S-Assimilation Influences in Carrageenan Biosynthesis Genes during Ethylene-Induced Carposporogenesis in Red Seaweed Grateloupia imbricata
Abstract
:1. Introduction
2. Results
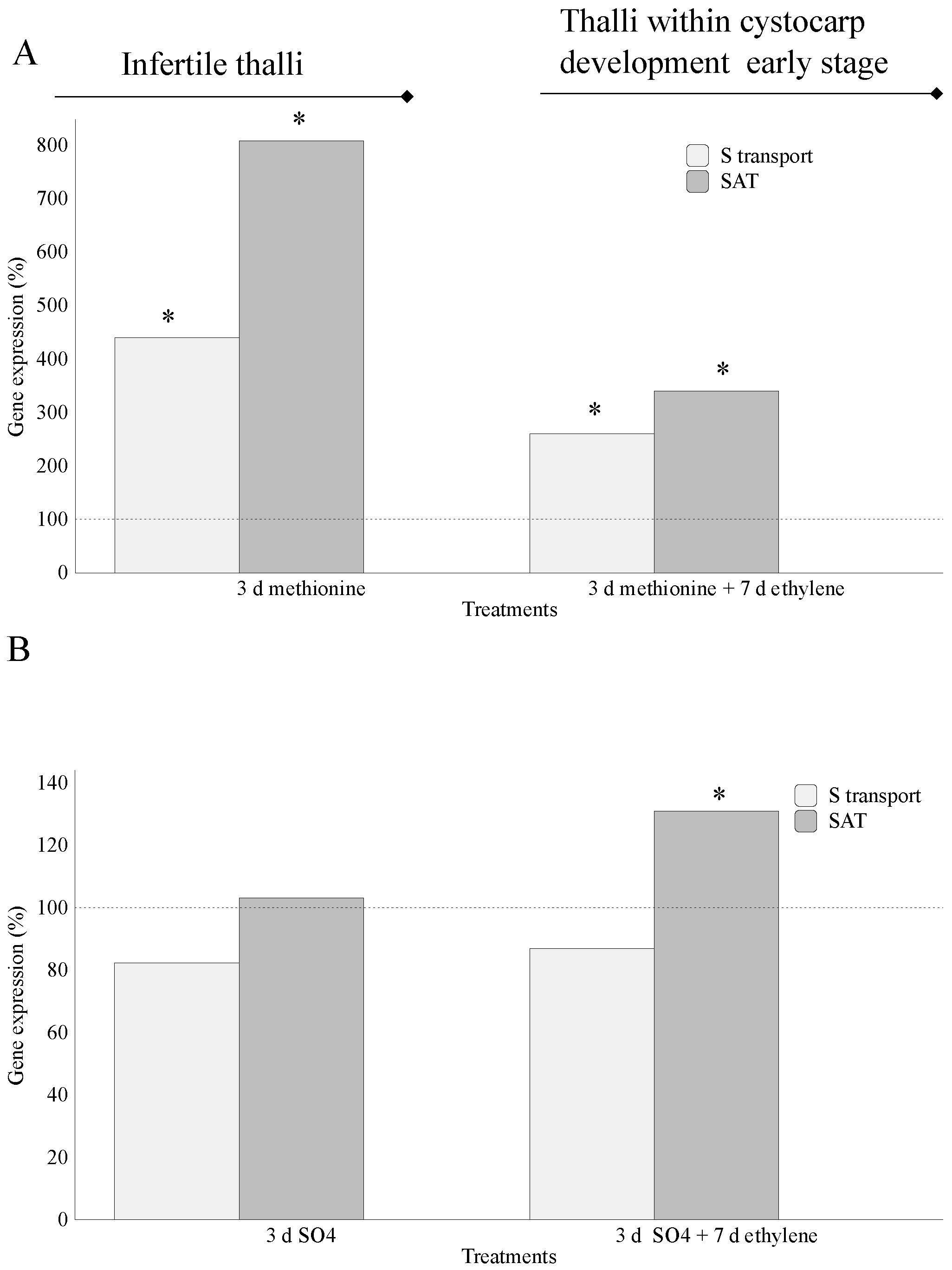
2.1. Assimilation of S-Source
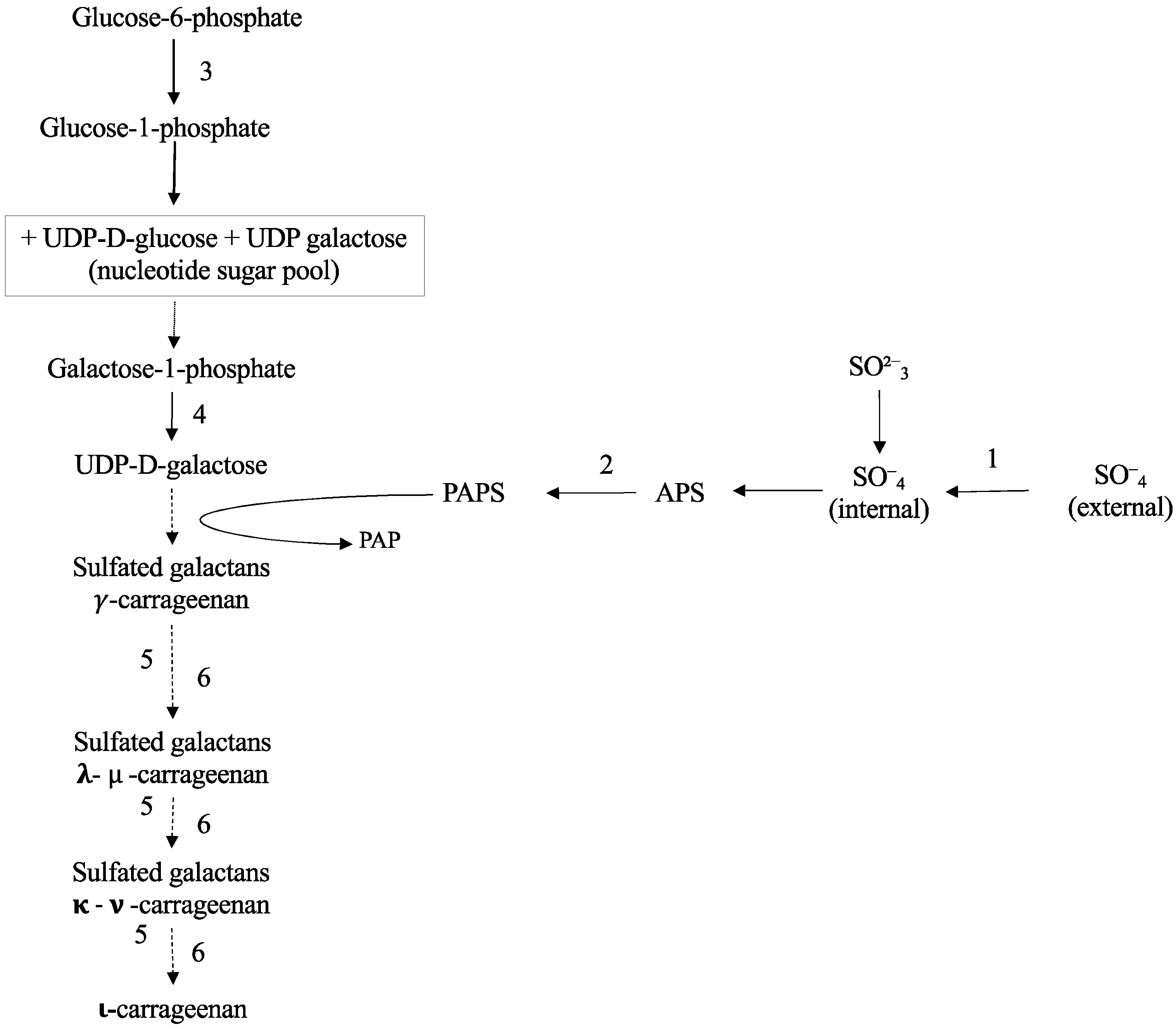
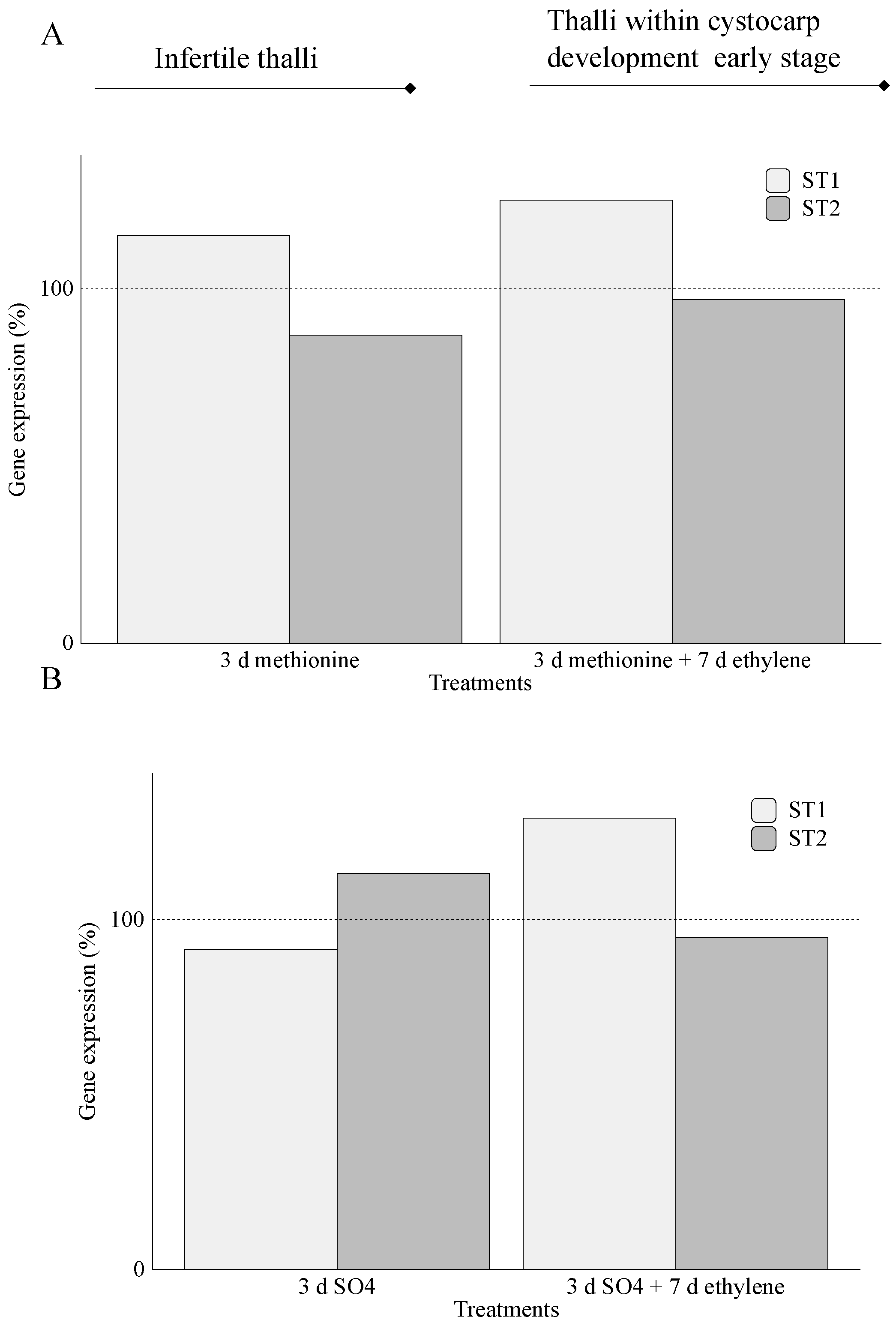
2.2. Carrageenan Synthesis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Changes in Gene Expression According to S-Source and to Elicitation of Cystocarps by Ethylene
4.3. RNA Extraction
4.4. Droplet Digital PCR (ddPCR) Primers and Protocol Implementation
4.5. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Shao, Z.; Duan, D. The Cell Wall Polysaccharides Biosynthesis in Seaweeds: A Molecular Perspective. Front. Plant Sci. 2022, 13, 902823. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ma, C.; Lou, Z.; Wang, A.; Wang, H. Purification and characterization of d-Gal-6-sulfurylase from Eucheuma striatum. Carbohydr. Polym. 2013, 96, 9–14. [Google Scholar] [CrossRef]
- La Barre, S.; Bates, S.S. Blue Biotechnology. Carrageenans: New Tools for New Applications. In Blue Biotechnology: Production and Use of Marine Molecules; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; Volume 1, pp. 371–416. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Mantesa, S.R.; Robaina, R.R. Expression of Genes Related to Carrageenan Synthesis during Carposporogenesis of the Red Seaweed Grateloupia imbricata. Mar. Drugs 2020, 18, 432. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural Diversity in Galactans from Red Seaweeds and Its Influence on Rheological Properties. Front. Plant Sci. 2020, 11, 559986. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, C.; Schnarrenberger, C.; Gross, W. Phosphomannomutase and phosphoglucomutase in the red alga Galdieria sulphuraria. Plant Sci. 1996, 121, 19–27. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, Z.; Li, L.; Liu, S.; Yao, J.; Duan, D. Molecular characterisation and biochemical properties of phosphomannomutase/phosphoglucomutase (PMM/PGM) in the brown seaweed Saccharina japonica. J. Appl. Phycol. 2018, 30, 2687–2696. [Google Scholar] [CrossRef]
- McQueney, M.S.; Anderson, K.S.; Markham, G.D. Energetics of S-Adenosylmethionine Synthetase Catalysis. Biochemistry 2000, 39, 4443–4454. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Robaina, R.R. Effects of Ethylene on Tetrasporogenesis in Pterocladiella Capillacea (Rhodophyta). J. Phycol. 2012, 48, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, P.; Robaina, R.R. On reproduction in red algae: Further research needed at the molecular level. Front. Plant Sci. 2015, 6, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Jimenez, P.; Robaina, R.R.; Montero-Fernández, M. Molecular mechanisms underlying Grateloupia imbricata (Rhodophyta) carposporogenesis induced by methyl jasmonate. J. Phycol. 2017, 53, 1340–1344. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Montero-Fernández, M.; Robaina, R.R. Analysis of ethylene-induced gene regulation during carposporogenesis in the red seaweed Grateloupia imbricata (Rhodophyta). J. Phycol. 2018, 54, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, P.; Robaina, R.R. Volatiles in the Aquatic Marine Ecosystem: Ethylene and Related Plant Hormones and Sporulation in Red Seaweeds. In Systems Biology of Marine Ecosystems; Springer: Cham, Switzerland, 2017; pp. 99–116. [Google Scholar]
- Garcia-Jimenez, P.; Brito-Romano, O.; Robaina, R.R. Occurrence of jasmonates during cystocarp development in the red alga Grateloupia imbricata. J. Phycol. 2016, 52, 1085–1093. [Google Scholar] [CrossRef]
- Gojon, A.; Nacry, P.; Davidian, J.-C. Root uptake regulation: A central process for NPS homeostasis in plants. Curr. Opin. Plant Biol. 2009, 12, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Davidian, J.-C.; Kopriva, S. Regulation of Sulfate Uptake and Assimilation—The Same or Not the Same? Mol. Plant 2010, 3, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Algeo, T.J.; Luo, G.M.; Song, H.Y.; Lyons, T.W.; Canfield, D.E. Reconstruction of secular variation in seawater sulfate concentrations. Biogeosciences 2015, 12, 2131–2151. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Mentch, S.J.; Gao, X.; Nichenametla, S.N.; Locasale, J.W. Methionine metabolism influences genomic architecture and gene expression through H3K4me3 peak width. Nat. Commun. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Whitcomb, S.; Rakpenthai, A.; Brückner, F.; Fischer, A.; Parmar, S.; Erban, A.; Kopka, J.; Hawkesford, M.; Hoefgen, R. Cysteine and Methionine Biosynthetic Enzymes Have Distinct Effects on Seed Nutritional Quality and on Molecular Phenotypes Associated with Accumulation of a Methionine-Rich Seed Storage Protein in Rice. Front. Plant Sci. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Prioretti, L.; Gontero, B.; Hell, R.; Giordano, M. Diversity and regulation of ATP sulfurylase in photosynthetic organisms. Front. Plant Sci. 2014, 5, 597. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leustek, T. Sulfate Metabolism. Arab. Book 2002, 1, e0017. [Google Scholar] [CrossRef]
- Giordano, M.; Raven, J.A. Nitrogen and sulfur assimilation in plants and algae. Aquat. Bot. 2014, 118, 45–61. [Google Scholar] [CrossRef]
- Hopkins, L.; Parmar, S.; Błaszczyk, A.; Hesse, H.; Hoefgen, R.; Hawkesford, M.J. O-Acetylserine and the Regulation of Expression of Genes Encoding Components for Sulfate Uptake and Assimilation in Potato. Plant Physiol. 2005, 138, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Wawrzynska, A.; Moniuszko, G.; Sirko, A. Links Between Ethylene and Sulfur Nutrition—A Regulatory Interplay or Just Metabolite Association? Front. Plant Sci. 2015, 6, 1053. [Google Scholar] [CrossRef] [Green Version]
- Al Murad, M.; Razi, K.; Benjamin, L.; Lee, J.H.; Kim, T.H.; Muneer, S. Ethylene regulates sulfur acquisition by regulating the expression of sulfate transporter genes in oilseed rape. Physiol. Plant. 2021, 171, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Giordano, M. Allocation of Sulfur to Sulfonium Compounds in Microalgae. In Sulfur Assimilation and Abiotic Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 317–333. [Google Scholar] [CrossRef]
- Rotte, C.; Leustek, T. Differential Subcellular Localization and Expression of ATP Sulfurylase and 5′-Adenylylsulfate Reductase during Ontogenesis of Arabidopsis Leaves Indicates That Cytosolic and Plastid Forms of ATP Sulfurylase May Have Specialized Functions. Plant Physiol. 2000, 124, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Klonus, D.; Hofgen, R.; Willmitzer, L.; Riesmeier, J.W. Isolation and characterization of two cDNA clones encoding ATP-sulfurylases from potato by complementation of a yeast mutant. Plant J. 1994, 6, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, P.; Robaina, R.R. Insight into the Mechanism of Red Alga Reproduction. What Else Is Beyond Cystocarps Development? In Systems Biology; IntechOpen: London, UK, 2019. [Google Scholar]
- Garcia-Jimenez, P.; Garcia-Maroto, F.; Garrido-Cárdenas, J.A.; Ferrandiz, C.; Robaina, R.R. Differential expression of the ornithine decarboxylase gene during carposporogenesis in the thallus of the red seaweed Grateloupia imbricata (Halymeniaceae). J. Plant Physiol. 2009, 166, 1745–1754. [Google Scholar] [CrossRef]
- Egli, B.; Kölling, K.; Köhler, C.; Zeeman, S.C.; Streb, S. Loss of Cytosolic Phosphoglucomutase Compromises Gametophyte Development in Arabidopsis. Plant Physiol. 2010, 154, 1659–1671. [Google Scholar] [CrossRef] [Green Version]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Siow, R.-S.; Teo, S.-S.; Ho, W.-Y.; Shukor, M.Y.A.; Phang, S.-M.; Ho, C.-L. Molecular Cloning and Biochemical Characterization of Galactose-1-Phosphate URI-Dylyltransferase from Gracilaria Changii (Rhodophyta). J. Phycol. 2021, 48, 155–162. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Li, S.-Y.; Shabtai, Y.; Arad, S. Floridoside as a Carbon Precursor for the Synthesis of Cell-Wall Polysaccharide in the Red Microalga Porphyridium SP. (Rhodophyta). J. Phycol. 2002, 38, 931–938. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Cole, K.M.; Sheath, R.G. Biology of the Red Algae; Cambridge University Press: Cambridge, UK, 1990; p. 517. [Google Scholar]






| Gene | Primer Name | Sequence (5′-3′) |
|---|---|---|
| S transporter and assimilation | ||
| Sulfate transporter (S-transporter) | ST-2545F ST-2545R | GGAAGATCCGGACGAGATTATG GGGTACCTTCGTCTAGTGTTTC |
| Sulfate adenylyltransferase (SAT) | SAT-790F SAT-790R | GAGGAATGCTGATGCTGTCT ACCTCGGTTAATGAGTTCTTCC |
| Carrageenan precursor | ||
| Phosphoglucomutase (PGM) | PG-17368F PG-17368R | AGGTCGATAGCCGAGTTTAGA CCAGGATTGTGACTAGCTGTAAG |
| Galactose 1 phosphate uridyltransferase (GALT) | G1PU-1681F G1PU-1681R | GTAGTAGATGCCTGGTGTGATG CATATCTGGCCATGAGGATGAG |
| Carrageen synthesis | ||
| Galactose-6-Sulfurylase I (SYI.1) | GS1-136F GS1-136R | ACAACGAGAAGGCTGACAAG CCGCACATTTGTTTCGCTATC |
| Galactose-6-Sulfurylase I (SYI.2) | GS1-824F GS1-824R | GAAACGGAGGTCACTCTTGTAG GAAGTCGACCGAGTTGCTTAT |
| Galactose-6-Sulfurylase II (SYII.1) | GS2-5356F GS2-5356R | GGAGGATTCTTGTTCGAGGATG AGTAGCGAGACCCGAGTATT |
| Galactose-6-Sulfurylase II (SYII.2) | GS2-6049F GS2-6049R | ATAACCCAAGTCCTCCTCCT GCTATCCCGTTCTTGCATCT |
| Carbohydrate sulfotransferase (ST1) | CS-3064F CS-3064R | CTGCATCACTGCGTTACTATTTC CATCAGGTCCAGCCACATAA |
| Carbohydrate sulfotransferase (ST2) | CS-3265F CS-3265R | TGTCGGGTGATGCGTTAAA TCGTTCCACTTTGTCCAGATAC |
| Reproduction | ||
| Ornithine decarboxylase (ODC) | D2-ODCF D2-ODCR | 5′3′ CGCAGACGCGACACAGTA 5′3′ TCACCAGAATGTTTAGCGAAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Rosario-Santana, D.; Robaina, R.R.; Garcia-Jimenez, P. S-Assimilation Influences in Carrageenan Biosynthesis Genes during Ethylene-Induced Carposporogenesis in Red Seaweed Grateloupia imbricata. Mar. Drugs 2022, 20, 436. https://doi.org/10.3390/md20070436
del Rosario-Santana D, Robaina RR, Garcia-Jimenez P. S-Assimilation Influences in Carrageenan Biosynthesis Genes during Ethylene-Induced Carposporogenesis in Red Seaweed Grateloupia imbricata. Marine Drugs. 2022; 20(7):436. https://doi.org/10.3390/md20070436
Chicago/Turabian Styledel Rosario-Santana, Diana, Rafael R. Robaina, and Pilar Garcia-Jimenez. 2022. "S-Assimilation Influences in Carrageenan Biosynthesis Genes during Ethylene-Induced Carposporogenesis in Red Seaweed Grateloupia imbricata" Marine Drugs 20, no. 7: 436. https://doi.org/10.3390/md20070436
APA Styledel Rosario-Santana, D., Robaina, R. R., & Garcia-Jimenez, P. (2022). S-Assimilation Influences in Carrageenan Biosynthesis Genes during Ethylene-Induced Carposporogenesis in Red Seaweed Grateloupia imbricata. Marine Drugs, 20(7), 436. https://doi.org/10.3390/md20070436








