Growth Factor-Free Vascularization of Marine-Origin Collagen Sponges Using Cryopreserved Stromal Vascular Fractions from Human Adipose Tissue
Abstract
1. Introduction
2. Results
2.1. Generation of Prevascularized Collagen Sponges in an Extrinsic Angiogenic Growth Factor Free Manner
2.2. Profiling of Angiogenesis-Related Proteins in Prevascularized Collagen Sponges Secretome
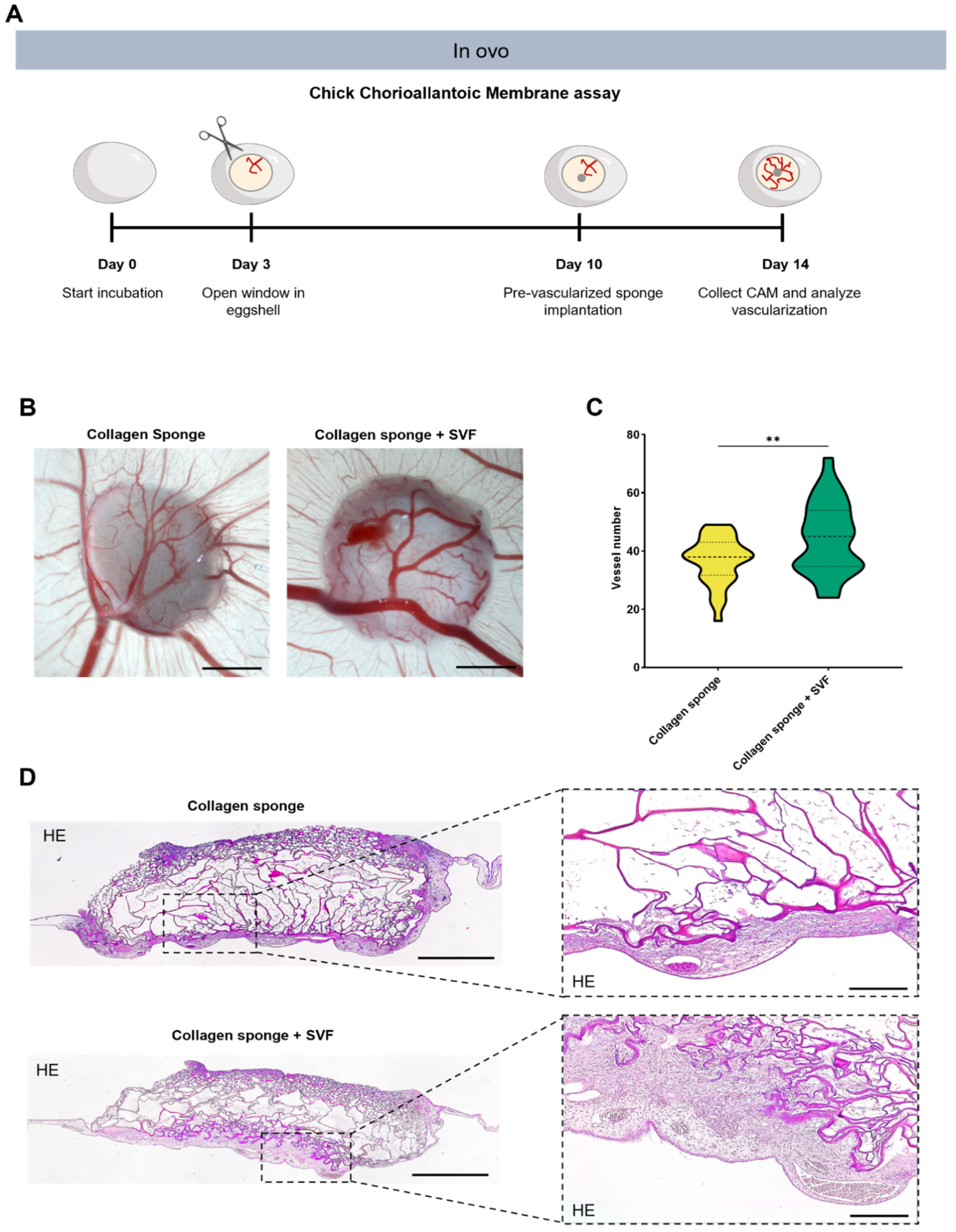
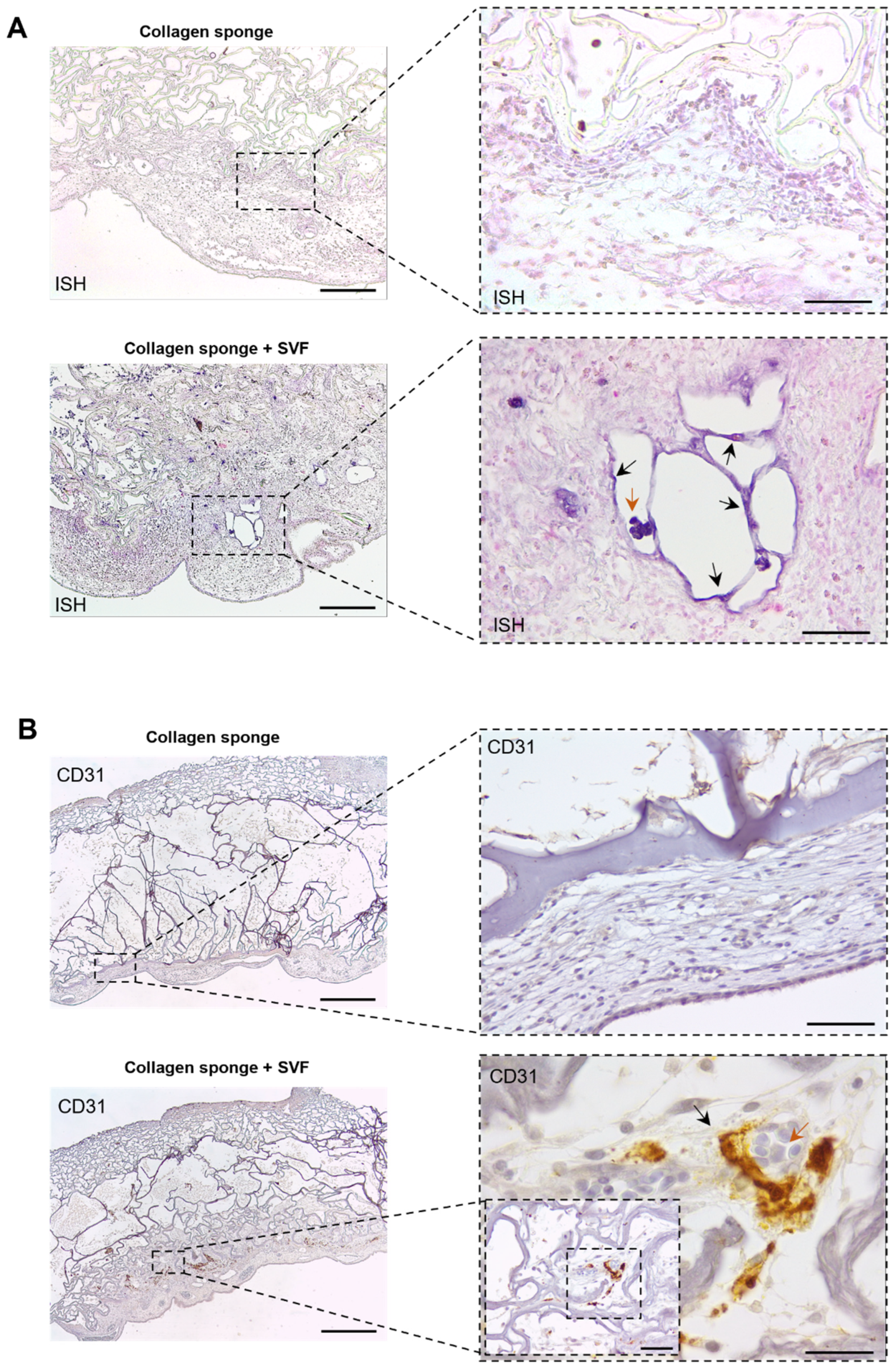
2.3. In Ovo Evaluation of Angiogenic Potential
3. Discussion
4. Materials and Methods
4.1. Collagen Acid Extraction
4.2. Collagen Sponge Fabrication
4.3. Isolation and Cryopreservation of Human Adipose-Derived SVF Cells
4.4. Cell Seeding
4.5. Immunocytochemistry
4.6. Secretome Analysis
4.7. In ovo Implantation
4.8. New Vessel Quantification
4.9. Histological Analysis
4.10. Hematoxylin and Eosin Staining
4.11. Immunohistochemistry
4.12. In Situ Hybridization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouwkema, J.; Koopman, B.F.; van Blitterswijk, C.; Dhert, W.; Malda, J. Supply of Nutrients to Cells in Engineered Tissues. Biotechnol. Genet. Eng. Rev. 2009, 26, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.R.; Clark, E.L. Microscopic observations on the growth of blood capillaries in the living mammal. Am. J. Anat. 1939, 64, 251–301. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Miyagawa, S.; Sawa, Y.; Sakakida, S.; Taketani, S.; Kondoh, H.; Memon, I.A.; Imanishi, Y.; Shimizu, T.; Okano, T.; Matsuda, H. Tissue Cardiomyoplasty Using Bioengineered Contractile Cardiomyocyte Sheets to Repair Damaged Myocardium: Their Integration with Recipient Myocardium. Transplantation 2005, 80, 1586–1595. [Google Scholar] [CrossRef]
- Shafiee, S.; Shariatzadeh, S.; Zafari, A.; Majd, A.; Niknejad, H. Recent Advances on Cell-Based Co-Culture Strategies for Prevascularization in Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kniebs, C.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Jockenhoevel, S.; Thiebes, A.L. Influence of Different Cell Types and Sources on Pre-Vascularisation in Fibrin and Agarose–Collagen Gels. Organogenesis 2020, 16, 14–26. [Google Scholar] [CrossRef]
- Qian, Z.; Sharma, D.; Jia, W.; Radke, D.; Kamp, T.; Zhao, F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics 2019, 9, 2143–2157. [Google Scholar] [CrossRef]
- Jackson, C.; Nguyen, M. Human microvascular endothelial cells differ from macrovascular endothelial cells in their expression of matrix metalloproteinases. Int. J. Biochem. Cell Biol. 1997, 29, 1167–1177. [Google Scholar] [CrossRef]
- Sacharidou, A.; Stratman, A.N.; Davis, G.E. Molecular Mechanisms Controlling Vascular Lumen Formation in Three-Dimensional Extracellular Matrices. Cells Tissues Organs 2012, 195, 122–143. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arter. Thromb. Vasc. Biol. 2019, 39, 1034–1044. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Cerqueira, M.T.; Santos, T.C.; Sampaio-Marques, B.; Ludovico, P.; Marques, A.P.; Pirraco, R.P.; Reis, R.L. Cell sheet engineering using the stromal vascular fraction of adipose tissue as a vascularization strategy. Acta Biomater. 2017, 55, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Mytsyk, M.; Cerino, G.; Reid, G.; Sole, L.; Eckstein, F.; Santer, D.; Marsano, A. Long-Term Severe In Vitro Hypoxia Exposure Enhances the Vascularization Potential of Human Adipose Tissue-Derived Stromal Vascular Fraction Cell Engineered Tissues. Int. J. Mol. Sci. 2021, 22, 7920. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Heino, J. The collagen family members as cell adhesion proteins. BioEssays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Marino, D.; Luginbühl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering Dermo-Epidermal Skin Grafts with Blood and Lymphatic Capillaries. Sci. Transl. Med. 2014, 6, 221ra14. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Amirkhani, M.A.; Seifalian, A.M.; Banafshe, H.R.; Verdi, J.; Nouradini, M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin–collagen hydrogel: A clinical study for diabetic wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Ayuso, J.M.; White, B.R.; Harari, P.M.; Ponik, S.M.; Beebe, D.J.; Gong, M.M.; Virumbrales-Muñoz, M. Matrix density drives 3D organotypic lymphatic vessel activation in a microfluidic model of the breast tumor microenvironment. Lab Chip 2020, 20, 1586–1600. [Google Scholar] [CrossRef]
- McCoy, M.G.; Seo, B.R.; Choi, S.; Fischbach, C. Collagen I hydrogel microstructure and composition conjointly regulate vascular network formation. Acta Biomater. 2016, 44, 200–208. [Google Scholar] [CrossRef]
- Montaño, I.; Schiestl, C.; Schneider, J.; Pontiggia, L.; Luginbühl, J.; Biedermann, T.; Böttcher-Haberzeth, S.; Braziulis, E.; Meuli, M.; Reichmann, E. Formation of Human Capillaries In Vitro: The Engineering of Prevascularized Matrices. Tissue Eng. Part A 2010, 16, 269–282. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (EMA/410/01 rev.3). Off. J. Eur. Union 2011, 73, 1–18. [Google Scholar]
- Sotelo, C.G.; Comesaña, M.B.; Ariza, P.R.; Pérez-Martín, R.I. Characterization of Collagen from Different Discarded Fish Species of the West Coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2015, 25, 388–399. [Google Scholar] [CrossRef]
- Fowler, G.M.; Campana, S.E. Commercial by-catch rates of blue shark (prionace glauca) from longline fisheries in the Canadian Atlantic. Collect. Vol. Sci. Pap. ICCAT 2009, 64, 1650–1667. [Google Scholar]
- Elango, J.; Lee, J.W.; Wang, S.; Henrotin, Y.; De Val, J.E.M.S.; Regenstein, J.M.; Lim, S.Y.; Bao, B.; Wu, W. Evaluation of Differentiated Bone Cells Proliferation by Blue Shark Skin Collagen via Biochemical for Bone Tissue Engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef]
- Nomura, Y.; Yamano, M.; Hayakawa, C.; Ishii, Y.; Shirai, K. Structural Property and in Vitro Self-assembly of Shark Type I Collagen. Biosci. Biotechnol. Biochem. 1997, 61, 1919–1923. [Google Scholar] [CrossRef]
- Diogo, G.S.; Carneiro, F.; Freitas-Ribeiro, S.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Prionace glauca skin collagen bioengineered constructs as a promising approach to trigger cartilage regeneration. Mater. Sci. Eng. C 2020, 120, 111587. [Google Scholar] [CrossRef]
- Joshi, V.S.; Lei, N.Y.; Walthers, C.M.; Wu, B.; Dunn, J.C. Macroporosity enhances vascularization of electrospun scaffolds. J. Surg. Res. 2013, 183, 18–26. [Google Scholar] [CrossRef]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, G.; Cohen, S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. 2003, 65A, 489–497. [Google Scholar] [CrossRef]
- Oliviero, O.; Ventre, M.; Netti, P. Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomater. 2012, 8, 3294–3301. [Google Scholar] [CrossRef]
- Sharma, D.; Ross, D.; Wang, G.; Jia, W.; Kirkpatrick, S.J.; Zhao, F. Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater. 2019, 95, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; He, H.; Chen, Y.; Zeng, F.; Huang, J.; Wu, L.; Chen, Y. Effects of long-term serial cell passaging on cell spreading, migration, and cell-surface ultrastructures of cultured vascular endothelial cells. Cytotechnology 2014, 66, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, K.; Dietl, S.; Ludwig, N.; Berberich, O.; Hoefner, C.; Storck, K.; Blunk, T.; Bauer-Kreisel, P. Engineering Vascularized Adipose Tissue Using the Stromal-Vascular Fraction and Fibrin Hydrogels. Tissue Eng. Part A 2015, 21, 1343–1353. [Google Scholar] [CrossRef]
- Collen, D. The Plasminogen (Fibrinolytic) System. Thromb. Haemost. 1999, 82, 259–270. [Google Scholar] [CrossRef]
- Pepper, M.S.; Sappino, A.P.; Montesano, R.; Orci, L.; Vassalli, J.-D. Plasminogen activator inhibitor-1 is induced in migrating endothelial cells. J. Cell. Physiol. 1992, 153, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Montesano, R. Proteolytic balance and capillary morphogenesis. Cell Differ. Dev. 1990, 32, 319–327. [Google Scholar] [CrossRef]
- Kraling, B.; Wiederschain, D.; Boehm, T.; Rehn, M.; Mulliken, J.; Moses, M. The role of matrix metalloproteinase activity in the maturation of human capillary endothelial cells in vitro. J. Cell Sci. 1999, 112, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Taubman, M.B. Chemokines in the Pathogenesis of Vascular Disease. Circ. Res. 2004, 95, 858–866. [Google Scholar] [CrossRef]
- Jaffe, E.; Ruggiero, J.; Falcone, D. Monocytes and macrophages synthesize and secrete thrombospondin. Blood 1985, 65, 79–84. [Google Scholar] [CrossRef]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kim, S.; Egashira, K.; Takeya, M.; Ikeda, T.; Mimura, O.; Iwao, H. Molecular Mechanism and Role of Endothelial Monocyte Chemoattractant Protein-1 Induction by Vascular Endothelial Growth Factor. Arter. Thromb. Vasc. Biol. 2003, 23, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Q.; Fei, T.; Han, J.-D.J.; Chen, Y.-G. MCP-1 mediates TGF-β–induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood 2006, 109, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.F.F.; Pirraco, R.P.; Szymczyk, W.; Frias, A.M.; Santos, T.C.; Reis, R.L.; Marques, A.P. Perivascular-Like Cells Contribute to the Stability of the Vascular Network of Osteogenic Tissue Formed from Cell Sheet-Based Constructs. PLoS ONE 2012, 7, e41051. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, M.; O’Rourke, M.; McKeown, S.R.; Hirst, D.G.; Robson, T. Pericytes influence endothelial cell growth characteristics: Role of plasminogen activator inhibitor type 1 (PAI-1). Cardiovasc. Res. 2006, 69, 207–217. [Google Scholar] [CrossRef]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]
- Montesano, R.; Orci, L.; Vassalli, P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J. Cell Biol. 1983, 97, 1648–1652. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef]
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The Correlation Between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study. Tissue Eng. Part A 2010, 16, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Somo, S.I.; Akar, B.; Bayrak, E.S.; Larson, J.C.; Appel, A.A.; Mehdizadeh, H.; Cinar, A.; Brey, E.M. Pore Interconnectivity Influences Growth Factor-Mediated Vascularization in Sphere-Templated Hydrogels. Tissue Eng. Part C Methods 2015, 21, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, H.; Sumo, S.; Bayrak, E.S.; Brey, E.M.; Cinar, A. Three-dimensional modeling of angiogenesis in porous biomaterial scaffolds. Biomaterials 2013, 34, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, T.S.; Santos, C.F. A Review of Sustainability Concepts in Marine Spatial Planning and the Potential to Supporting the UN Sustainable Development Goal 14. Front. Mar. Sci. 2021, 8, 1244. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Kanczler, J.M.; Hulsart-Billstrom, G.; Inglis, S.; Oreffo, R.O. The Chorioallantoic Membrane Assay for Biomaterial Testing in Tissue Engineering: A Short-Term In Vivo Preclinical Model. Tissue Eng. Part C Methods 2017, 23, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Santos, L.F.; Mendes, M.C.; Mano, J.F. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials 2020, 231, 119664. [Google Scholar] [CrossRef]
- Feijão, T.; Neves, M.I.; Sousa, A.; Torres, A.L.; Bidarra, S.J.; Orge, I.D.; Carvalho, D.T.; Barrias, C.C. Engineering injectable vascularized tissues from the bottom-up: Dynamics of in-gel extra-spheroid dermal tissue assembly. Biomaterials 2021, 279, 121222. [Google Scholar] [CrossRef]
- Sumi, M.; Sata, M.; Toya, N.; Yanaga, K.; Ohki, T.; Nagai, R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007, 80, 559–565. [Google Scholar] [CrossRef]
- Koh, Y.J.; Koh, B.I.; Kim, H.; Joo, H.J.; Jin, H.K.; Jeon, J.; Choi, C.; Lee, D.H.; Chung, J.H.; Cho, C.-H.; et al. Stromal Vascular Fraction From Adipose Tissue Forms Profound Vascular Network Through the Dynamic Reassembly of Blood Endothelial Cells. Arter. Thromb. Vasc. Biol. 2011, 31, 1141–1150. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel Autologous Cell Therapy in Ischemic Limb Disease Through Growth Factor Secretion by Cultured Adipose Tissue–Derived Stromal Cells. Arter. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Klar, A.S.; Güven, S.; Zimoch, J.; Zapiórkowska, N.A.; Zapiórkowska, T.; Böttcher-Haberzeth, S.; Meuli-Simmen, C.; Martin, I.; Scherberich, A.; Reichmann, E.; et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr. Surg. Int. 2016, 32, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cerino, G.; Gaudiello, E.; Muraro, M.G.; Eckstein, F.; Martin, I.; Scherberich, A.; Marsano, A. Engineering of an angiogenic niche by perfusion culture of adipose-derived stromal vascular fraction cells. Sci. Rep. 2017, 7, 14252. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Granja, S.; Neves, N.; Reis, R.L.; Baltazar, F.; Silva, T.H.; Martins, A. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr. Polym. 2019, 223, 115034. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas-Ribeiro, S.; Diogo, G.S.; Oliveira, C.; Martins, A.; Silva, T.H.; Jarnalo, M.; Horta, R.; Reis, R.L.; Pirraco, R.P. Growth Factor-Free Vascularization of Marine-Origin Collagen Sponges Using Cryopreserved Stromal Vascular Fractions from Human Adipose Tissue. Mar. Drugs 2022, 20, 623. https://doi.org/10.3390/md20100623
Freitas-Ribeiro S, Diogo GS, Oliveira C, Martins A, Silva TH, Jarnalo M, Horta R, Reis RL, Pirraco RP. Growth Factor-Free Vascularization of Marine-Origin Collagen Sponges Using Cryopreserved Stromal Vascular Fractions from Human Adipose Tissue. Marine Drugs. 2022; 20(10):623. https://doi.org/10.3390/md20100623
Chicago/Turabian StyleFreitas-Ribeiro, Sara, Gabriela S. Diogo, Catarina Oliveira, Albino Martins, Tiago H. Silva, Mariana Jarnalo, Ricardo Horta, Rui L. Reis, and Rogério P. Pirraco. 2022. "Growth Factor-Free Vascularization of Marine-Origin Collagen Sponges Using Cryopreserved Stromal Vascular Fractions from Human Adipose Tissue" Marine Drugs 20, no. 10: 623. https://doi.org/10.3390/md20100623
APA StyleFreitas-Ribeiro, S., Diogo, G. S., Oliveira, C., Martins, A., Silva, T. H., Jarnalo, M., Horta, R., Reis, R. L., & Pirraco, R. P. (2022). Growth Factor-Free Vascularization of Marine-Origin Collagen Sponges Using Cryopreserved Stromal Vascular Fractions from Human Adipose Tissue. Marine Drugs, 20(10), 623. https://doi.org/10.3390/md20100623











