L. Enhanced wound healing activity of undenatured type I collagen isolated from discarded skin of black sea gilthead bream (Sparus aurata) conditioned as 3d porous dressing. Chem. Biodivers. 2021, 18, e2100293. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Shakir, K.A.; Walsh, M.K. Functional properties of collagen extracted from catfish (Silurus triostegus) waste. Foods 2022, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.C.; Du, H.; Wen, J.X.; Zhong, C.; Liu, G.M.; Miao, S.; Cao, M.J. Physicochemical properties of acid-soluble collagens from different tissues of large yellow croaker (Larimichthys crocea). Int. J. Food Sci. Technol. 2021, 56, 5371–5381. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, L.J.; Chen, A.L.; Wu, F.J. Improvement of mechanical property for PLA/TPU blend by adding pla-tpu copolymers prepared via in situ ring-opening polymerization. Polymers 2022, 14, 1530. [Google Scholar] [CrossRef]
- Džunuzović, J.V.; Stefanović, I.S.; Džunuzović, E.S.; Dapčević, A.; Šešlija, S.I.; Balanč, B.D.; Lama, G.C. Polyurethane networks based on polycaprolactone and hyperbranched polyester: Structural, thermal and mechanical investigation. Prog. Org. Coat. 2019, 137, 105305. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, L.; Yi, R.Z.; Xu, N.H.; Gao, R.; Hong, B.H. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT-Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Jalan, A.; Kastner, D.W.; Webber, K.G.I.; Smith, M.S.; Price, J.L.; Castle, S.L. Bulky dehydroamino acids enhance proteolytic stability and folding in beta-hairpin peptides. Org. Lett. 2017, 19, 5190–5193. [Google Scholar] [CrossRef]
- Lin, T.A.; Lin, J.-H.; Bao, L. A study of reusability assessment and thermal behaviors for thermoplastic composite materials after melting process: Polypropylene/thermoplastic polyurethane blends. J. Clean. Prod. 2021, 279, 123473. [Google Scholar] [CrossRef]
- Pei, Y.; Jordan, K.E.; Xiang, N.; Parker, R.N.; Mu, X.; Zhang, L.; Feng, Z.B.; Chen, Y.; Li, C.M.; Guo, C.C.; et al. Liquid-exfoliated mesostructured collagen from the bovine achilles tendon as building blocks of collagen membranes. ACS Appl. Mater. Interfaces 2021, 13, 3186–3198. [Google Scholar] [CrossRef]
- Fang, C.Q.; Yang, R.; Zhang, Z.S.; Zhou, X.; Lei, W.Q.; Cheng, Y.L.; Zhang, W.; Wang, D. Effect of multi-walled carbon nanotubes on the physical properties and crystallisation of recycled PET/TPU composites. RSC Adv. 2018, 8, 8920–8928. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Ci, M.Y.; Sui, S.Y.; Zhu, P. Study on the rheological properties of regenerated cellulose/thermoplastic polyurethane blend spinning solutions. Ferroelectrics 2020, 562, 104–113. [Google Scholar] [CrossRef]
- Lopes, M.S.; Catelani, T.A.; Nascimento, A.L.; Garcia, J.S.; Trevisan, M.G. Ketoconazole: Compatibility with pharmaceutical excipients using DSC and TG techniques. J. Therm. Anal. Calorim. 2019, 141, 1371–1378. [Google Scholar] [CrossRef]
- Yehia, A.A.; Mansour, A.A.; Stoll, B. Detection of compatibility of some rubber blends by DSC. J. Therm. Anal. Calorim. 1997, 48, 1299–1310. [Google Scholar] [CrossRef]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Saha, P.; Khomlaem, C.; Aloui, H.; Kim, B.S. Biodegradable polyurethanes based on castor oil and poly (3-hydroxybutyrate). Polymers 2021, 13, 1387. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Jing, X.; Li, X.; Jiang, Y.F.; Zhao, R.H.; Ding, Q.J.; Han, W.J. Excellent coating of collagen fiber/chitosan-based materials that is water- and oil-resistant and fluorine-free. Carbohydr. Polym. 2021, 266, 118173. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Qiu, D. Design of selective cell migration biomaterials and their applications for tissue regeneration. J. Mater. Sci. 2020, 56, 4080–4096. [Google Scholar] [CrossRef]
- Arhant, M.; Gall, M.L.; Gac, P.-Y.L. Fracture test to accelerate the prediction of polymer embrittlement during aging-case of PET hydrolysis. Polym. Degrad. Stab. 2022, 196, 109848. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Assanah, F.; Khan, Y. Cell responses to physical forces, and how they inform the design of tissue-engineered constructs for bone repair: A review. J. Mater. Sci. 2018, 53, 5618–5640. [Google Scholar] [CrossRef]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimovic, S.; Paul, A.; Camci-Unal, G.; Mofrad, M.R.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.M.; Walker, C.J.; Maples, M.M.; Randolph, M.A.; Bryant, S.J.; Anseth, K.S. Mechanobiological interactions between dynamic compressive loading and viscoelasticity on chondrocytes in hydrazone covalent adaptable networks for cartilage tissue engineering. Adv. Healthc. Mater. 2021, 10, 2002030. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Zhang, E.W.; Xu, L.; Wei, S.C. Electrospun PCL/PLA/HA based nanofibers as scaffold for osteoblast-like cells. J. Nanosci. Nanotechnol. 2010, 10, 7747–7751. [Google Scholar] [CrossRef]
- Zheng, X.; Ke, X.; Yu, P.; Wang, D.Q.; Pan, S.Y.; Yang, J.J.; Ding, C.M.; Xiao, S.M.; Luo, J.; Li, J.S. A facile strategy to construct silk fibroin based GTR membranes with appropriate mechanical performance and enhanced osteogenic capacity. J. Mater. Chem. B 2020, 8, 10407–10415. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, S.; Lu, J.F.; Jiang, S.T.; Lin, L. Characterization of polycaprolactone/collagen fibrous scaffolds by electrospinning and their bioactivity. Int. J. Biol. Macromol. 2015, 76, 94–101. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.J.; Luo, B.H.; Wen, W.; Liu, M.X.; Wang, X.Y.; Zhou, C.R. Electrospun composite nanofiber membrane of poly(l-lactide) and surface grafted chitin whiskers: Fabrication, mechanical properties and cytocompatibility. Carbohydr. Polym. 2016, 147, 216–225. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Bian, T.R.; Zhang, H.; Xing, H.Y. Preparation and biological properties of collagen/nano-hydroxyapatite composite nanofibers based on ordered nano-hydroxyapatite ceramic fibers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 124802. [Google Scholar] [CrossRef]
- Li, L.Y.; Zhao, Y.Q.; He, Y.; Chi, C.F.; Wang, B. Physicochemical and antioxidant properties of acid- and pepsin-soluble collagens from the scales of miiuy croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Li, L.; Yi, R.Z.; Gao, R.; He, J.L. Release kinetics of tilapia scale collagen I peptides during tryptic hydrolysis. Food Hydrocoll. 2018, 77, 931–936. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Y.C.; Stiadle, J.; Wang, X.F.; Wang, L.X.; Li, Q.; Shen, C.Y.; Thibeault, S.L.; Turng, L.S. Electrospun nanofibrous thermoplastic polyurethane/poly(Glycerol sebacate) hybrid scaffolds for vocal fold tissue engineering applications. Mater. Sci. Eng. C 2019, 94, 740–749. [Google Scholar] [CrossRef]
- Cong, K.; He, J.Y.; Yang, R.J. Using twin screw extrusion reaction (TSER) to produce thermoplastic polyurethane (TPU): Tunable, stoichiometric and eco-friendly. Polym. Adv. Technol. 2021, 32, 3495–3504. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of different crosslinking agents on hybrid biomimetic collagen-hydroxyapatite composites for regenerative medicine. Int. J. Biol. Macromol. 2018, 106, 739–748. [Google Scholar] [CrossRef]
- Lalia, B.S.; Janajreh, I.; Hashaikeh, R. A facile approach to fabricate superhydrophobic membranes with low contact angle hysteresis. J. Membr. Sci. 2017, 539, 144–151. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Wang, W.; Qi, D.P.; Luo, Y.F.; Liu, Y.R.; Xu, Y.; Cui, F.Y.; Wang, C.; Chen, X.D. Calcinable polymer membrane with revivability for efficient oily-water remediation. Adv. Mater. 2018, 30, 1801870. [Google Scholar] [CrossRef]
- Yuan, M.Q.; Dai, F.F.; Li, D.; Fan, Y.Q.; Xiang, W.; Tao, F.H.; Cheng, Y.X.; Deng, H.B. Lysozyme/collagen multilayers layer-by-layer deposited nanofibers with enhanced biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2020, 112, 110868. [Google Scholar] [CrossRef]
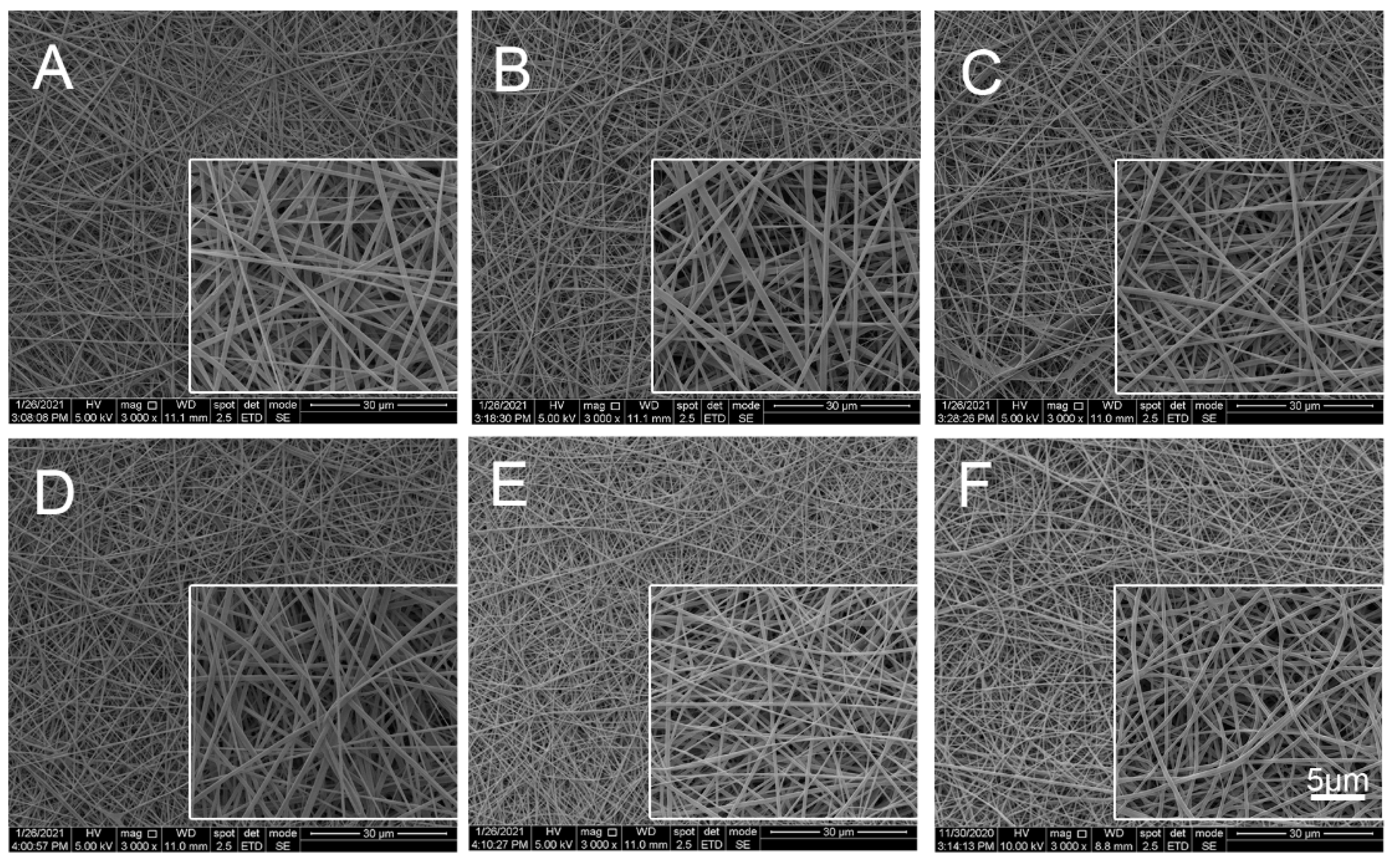



| Ratio (Col:TPU) | Abbreviation | Average Diameter (nm) | Porosity (%) |
|---|---|---|---|
| 100:0 | Col00 | 379.96 ± 134.28 | 46.59 |
| 95:5 | Col95 | 378.40 ± 151.87 | 47.29 |
| 90:10 | Col90 | 316.80 ± 94.51 | 48.81 |
| 80:20 | Col80 | 313.80 ± 102.88 | 49.15 |
| 60:40 | Col60 | 232.94 ± 87.82 | 52.89 |
| Samples | Thickness (mm) | Width (mm) | Breaking Strength (cN) | Tensile Strain (%) | Tensile Strength (MPa) |
|---|---|---|---|---|---|
| Col100 | 10.50 | 0.0242 | 36.21 | 3.20 | 1.40 |
| Col95 | 9.80 | 0.0264 | 43.26 | 8.90 | 1.64 |
| Col90 | 10.20 | 0.0200 | 44.06 | 16.50 | 2.12 |
| Col80 | 10.60 | 0.0190 | 51.10 | 41.90 | 2.49 |
| Col60 | 10.40 | 0.0174 | 56.28 | 48.30 | 3.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Yang, L.; Chen, J. Preparation and Characterization of Tilapia Collagen-Thermoplastic Polyurethane Composite Nanofiber Membranes. Mar. Drugs 2022, 20, 437. https://doi.org/10.3390/md20070437
Wu S, Yang L, Chen J. Preparation and Characterization of Tilapia Collagen-Thermoplastic Polyurethane Composite Nanofiber Membranes. Marine Drugs. 2022; 20(7):437. https://doi.org/10.3390/md20070437
Chicago/Turabian StyleWu, Sijia, Longhe Yang, and Junde Chen. 2022. "Preparation and Characterization of Tilapia Collagen-Thermoplastic Polyurethane Composite Nanofiber Membranes" Marine Drugs 20, no. 7: 437. https://doi.org/10.3390/md20070437
APA StyleWu, S., Yang, L., & Chen, J. (2022). Preparation and Characterization of Tilapia Collagen-Thermoplastic Polyurethane Composite Nanofiber Membranes. Marine Drugs, 20(7), 437. https://doi.org/10.3390/md20070437