Cyclodepsipeptides: Isolation from Endophytic Fungi of Sarcophyton ehrenbergi and Verification of Their Larvicidal Activity via In-Vitro and In-Silico Studies
Abstract
1. Introduction
2. Results
2.1. Compound Identification
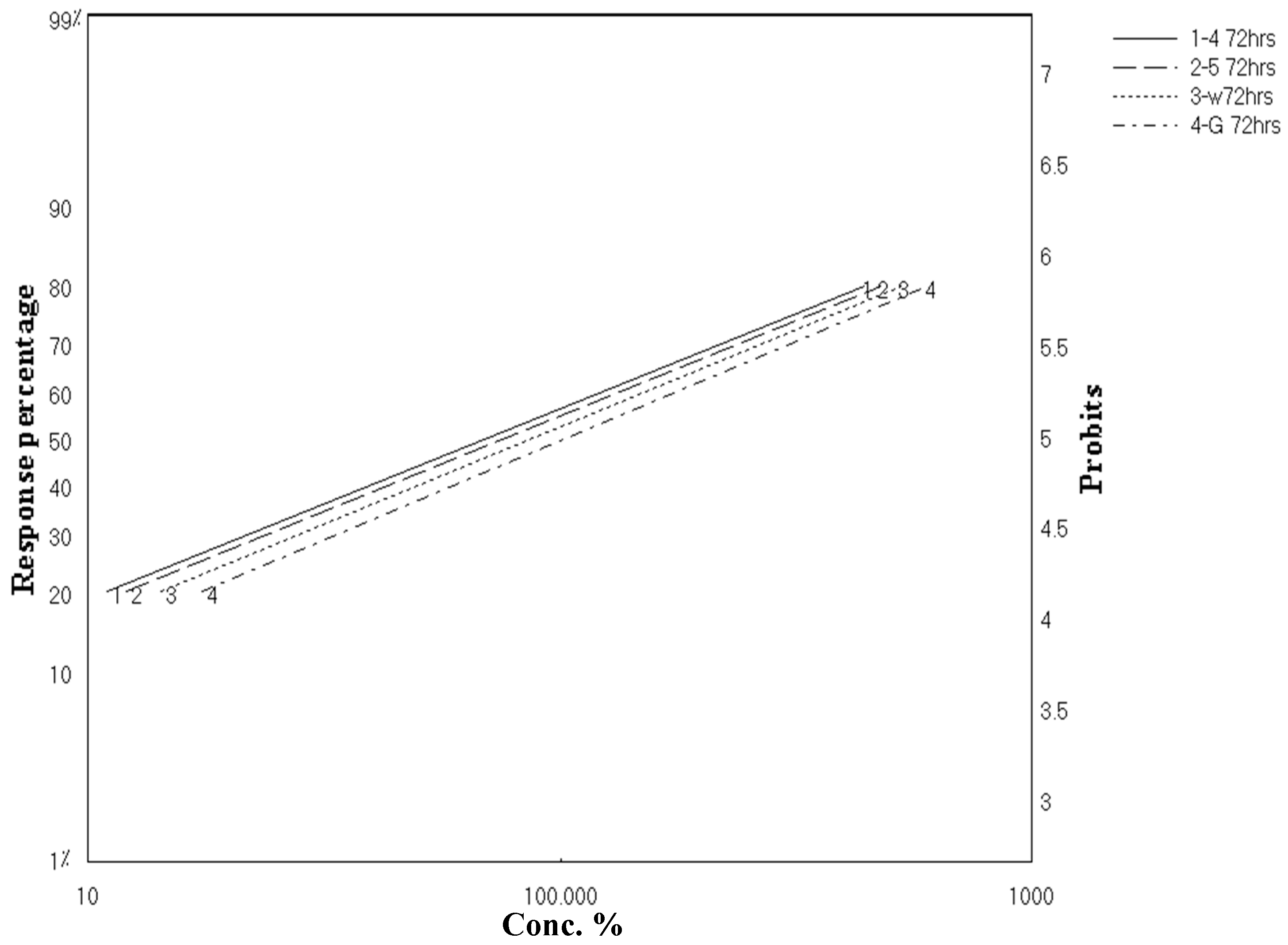
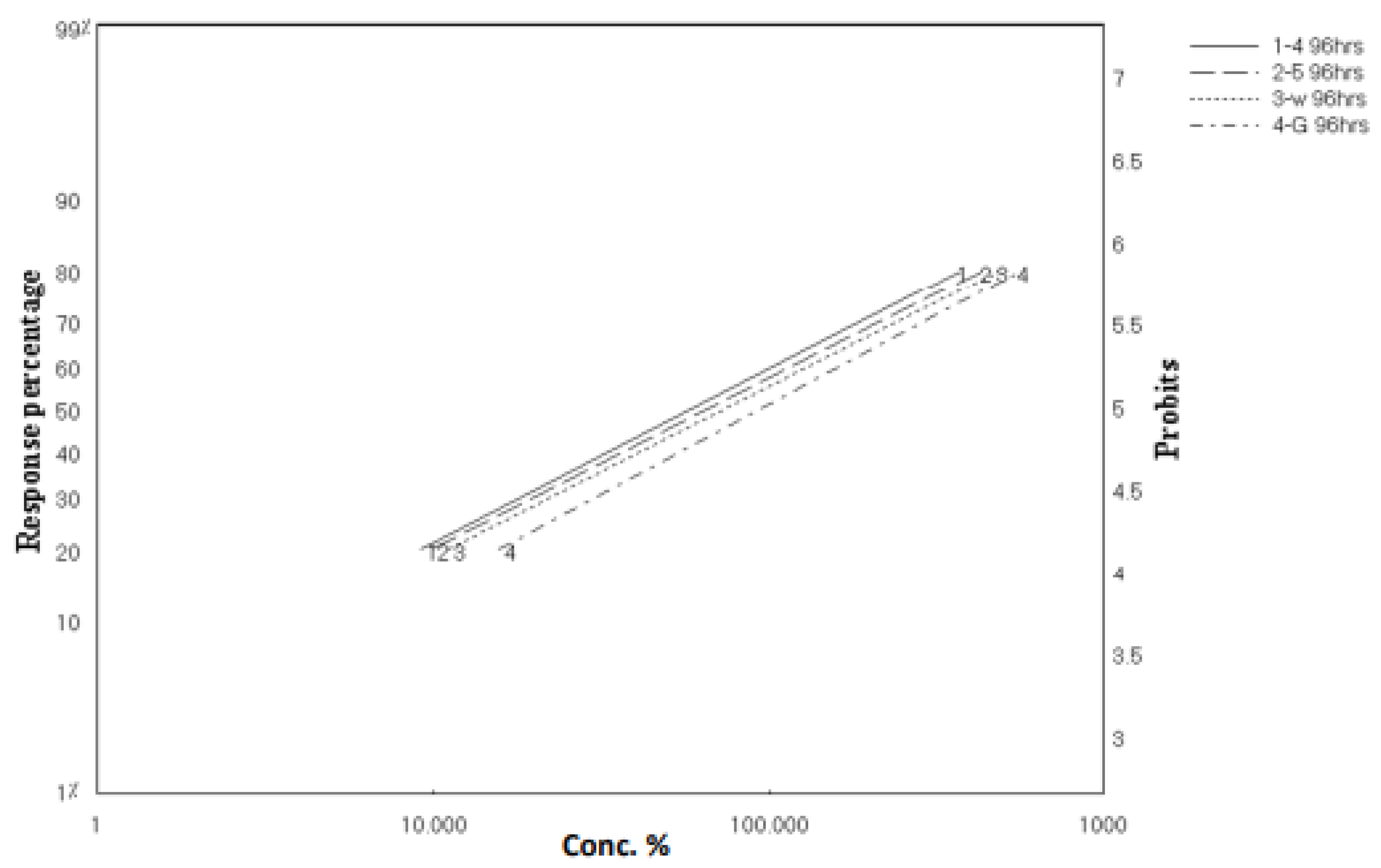
2.2. Larvicidal Activity
2.3. Biochemical Effects of A. flavus, A. nomius Extracts, and Isolated Compounds against C. pipiens Larvae
2.3.1. Quantitative Analysis (Spectroscopic Analysis)
2.3.2. Determination of Chitinase
2.3.3. Determination of Phenoloxidases
2.3.4. Determination of Lipase
2.3.5. Determination of Protease
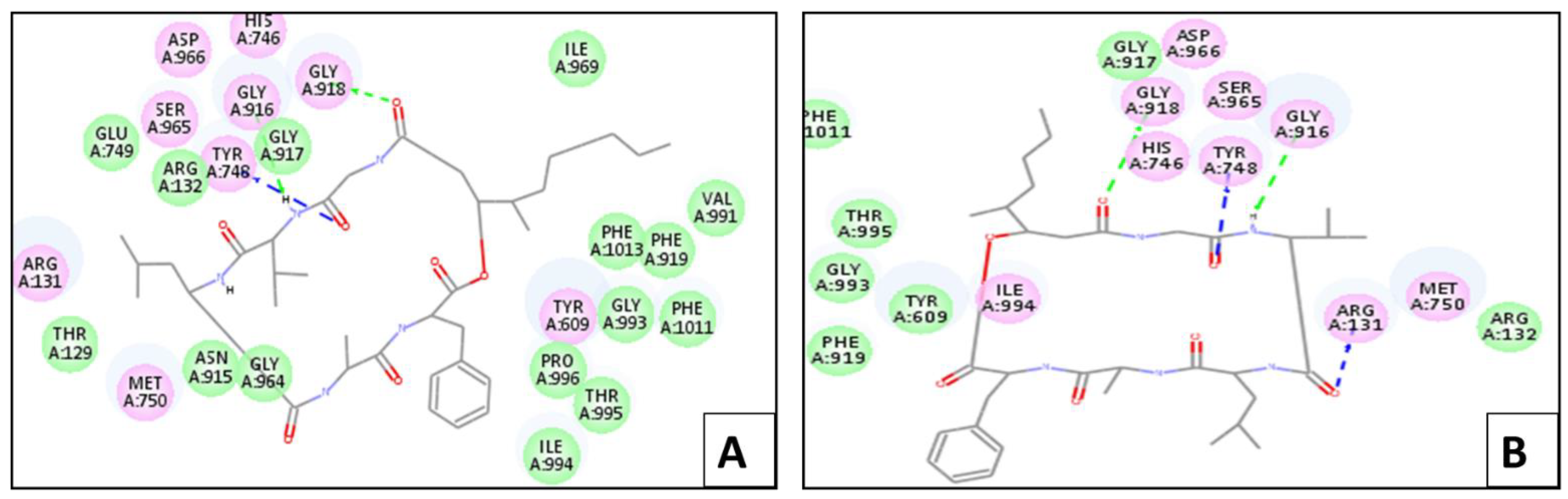
2.4. Molecular Docking Simulation
2.4.1. In Silico Molecular Docking Studies for Larvicidal Enzymatic Activity
Chitinase Enzyme
Lipase Enzyme
Protease Enzyme
Phenoloxidase Enzyme
3. Discussion
4. Materials and Methods
4.1. Fungal Material and Identification
4.2. Cultivation, Extraction, and Isolation
4.3. Nuclear Magnetic Resonance (NMR) Spectrometer
4.4. Investigation of the Molecular Target Involved in the Larvicidal Activity of the Isolated Compounds
4.5. Larvicidal Activity
4.6. Method for Larvicidal Activity Using Biochemical Analysis
4.7. Method for Determination of Total Proteins (Spectroscopic Analysis)
4.7.1. Protein Preparation
4.7.2. Preparation of Protein Reagent
4.8. Mechanism of Action of Crude Extracts of Both A. flavus and A. nomius and the Isolated Compounds Scopularide A and B in Larvicidal Activity
4.8.1. Chitinase Activity
4.8.2. Lipase Activity
4.8.3. Proteolytic Activity
4.8.4. Phenol Oxidase Activity
4.8.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yaseen, M.; Kausar, T.; Praween, B.; Shah, S.J.; Jan, Y.; Shekhawat, S.S.; Malik, M.; Azaz Ahmad Azad, Z. Insect pest infestation during storage of cereal grains, pulses and oilseeds. In Health and Safety Aspects of Food Processing Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 209–234. [Google Scholar]
- Wyckhuys, K.A.; Lu, Y.; Zhou, W.; Cock, M.J.; Naranjo, S.E.; Fereti, A.; Williams, F.E.; Furlong, M.J. Ecological pest control fortifies agricultural growth in Asia–Pacific economies. Nat. Ecol. Evol. 2020, 4, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Kuttithodi, A.M.; Alfarhan, A.; Rajagopal, R.; Barcelo, D. Chemical Composition, Insecticidal and Mosquito Larvicidal Activities of Allspice (Pimenta dioica) Essential Oil. Molecules 2021, 26, 6698. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Laboratory and Field Testing of mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, E. Predation Capacity of Culiseta longiareolata Mosquito Larvae against Some Mosquitoes Species Larvae. J. Entomol. 2012, 9, 183–186. [Google Scholar] [CrossRef][Green Version]
- Bilal, H.; Khan, I.; Hassan, S.; Akram, W.; Arshad, M.; Din, S. Larvicidal activity of selected plant extracts against Aedes albopictus Skuse (Diptera: Culicidae). Afr. Entomol. 2012, 20, 8–12. [Google Scholar] [CrossRef]
- Santra, H.K.; Maity, S.; Banerjee, D. Production of Bioactive Compounds with Broad Spectrum Bactericidal Action, Bio-Film Inhibition and Antilarval Potential by the Secondary Metabolites of the Endophytic Fungus Cochliobolus sp. APS1 Isolated from the Indian Medicinal Herb Andrographis paniculata. Molecules 2022, 27, 1459. [Google Scholar]
- Narayanankutty, A.; Sasidharan, A.; Job, J.T.; Rajagopal, R.; Alfarhan, A.; Kim, Y.O.; Kim, H.-J. Mango ginger (Curcuma amada Roxb.) rhizome essential oils as source of environmental friendly biocides: Comparison of the chemical composition, antibacterial, insecticidal and larvicidal properties of essential oils extracted by different methods. Environ. Res. 2021, 202, 111718. [Google Scholar] [CrossRef]
- Antonelli, F.; Bartolini, M.; Plissonnier, M.-L.; Esposito, A.; Galotta, G.; Ricci, S.; Davidde Petriaggi, B.; Pedone, C.; Di Giovanni, A.; Piazza, S.; et al. Essential Oils as Alternative Biocides for the Preservation of Waterlogged Archaeological Wood. Microorganisms 2020, 8, 2015. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Shazly, M.; Eldahshan, O.A. The Role of Plant-Derived Compounds in Managing Diabetes Mellitus: A Review of Literature from 2014 to 2019. Curr. Med. Chem. 2021, 28, 4694–4730. [Google Scholar] [CrossRef]
- Al-Madhagy, S.A.; Mostafa, N.M.; Youssef, F.S.; Awad, G.E.A.; Eldahshan, O.A.; Singab, A.N.B. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019, 10, 6267–6275. [Google Scholar] [CrossRef]
- Abdallah, S.H.; Mostafa, N.M.; Mohamed, M.; Nada, A.S.; Singab, A.N.B. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat. Prod. Res. 2021, 1–7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Kamal, E.M.; Bishr, M.M.; Singab, A.B.; Salama, O.M. Chemical Diversity in Species Belonging to Soft Coral Genus Sacrophyton and Its Impact on Biological Activity: A Review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.S.; Ravikumar, S.; Beula, J.M. Mosquito larvicidal activity of seaweeds extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Asian Pac. J. Trop. Dis. 2013, 3, 196–201. [Google Scholar] [CrossRef]
- Müller, P.; Döring, M. Isothermal DNA amplification facilitates the identification of a broad spectrum of bacteria, fungi and pro-tozoa in Eleutherococcus sp. plant tissue cultures. Plant Cell Tissue Organ Cult. 2009, 98, 35–45. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Mostafa, N.M.; Singab, A.N.B. Pulchranin A: First report of isolation from an endophytic fungus and its inhibitory activity on cyclin dependent kinases. Nat. Prod. Res. 2020, 34, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Wilson, M.E. Dengue and chikungunya infections in travelers. Curr. Opin. Infect. Dis. 2010, 23, 438–444. [Google Scholar] [CrossRef]
- Bazes, A.; Silkina, A.; Douzenel, P.; Faÿ, F.; Kervarec, N.; Morin, D.; Berge, J.-P.; Bourgougnon, N. Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2009, 21, 395–403. [Google Scholar] [CrossRef]
- Elissawy, A.; Ebada, S.; Ashour, M.; El-Neketi, M.; Ebrahim, W.; Singab, A.N. New Secondary Metabolites from the Mangrove-Derived Fungus Aspergillus sp. AV-2. Phytochem. Lett. 2019, 29, 1–5. [Google Scholar] [CrossRef]
- Elissawy, A.; Ebada, S.; Ashour, M.; Özkaya, F.C.; Ebrahim, W.; Singab, A.N.; Proksch, P. Spiroarthrinols A and B, Two Novel Meroterpenoids Isolated from the Sponge- Derived Fungus Arthrinium sp. Phytochem. Lett. 2017, 20, 246–251. [Google Scholar] [CrossRef]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Bael, S.V.; Aenold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control 2008, 4, 4–14. [Google Scholar] [CrossRef]
- Mustapha, A.; Adeniji, S.E. In-silico Molecular Docking and ADME/Pharmacokinetic Prediction Studies of Some Novel Carboxamide Derivatives as Anti-tubercular Agents. Chem. Afr. 2020, 3, 989–1000. [Google Scholar]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Ismail, M.I.; El-Araby, A.M.; Bahgat, D.M.; Elissawy, A.M.; Mostafa, A.M.; Eldahshan, O.A.; Singab, A.N.B. Investigation of SARS-CoV-2 Main Protease Potential Inhibitory Activities of Some Natural Antiviral Compounds Via Molecular Docking and Dynamics Approaches. Phyton Int. J. Exp. Bot. 2022, 91, 1089–1104. [Google Scholar] [CrossRef]
- Dent, S. Purity and Identification of Solids Using Melting Points; Portland State University: Portland, OR, USA, 2006. [Google Scholar]
- Yu, Z.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, Cyclodepsipeptides from a Marine Sponge-Derived Fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Meegan, J.M.; Khalil, G.M.; Adham, F.K. The Rift Valley fever epizootic in Egypt 1977-78. 2. Ecological and entomological studies. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 624–629. [Google Scholar] [CrossRef]
- Thangam, T.S.; Kathiresan, K. Marine Plants for Mosquito Control. In Proceeding of the Second International Conference on Urban Pests, Edinburgh, Scotland, 7–10 July 1996. [Google Scholar]
- Bahgat, I.M.; El Kady, G.A.; Ismail, M.M.; Shoukry, A. Toxicity of spinosadas a novel bioinsecicids against larval instars of Culex pipiens (Diptera: Culicidae). Bull. Entomol. Soc. Egypt 2001, 28, 81–89. [Google Scholar]
- Kathiresan, K.; Thangam, T.S. Light induced effects of latex of Excoecaria agallocha L. on salt marsh mosquito Culex sitiens. J. Mar. Biol. Ass. India 1987, 29, 378–380. [Google Scholar]
- Yan, X.-N.; Sikora, R.A.; Zheng, J.-W. Potential use of cucumber (Cucumis sativus L.) endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita. J. Zhejiang Univ. Sci. B. 2011, 12, 219–225. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Elhawary, E.A.; Mostafa, N.M.; Labib, R.M.; Singab, A.N. Metabolomic Profiles of Essential Oils from Selected Rosa Varieties and Their Antimicrobial Activities. Plants 2021, 10, 1712. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Badry, M.A.; Eldahshan, O.A.; Singab, A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2021, 35, 5369–5372. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Singab, A.N.; Mostafa, N.; Schultze, W. Volatile Constituents of Leaves of Ficus carica Linn. Grown in Egypt. J. Essent. Oil Bear. Plants 2010, 13, 316–321. [Google Scholar] [CrossRef]
- Mostafa, N. β-amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. Rec. Nat. Prod. 2018, 12, 480–492. [Google Scholar] [CrossRef]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Müller, W.E.G.; Kalscheuer, R.; Singab, A.; Lin, W.; Liu, Z.; Proksch, P. Hydroquinone derivatives from the marine-derived fungus Gliomastix sp. RSC Adv. 2017, 7, 30640–30649. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.G.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and sesquiterpene derivatives from a marine-derived fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N.B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat. Prod. Res. 2022, 36, 1182–1190. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Edmond, M.P.; El-Shazly, M.; Fahmy, H.A.; Sherif, N.H.; Singab, A.N.B. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat. Prod. Res. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- El-Zahar, H.; Menze, E.T.; Handoussa, H.; Osman, A.K.; El-Shazly, M.; Mostafa, N.M.; Swilam, N. UPLC-PDA-MS/MS Profiling and Healing Activity of Polyphenol-Rich Fraction of Alhagi maurorum against Oral Ulcer in Rats. Plants 2022, 11, 455. [Google Scholar] [CrossRef]
- Andersen, O.A.; Nathubhai, A.; Dixon, M.J.; Eggleston, I.M.; van Aalten, D.M. Structure-based dissection of the natural product cyclopentapeptide chitinase inhibitor argifin. Chem. Biol. 2008, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yapoudjian, S.; Ivanova, M.G.; Brzozowski, A.M.; Patkar, S.A.; Vind, J.; Svendsen, A.; Verger, R. Binding of Thermomyces (Humicola) lanuginosa lipase to the mixed micelles of cis-parinaric acid/NaTDC: Fluorescence resonance energy transfer and crystallographic study. Eur. J. Biochem. 2002, 269, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Groll, M.; Musiol, H.J.; Behrendt, R.; Kaiser, M.; Moroder, L.; Huber, R.; Brandstetter, H. Navigation inside a protease: Substrate selection and product exit in the tricorn protease from Thermoplasma acidophilum. J. Mol. Biol. 2002, 324, 1041–1050. [Google Scholar] [CrossRef]
- Fujdiarová, E.; Houser, J.; Dobeš, P.; Paulíková, G.; Kondakov, N.; Kononov, L.; Hyrsl, P.; Wimmerová, M. Heptabladed β-propeller lectins PLL2 and PHL from Photorhabdus spp. recognize O-methylated sugars and influence the host immune system. FEBS J. 2020, 288, 1343–1365. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.R. Biochemical and Physiological Studies of Some Insect Growth Regulators on the Cotton Leafworm, Spodoptera littoralis (Boisd.). Ph.D. Thesis, Faculty of Science, Cairo University,, Cairo, Egypt, 1998. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bade, M.L.; Stinson, A. Biochemistry of insect differentiation: A system for studying the mechanism of chitinase activity in vitro. Arch. Biochem. Biophys. 1981, 206, 213–221. [Google Scholar] [CrossRef]
- Ishaaya, I.; Casida, J.E. Dietary TH- 6040 alters composition and enzyme activity of housefly larval cuticle, pestic. J. Physiol. Biochem. 1974, 4, 484–490. [Google Scholar]
- Waterhouse, D.F.; Hockman, R.H.; Mckellar, J.W. An investigation of chitinase activity in cockroach and termite extracts. J. Insect Physiol. 1961, 6, 96–112. [Google Scholar] [CrossRef]
- Tahoun, M.K.; Abdel-Ghaffar, M. A modified colourimetric method for assay of lipase activity. Alex. Sci. Exch. J. 1986, 7, 235–244. [Google Scholar]
- Tatchell, R.J.; Araman, S.F.; Boctor, F.N. Biochemical and physiological studies of certain Ticks (Ixodoidea). Z. Für Parasitenkd. 1972, 39, 345–350. [Google Scholar] [CrossRef]
- Lee, Y.P.; Takabashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Ishaaya, I. In the armored scale Aonidiella aurantii and Observation on the phenoloxidase system Chrysomphalus aonidum. Comp. Biochem. Physiol. 1971, 39, 935–943. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK; New York, NY, USA,, 1971; p. 1432. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
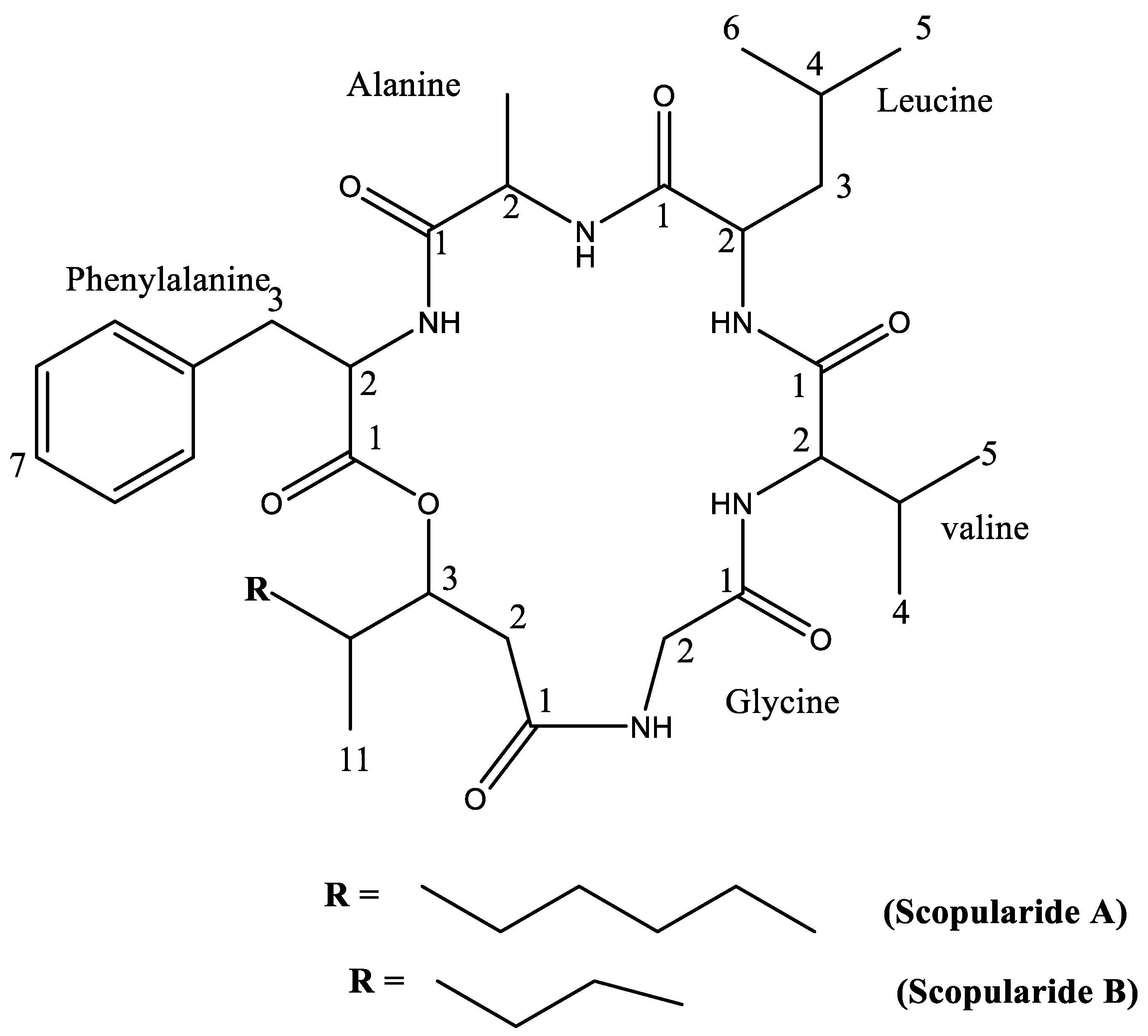

| Position | δC (ppm) | δH (ppm), Multiplicity, and J (Hz) | COSY | HMBC | Position | δC (ppm) | δH (ppm), Multiplicity, and J (Hz) | COSY | HMBC |
|---|---|---|---|---|---|---|---|---|---|
| Phenylalanine | Valine | ||||||||
| 1 | 172.66 | -- | 1 | 171.79 | -- | ||||
| 2 | 53.25 | 4.83 (m) | 3 | 1, 3, 4, Ala-1 | 2 | 57.93 | 4.21 (m) | 3 | 1, 3, 4, 5 |
| 3 | 38.55 | 3.12 (d, J = 8.1 Hz) 3.09 (d, J = 8.1 Hz) | 2 | 1, 2, 4, 5/9 | 3 | 31.11 | 2.18 (m) | 2, 4, 5 | 1, 2, 4, 5 |
| 4 | 135.76 | -- | 4 | 18.21 | 0.93 (d, J = 3.5 Hz) | 3 | 2, 3, 5 | ||
| 5/9 | 129.10 | 7.16 (m) | 6/8 | 3, 5/9, 7 | 5 | 19.60 | 0.93 (d, J = 3.5 Hz) | 3 | 2, 3, 4 |
| 6/8 | 128.52 | 7.28 (m) | 5/9 | 4, 6/8 | Glycine | ||||
| 7 | 127.07 | 7.21 (m) | 6/8 | 5/9 | 1 | 171.17 | -- | ||
| Alanine | 2 | 43.14 | 3.44 (dd, J = 17.2, 4.1 Hz) 4.43 (d, J = 8.0 Hz) | 1, HMDA-1 | |||||
| 1 | 172.70 | -- | Hydroxy methyl decanoic acid (HMDA) | ||||||
| 2 | 49.53 | 4.24 (m) | 3 | 1, 3, Leu-1 | 1 | 172.81 | -- | ||
| 3 | 17.50 | 1.29 (d, J = 7.3 Hz) | 2 | 2, 3 | 2 | 40.98 | 2.40 (m) | 3 | |
| Leucine | 3 | 78.14 | 4.64 (m) | 2, 4 | |||||
| 1 | 173.28 | -- | 4 | 37.92 | 1.58 (m) | 3, 5, 11 | 3, 5, 6, 11 | ||
| 2 | 54.00 | 4.16 (m) | 3 | 5 | 32.12 | 0.91 (m) 1.21 (m) | 4, 6 | 3, 4, 7, 11 | |
| 3 | 39.15 | 1.58 (m) | 2,4 | 6 | 29.24 | 1.21 (m) | 5, 7 | 4, 8 | |
| 4 | 24.66 | 1.68 (m) | 3, 5, 6 | 7 | 29.06 | 1.21 (m) | 6, 8 | 5, 6, 8, 9 | |
| 5 | 22.43 | 0.95 (d, J = 2.5 Hz) | 4 | 8 | 33.20 | 1.28 (m) | 7, 9 | 6, 7, 9, 10 | |
| 6 | 22.01 | 0.91 (d, J = 2.2 Hz) | 4 | 9 | 22.69 | 1.22 (m) | 8, 10 | 7, 8, 10 | |
| 10 | 13.92 | 0.85 (t) | 9 | 8, 9 | |||||
| 11 | 13.52 | 0.78 (d, J = 6.7 Hz) | 4 | 3, 4, 5 | |||||
| Position | δC (ppm) | δH (ppm), Multiplicity, and J (Hz) | HMBC | Position | δC (ppm) | δH (ppm), Multiplicity, and J (Hz) | HMBC |
|---|---|---|---|---|---|---|---|
| Phenylalanine | Valine | ||||||
| 1 | 172.29 | -- | 1 | 171.81 | -- | ||
| 2 | 53.49 | 4.72–4.64 (m) | 1, 3, 4, Ala-1 | 2 | 58.05 | 4.21 (t) | 1, 3, 4, 5 |
| 3 | 40.93 | 2.98 (dd, J = 13.5, 7.5 Hz) 3.12 (dd, J = 13.5, 8.5 Hz) | 1, 2, 4, 5/9 | 3 | 28.25 | 2.27 (m) | 1, 2, 4, 5 |
| 4 | 135.74 | -- | 4 | 18.39 | 0.97 (d, J = 2.3 Hz) | 2, 3, 5 | |
| 5/9 | 129.13 | 7.17 (m) | 3, 5/9, 7 | 5 | 19.65 | 0.97 (d, J = 2.3 Hz) | 2, 3, 4 |
| 6/8 | 128.55 | 7.26 (m) | 4, 6/8 | Glycine | |||
| 7 | 127.09 | 7.22 (m) | 5/9 | 1 | 1711.11 | -- | |
| Alanine | 2 | 43.21 | 4.46 (d, J = 8.0 Hz) 3.47 (dd, J = 17.2, 4.0 Hz) | 1, HMOA-1 | |||
| 1 | 172.64 | -- | Hydroxy methyl lactonic acid (HMOA) | ||||
| 2 | 49.43 | 4.14 (q) | 1, 3, Leu-1 | 1 | 172.91 | -- | |
| 3 | 17.89 | 1.29 (d, J = 7.3 Hz) | 2,3 | 2 | 41.2 | 2.41(m) | |
| Leucine | 3 | 77.98 | 4.80 (m) | 2, 5, 9 | |||
| 1 | 172.94 | -- | 4 | 37.74 | 1.50 (m) 1.2 (m) | 3, 5, 6, 9 | |
| 2 | 53.23 | 4.27 (t) | 1, 3, 4, Val-1 | 5 | 32.48 | 1.19 (m) 0.91 (m) | 3, 4, 7, 9 |
| 3 | 39.06 | 1.60–1.57 (m) | 1, 2, 4, 5, 6 | 6 | 29.34 | 1.10 (m) | 4, 7, 8 |
| 4 | 24.52 | 1.64 (m) | 2, 3, 5, 6 | 7 | 22.59 | 1.24 (m) | 5, 6, 8 |
| 5 | 22.02 | 0.97 (d, J = 2.2 Hz) | 3, 4, 6 | 8 | 13.67 | 0.87 (t) | 6, 7 |
| 6 | 22.55 | 0.95 (d, J = 2.2 Hz) | 3, 4, 5 | 9 | 14.05 | 0.75 (d, J = 6.7 Hz) | 3, 4, 5 |
| Percentage Mortality (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Concentrations (ppm) | Scopularide A | Scopularide B | ||||||
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | |
| 300 | 21.33 | 25.33 | 80 | 82.66 | 20 | 24 | 78.66 | 80 |
| 200 | 17.33 | 20 | 69.33 | 72 | 16 | 18.66 | 68 | 70.66 |
| 100 | 13.33 | 16 | 53.33 | 56 | 12 | 14.66 | 52 | 53.33 |
| 50 | 10.66 | 13.33 | 34.66 | 37.33 | 9.33 | 12 | 33.33 | 36 |
| 10 | 9.33 | 12 | 24 | 26.66 | 8 | 10.66 | 22.66 | 25.33 |
| LC50 (ppm) | 74,714.96 | 51,225.03 | 69.96 | 58.96 | 65,489.96 | 50,583.51 | 76.17 | 66.14 |
| LC90 (ppm) | 254,765,445.4 | 306,771,168.1 | 1167.2097 | 994.31 | 121,222,443.6 | 183,614,892.8 | 1256.21 | 1180.08 |
| Slope ± SE | 0.3628 ± 0.14 | 0.3393 ± 0.13 | 1.0485 ± 0.1203 | 1.0446 ± 0.1193 | 0.3922 ± 0.1493 | 0.36 ± 0.13 | 1.0529 ± 0.12 | 1.0241 ± 0.11 |
| Percentage Mortality (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Conc (ppm) | A. nomius Extract | A. flavus Extract | ||||||
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | |
| 300 | 18.66 | 22.66 | 76 | 78.66 | 17.33 | 20 | 73.33 | 74.66 |
| 200 | 14.66 | 17.33 | 66.66 | 69.33 | 13.33 | 14.66 | 64 | 65.33 |
| 100 | 10.66 | 13.33 | 49.33 | 49.33 | 9.33 | 12 | 46.66 | 48 |
| 50 | 8 | 10.66 | 32 | 34.66 | 6.66 | 8 | 29.33 | 30.66 |
| 10 | 6.66 | 9.33 | 20 | 24 | 5.33 | 6.66 | 17.33 | 18.66 |
| LC50 (ppm) | 52,659.3 | 46,847.74 | 87.2 | 74.3 | 40,102.7 | 32,051.5 | 102.8 | 94.8 |
| LC90 (ppm) | 49,875,033.8 | 98,192,084.9 | 1366.5 | 1328.8 | 19,084,973.0 | 20,417,679.0 | 1522.9 | 1449.9 |
| Slope ± SE | 0.4306 ± 0.15 | 0.3859 ± 0.14 | 0.1437 ± 0.12 | 1.0232 ± 0.12 | 0.4787 ± 0.16 | 0.457 ± 0.15 | 1.0947 ± 0.12 | 1.0818 ± 0.12 |
| Sample | (mg N-Acetylglucoseamine/min/mg Protein) Mean ± SD | % Change |
|---|---|---|
| A. flavuscrude extract | 118 ± 8.6 a | 9.3 |
| A. nomiuscrude extract | 95 ± 3.2 b | −12.0 |
| Scopularide B | 86 ± 2.5 b | −20.4 |
| Scopularide A | 82 ± 2.4 b | −24.1 |
| Control | 108 ± 5.1 a |
| Sample | (O.D. Units ×103/min/mg Protein) Mean ± SD | % Change |
|---|---|---|
| A. flavuscrude extract | 1579 ± 14.5 c | −11.88 |
| A. nomiuscrude extract | 1772 ± 15.9 a | −1.11 |
| Scopularide B | 1598 ± 13 bc | −10.82 |
| Scopularide A | 1631 ± 9 b | −8.98 |
| Control | 1792 ± 15.6 a |
| Sample | (µM Oleic Acid Liberated/min/gram Body Weight) Mean ± SD | % Change |
|---|---|---|
| A. flavuscrude extract | 4.6 ± 0.22 a | −52.7 |
| A. nomiuscrude extract | 2.9 ± 0.1 c | 0.3 |
| Scopularide B | 3.8 ± 0.16 b | −26 |
| Scopularide A | 3.9 ± 0.15 b | −28.3 |
| Control | 3 ± 0.12 c |
| Sample | (ng D,L Alanine/min/mg Protein) Mean ± SD | % Change |
|---|---|---|
| A. flavuscrude extract | 3235 ± 106 ab | 5.6 |
| A. nomiuscrude extract | 2873 ± 61 c | 16.1 |
| Scopularide A | 3116 ± 78 b | 9.0 |
| Scopularide B | 2277 ± 42 d | 33.5 |
| Control | 3426 ± 88 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singab, A.N.B.; Mostafa, N.M.; Elkhawas, Y.A.; Al-Sayed, E.; Bishr, M.M.; Elissawy, A.M.; Elnaggar, M.S.; Fawzy, I.M.; Salama, O.M.; Tsai, Y.-H.; et al. Cyclodepsipeptides: Isolation from Endophytic Fungi of Sarcophyton ehrenbergi and Verification of Their Larvicidal Activity via In-Vitro and In-Silico Studies. Mar. Drugs 2022, 20, 331. https://doi.org/10.3390/md20050331
Singab ANB, Mostafa NM, Elkhawas YA, Al-Sayed E, Bishr MM, Elissawy AM, Elnaggar MS, Fawzy IM, Salama OM, Tsai Y-H, et al. Cyclodepsipeptides: Isolation from Endophytic Fungi of Sarcophyton ehrenbergi and Verification of Their Larvicidal Activity via In-Vitro and In-Silico Studies. Marine Drugs. 2022; 20(5):331. https://doi.org/10.3390/md20050331
Chicago/Turabian StyleSingab, Abdel Nasser B., Nada M. Mostafa, Yasmin A. Elkhawas, Eman Al-Sayed, Mokhtar M. Bishr, Ahmed M. Elissawy, Mohamed S. Elnaggar, Iten M. Fawzy, Osama M. Salama, Yi-Hong Tsai, and et al. 2022. "Cyclodepsipeptides: Isolation from Endophytic Fungi of Sarcophyton ehrenbergi and Verification of Their Larvicidal Activity via In-Vitro and In-Silico Studies" Marine Drugs 20, no. 5: 331. https://doi.org/10.3390/md20050331
APA StyleSingab, A. N. B., Mostafa, N. M., Elkhawas, Y. A., Al-Sayed, E., Bishr, M. M., Elissawy, A. M., Elnaggar, M. S., Fawzy, I. M., Salama, O. M., Tsai, Y.-H., & Chang, F.-R. (2022). Cyclodepsipeptides: Isolation from Endophytic Fungi of Sarcophyton ehrenbergi and Verification of Their Larvicidal Activity via In-Vitro and In-Silico Studies. Marine Drugs, 20(5), 331. https://doi.org/10.3390/md20050331










