Monkfish Peptides Mitigate High Fat Diet-Induced Hepatic Steatosis in Mice
Abstract
:1. Introduction
2. Results
2.1. Identification and Protein Analysis of LPs
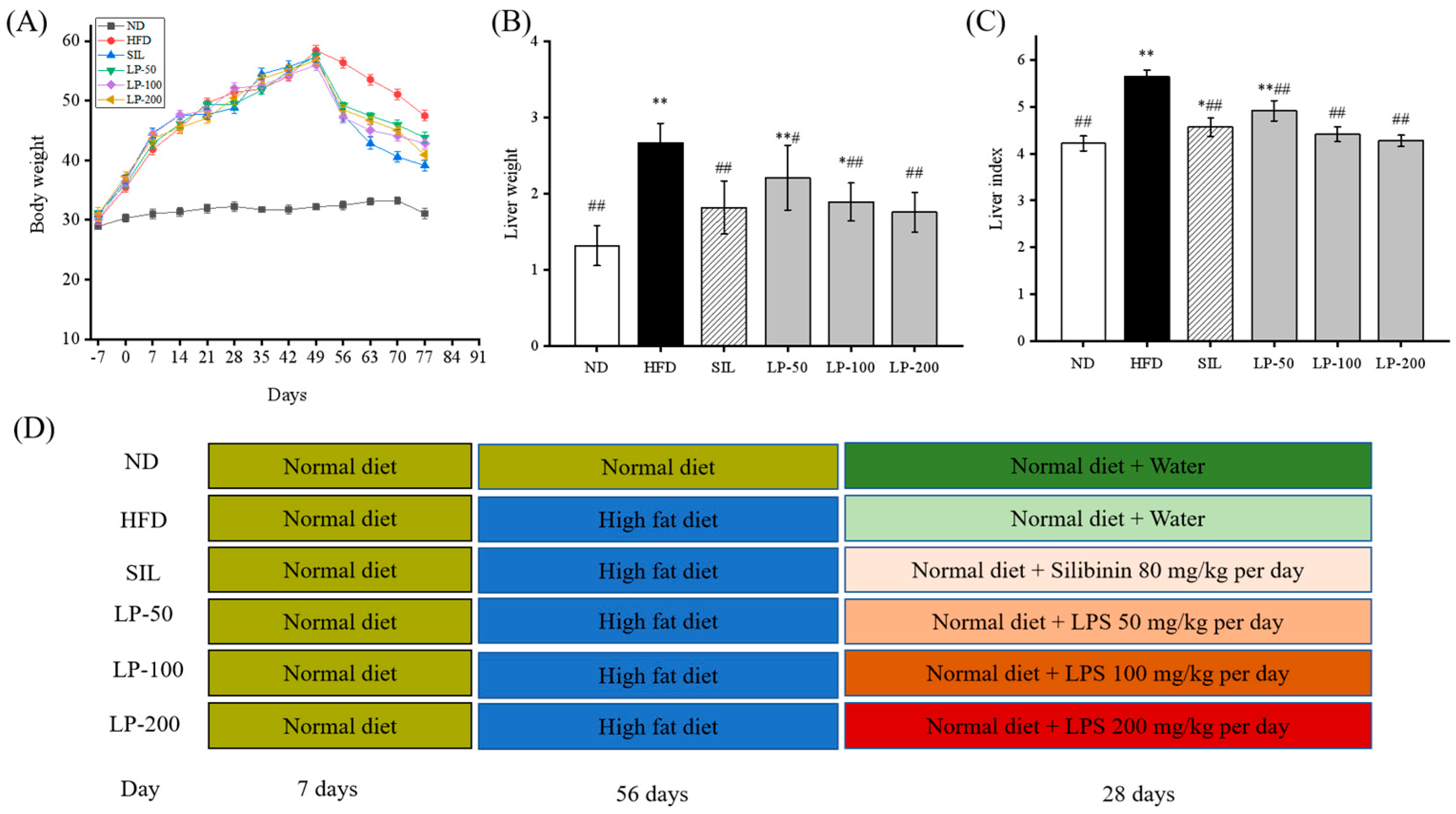
2.2. LPs Lowered Body Weight and Liver Weight
2.3. Effects of LPs on Blood Lipids
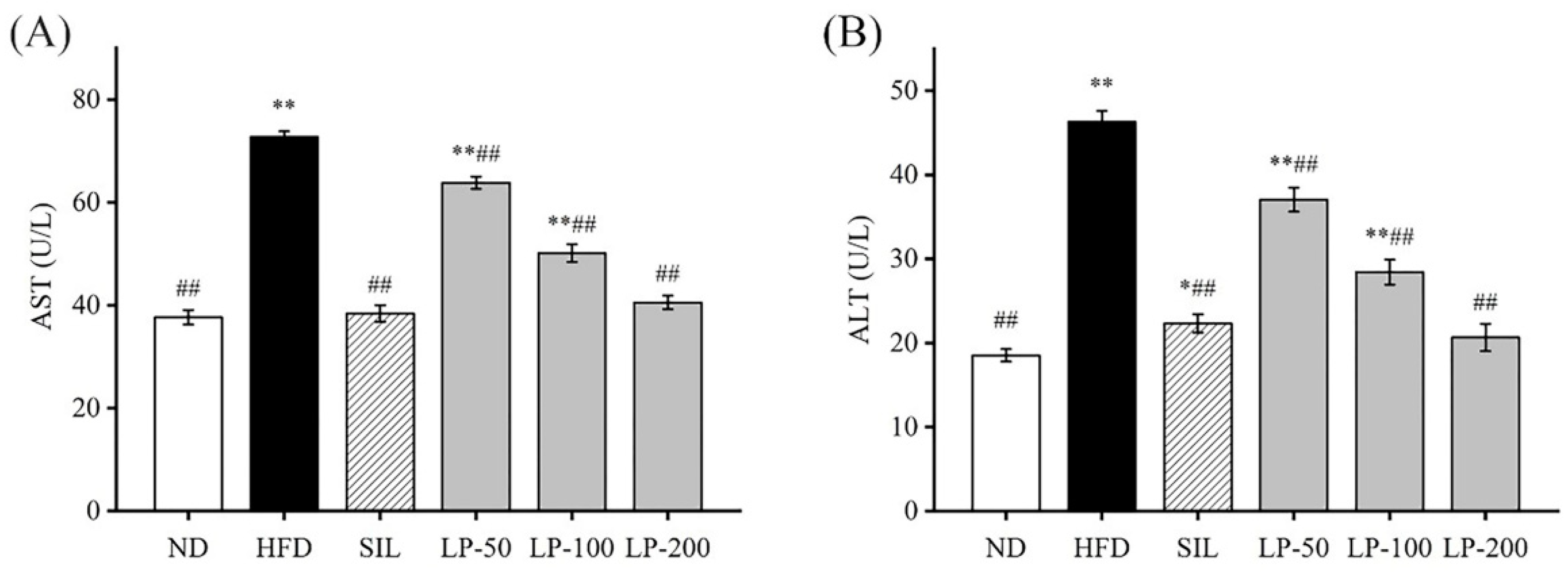
2.4. Effects of LPs on Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT)
2.5. Effects of LPs on Antioxidant Capacity
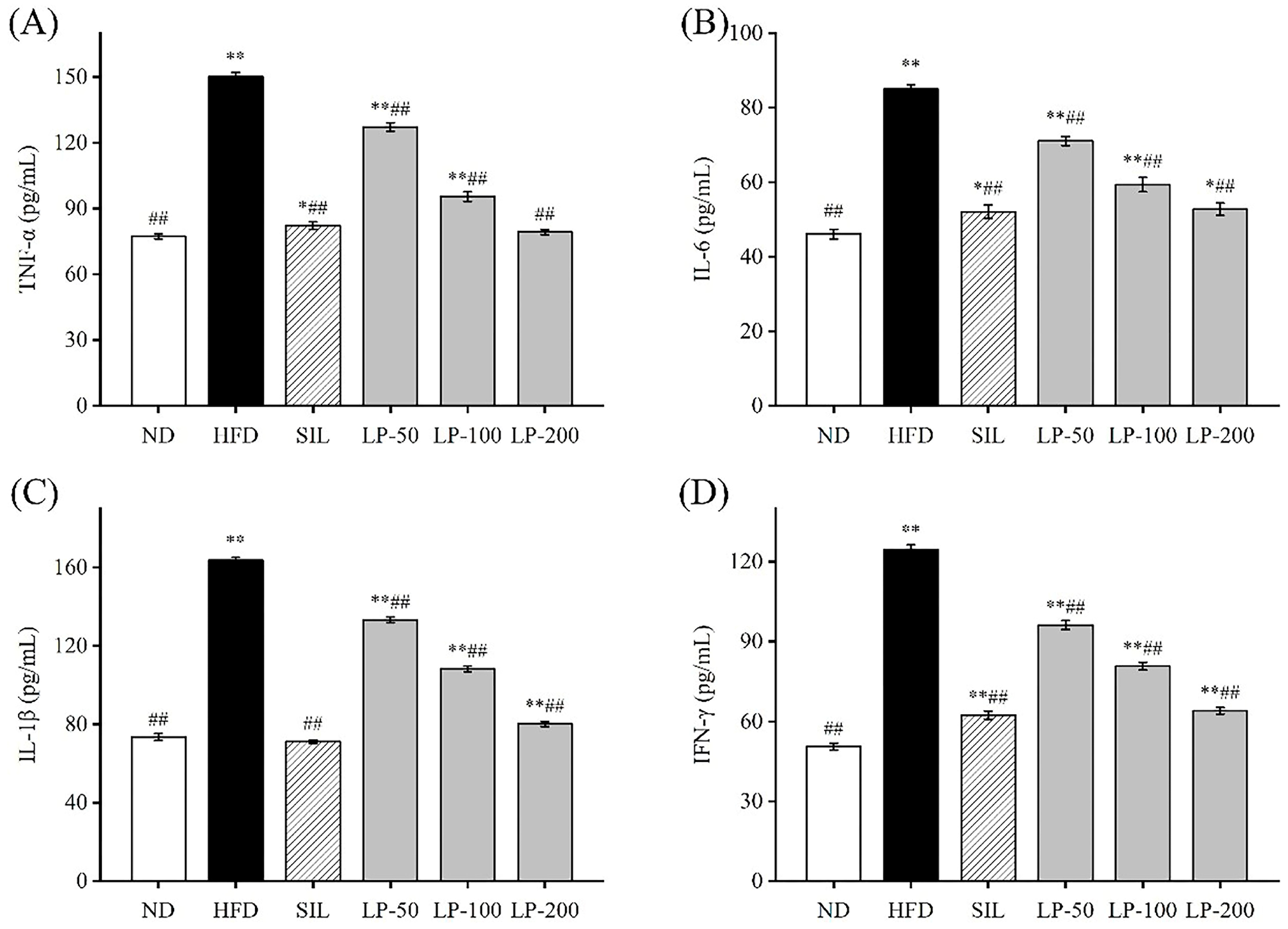
2.6. Effects of LPs on the Level of Inflammatory Factors
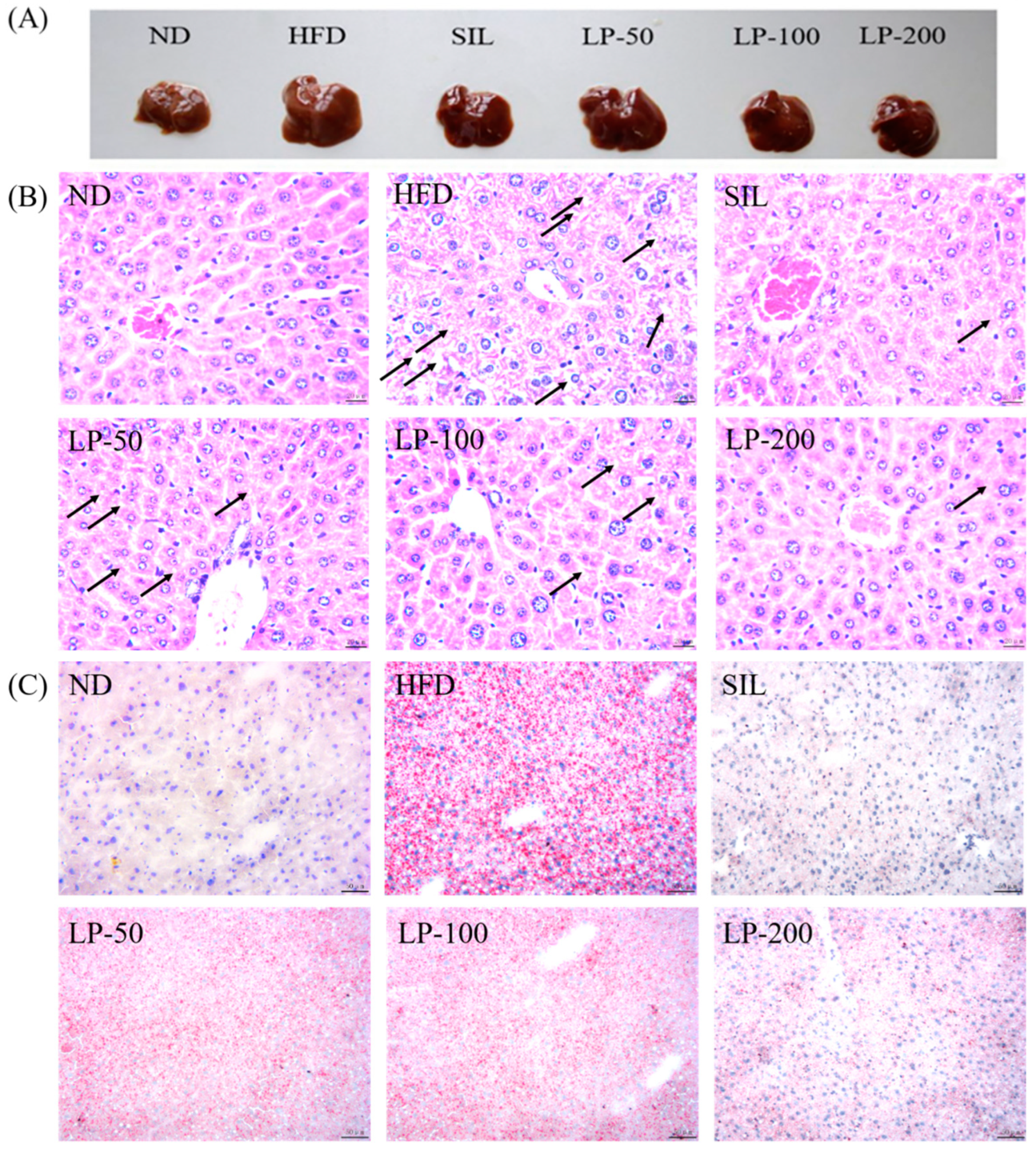
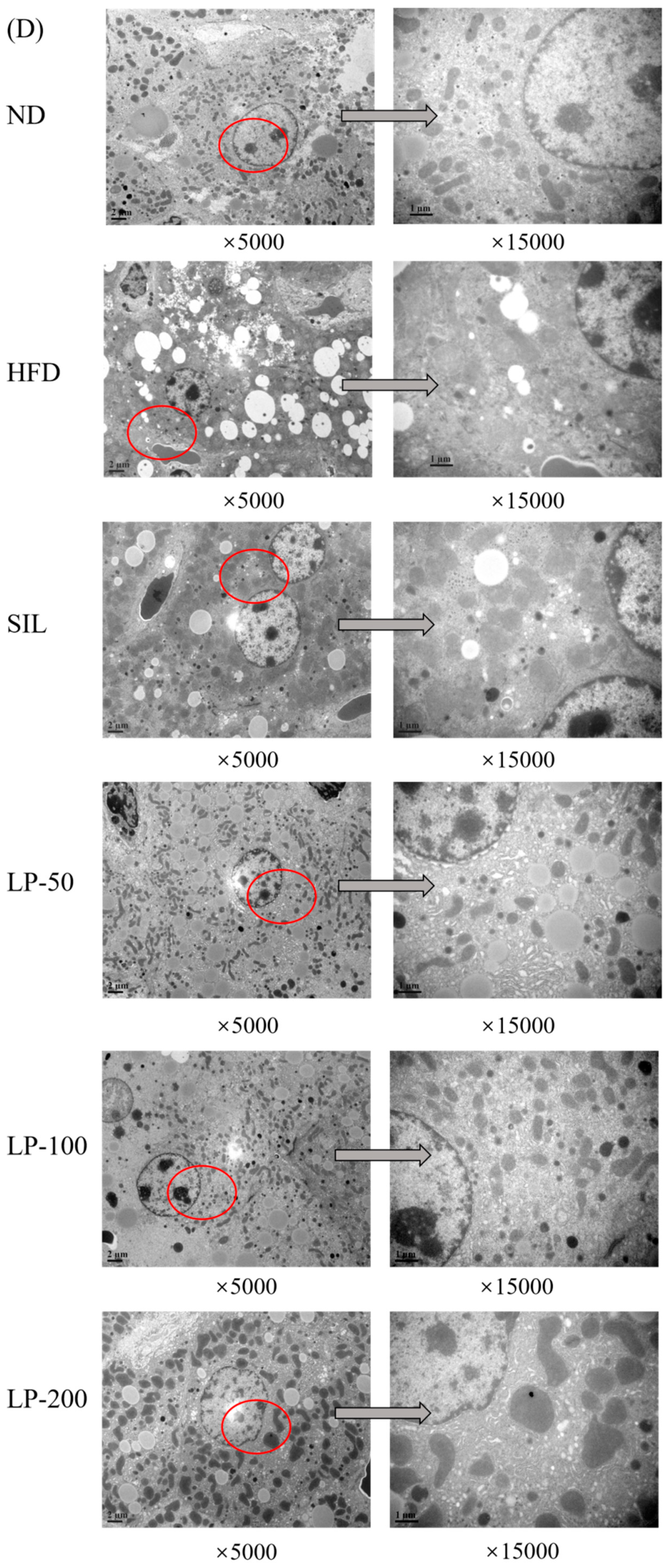
2.7. Effects of LPs on Hepatic Histopathology
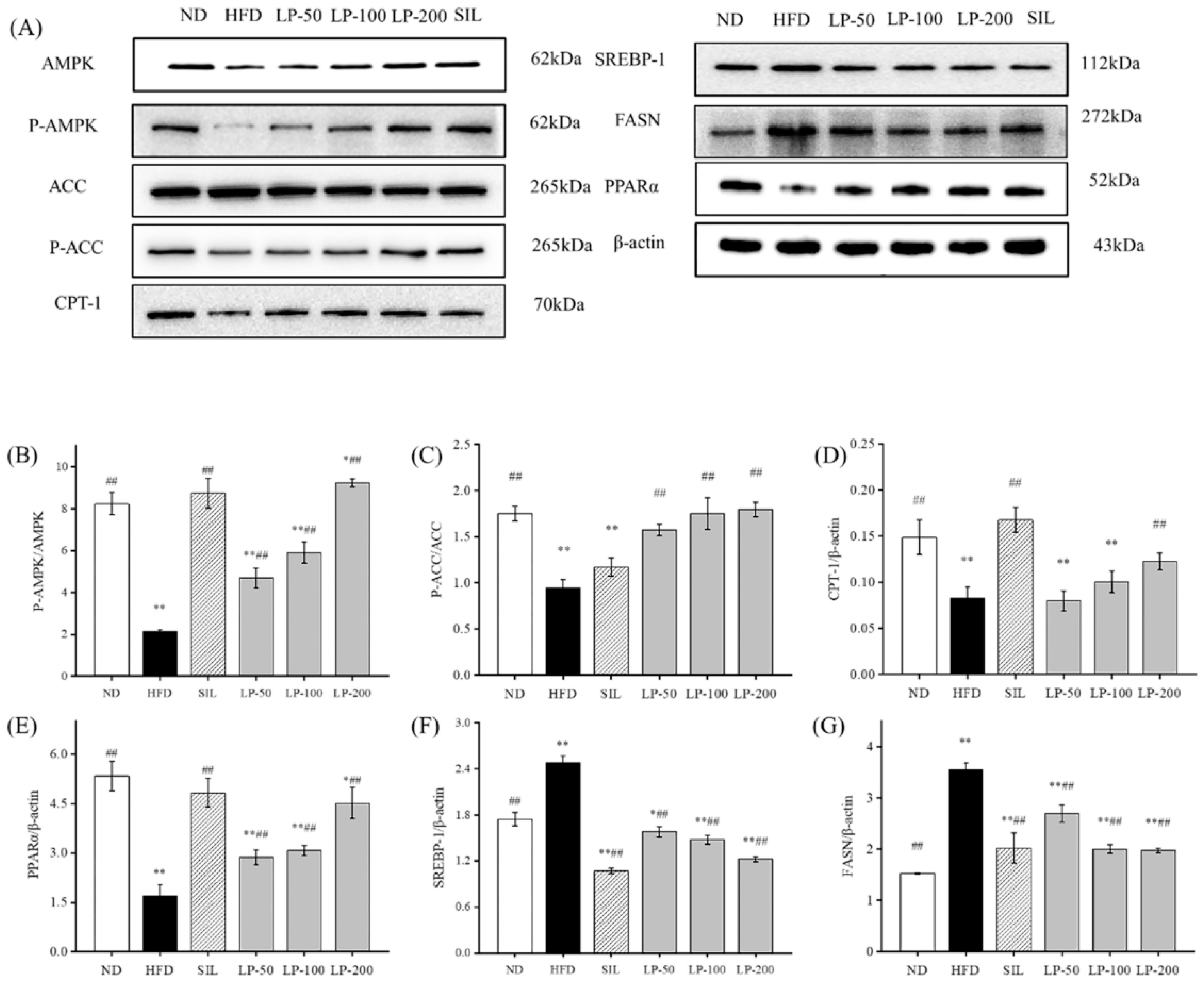
2.8. Effects of LPs on the Protein Expressions of AMPK Pathways
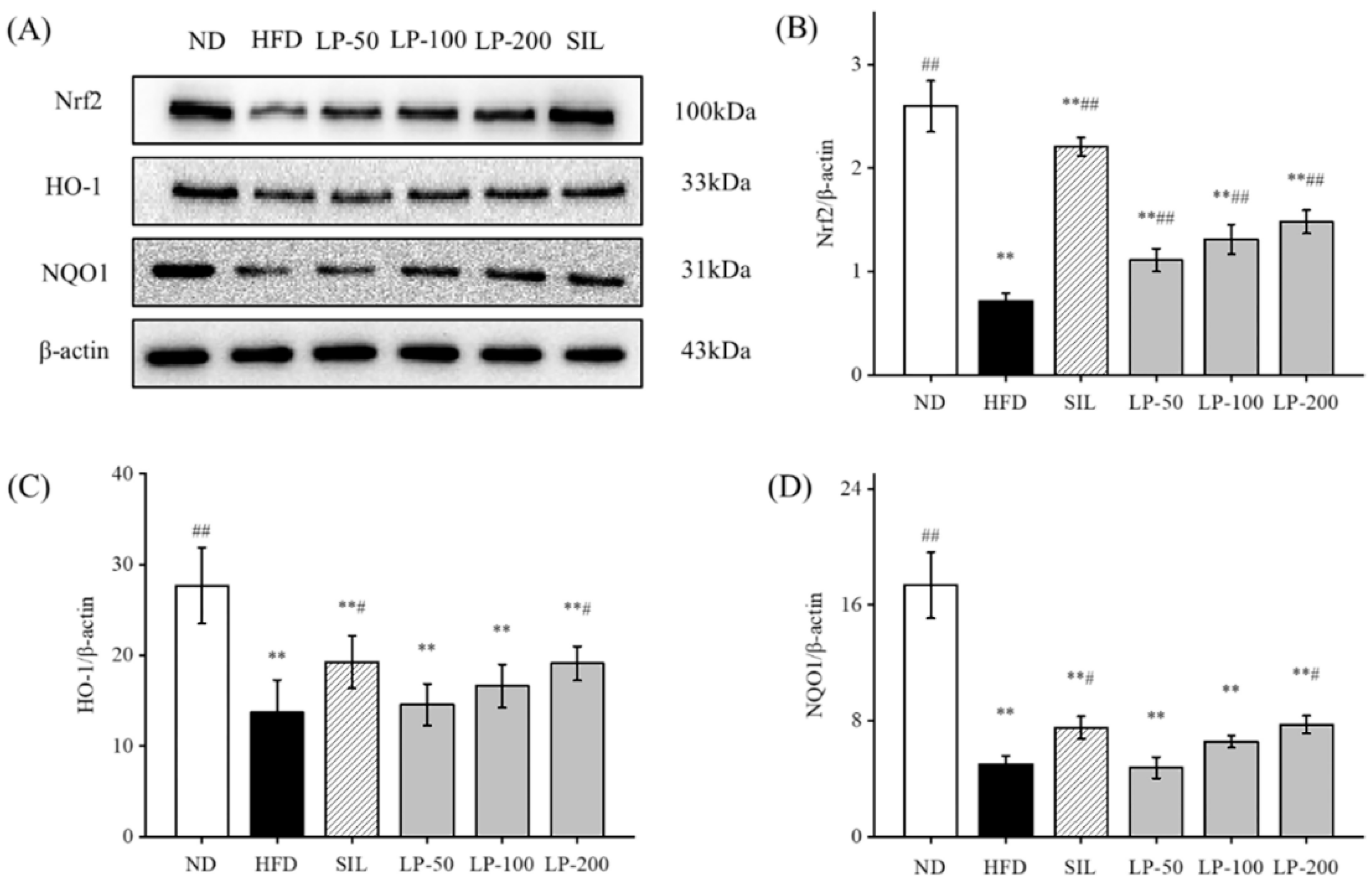
2.9. Effects of LPs on the Expressions of Hepatic Nrf2 Pathway Proteins
3. Discussion
4. Materials and Methods
4.1. Preparation of Monkfish Muscle Peptides
4.2. Animals and Treatments
4.3. Determination of the Serum Lipid Index, AST, ALT, and Liver Oxidative Stress Index
4.4. Pro-Inflammatory Factors Analysis
4.5. Histopathological Examination of the Liver
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, M.; Lim, S.-J.; Joung, E.-J.; Lee, B.; Oh, C.-W.; Kim, H.-R. Meroterpenoid-rich fraction of an ethanolic extract from Sargassum serratifolium alleviates obesity and non-alcoholic fatty liver disease in high fat-fed C57BL/6J mice. J. Funct. Foods 2018, 47, 288–298. [Google Scholar] [CrossRef]
- Xu, L.; Yan, L.; Tao, W. Pilose antler peptide attenuates high-fat-diet-induced liver injury. Toxicol. Mech. Methods 2018, 28, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Xu, M.; Ge, C.; Qin, Y.; Gu, T.; Lv, J.; Wang, S.; Ma, Y.; Lou, D.; Li, Q.; Hu, L.; et al. Activated TNF-α/RIPK3 signaling is involved in prolonged high fat diet-stimulated hepatic inflammation and lipid accumulation: Inhibition by dietary fisetin intervention. Food Funct. 2019, 10, 1302–1316. [Google Scholar] [CrossRef]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Ding, C.; Zhang, G.; Pan, R.; Liu, Y.; Huang, N.; Hou, N.; Han, F.; Xu, W.; Sun, X. Liraglutide ameliorates obesity-related nonalcoholic fatty liver disease by regulating Sestrin2-mediated Nrf2/HO-1 pathway. Biochem. Biophys. Res. Commun. 2020, 525, 895–901. [Google Scholar] [CrossRef]
- Gao, F.; Chen, M.; Yu, J.; Xu, L.; Yu, L.; Jiang, H.; Gu, Z. Tetrahydrocurcumin protects against nonalcoholic fatty liver disease by improving lipid metabolism and redox homeostasis. J. Funct. Foods 2022, 89, 104957. [Google Scholar] [CrossRef]
- Kim, S.; Hong, J.; Jeon, R.; Kim, H.S. Adzuki bean ameliorates hepatic lipogenesis and proinflammatory mediator expression in mice fed a high-cholesterol and high-fat diet to induce nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, F.; Jiang, H.; Wang, Z.; Hua, C.; Zhang, Y. Chicory (Cichorium intybus L.) polysaccharides attenuate high-fat diet induced non-alcoholic fatty liver disease via AMPK activation. Int. J. Biol. Macromol. 2018, 118, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ye, S.; Jiang, R.; Zhou, X.; Zhou, J.; Meng, S. Liensinine alleviates high fat diet (HFD)-induced non-alcoholic fatty liver disease (NAFLD) through suppressing oxidative stress and inflammation via regulating TAK1/AMPK signaling. Int. Immunopharmacol. 2022, 104, 108306. [Google Scholar] [CrossRef]
- Vanani, A.R.; Kalantari, H.; Mahdavinia, M.; Rashno, M.; Khorsandi, L.; Khodayar, M.J. Dimethyl fumarate reduces oxidative stress, inflammation and fat deposition by modulation of Nrf2, SREBP-1c and NF-kappaB signaling in HFD fed mice. Life Sci. 2021, 283, 119852. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bindels, L.B.; Segura Munoz, R.R.; Martínez, I.; Walter, J.; Ramer-Tait, A.E.; Rose, D.J. Disparate Metabolic Responses in Mice Fed a High-Fat Diet Supplemented with Maize-Derived Non-Digestible Feruloylated Oligo- and Polysaccharides Are Linked to Changes in the Gut Microbiota. PLoS ONE 2016, 11, e0146144. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kitts, D.D.; Weiler, K. Bioactive Proteins and Peptides from Food Sources. Applications of Bioprocesses used in Isolation and Recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Kim, J.W.; Park, J.B.; Hong, Y.K.; Ku, S.K.; Choi, J.S. Anti-obesity effects of yellow catfish protein hydrolysate on mice fed a 45% kcal high-fat diet. Int. J. Mol. Med. 2017, 40, 784–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gagnon, J.; Nair, S.; Sha, S. Herring Milt Protein Hydrolysate Improves Insulin Resistance in High-Fat-Diet-Induced Obese Male C57BL/6J Mice. Mar. Drugs 2019, 17, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drotningsvik, A.; Mjøs, S.A.; Pampanin, D.M.; Slizyte, R.; Carvajal, A.; Remman, T.; Høgøy, I.; Gudbrandsen, O.A. Dietary fish protein hydrolysates containing bioactive motifs affect serum and adipose tissue fatty acid compositions, serum lipids, postprandial glucose regulation and growth in obese Zucker fa/fa rats. Br. J. Nutr. 2016, 116, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.A.; Lied, E.; Espe, M.; Muna, Z.; Mørk, S.; Berge, R.K. Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol, and lowers acyl-CoA:cholesterol acyltransferase activity in liver of Zucker rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Zheng, J.; Xu, B.; Ye, J.; Yang, Z.; Yuan, F. Optimization of Extraction of Bioactive Peptides from Monkfish (Lophius litulon) and Characterization of Their Role in H(2)O(2)-Induced Lesion. Mar. Drugs 2020, 18, 468. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Deng, Y.-Y.; Wang, Y.-M.; Deng, S.-G.; Ma, J.-Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Greyling, N.; Bordoloi, A.; Goosen, N.J. Optimising enzymatic conditions of monkfish (Lophius vomerinus) heads hydrolysis towards potential waste biomass valorisation. Biomass Convers. Biorefinery 2020, 11, 2711–2722. [Google Scholar] [CrossRef]
- Nakamaru-Ogiso, E.; Han, H.; Matsuno-Yagi, A.; Keinan, E.; Sinha, S.C.; Yagi, T.; Ohnishi, T. The ND2 subunit is labeled by a photoaffinity analogue of asimicin, a potent complex I inhibitor. FEBS Lett 2010, 584, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Sadia, H.; Akter, Q.S.; Afroz, R.; Siddika, T. Effect of Punica Granatum (Pomegranate) on serum ALT and AST in Carbon tetrachloride induced liver damage in Wistar Albino Rats. J. Bangladesh. Soc. Physiol. 2016, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Braud, L.; Battault, S.; Meyer, G.; Nascimento, A.; Gaillard, S.; de Sousa, G.; Rahmani, R.; Riva, C.; Armand, M.; Maixent, J.M.; et al. Antioxidant properties of tea blunt ROS-dependent lipogenesis: Beneficial effect on hepatic steatosis in a high fat-high sucrose diet NAFLD obese rat model. J. Nutr. Biochem. 2017, 40, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharm. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.C.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Liu, K.L.; Chen, H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 2011, 51, 2073–2081. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive Peptides from Marine Ascidians and Future Drug Development—A Review. Int. J. Pept. Res. Ther. 2017, 24, 13–18. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Malhi, H.; Gores, G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008, 28, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Song, J.; Du, M.; Mao, X. Bovine α-lactalbumin hydrolysates (α-LAH) attenuate high-fat diet induced nonalcoholic fatty liver disease by modulating hepatic lipid metabolism in C57BL/6J mice. J. Funct. Foods 2019, 54, 254–262. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Zhang, Y.; Gong, X.; Cheng, G.; Pu, S.; Cai, S. The preventive effect of phenolic-rich extracts from Chinese sumac fruits against nonalcoholic fatty liver disease in rats induced by a high-fat diet. Food Funct. 2020, 11, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Hendy, O.M.; Elsabaawy, M.M.; Aref, M.M.; Khalaf, F.M.; Oda, A.M.A.; El Shazly, H.M. Evaluation of circulating zonulin as a potential marker in the pathogenesis of nonalcoholic fatty liver disease. Apmis 2017, 125, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, Z.; Chen, J.M.; Xu, Y.Y.; Ye, H.R.; Cui, J.; Fang, Y.; Jin, Y.; Zhu, D.R.; Yuan, L. Role of APN and TNF-α in type 2 diabetes mellitus complicated by nonalcoholic fatty liver disease. Genet. Mol. Res. 2015, 14, 2940–2946. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Liu, L.; Song, Z.; Sheikhahmadi, A.; Wang, Y.; Buyse, J. Effects of feed deprivation on the AMPK signaling pathway in skeletal muscle of broiler chickens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 191, 146–154. [Google Scholar] [CrossRef]
- Brown, Z.J.; Fu, Q.; Ma, C.; Kruhlak, M.; Zhang, H.; Luo, J.; Heinrich, B.; Yu, S.J.; Zhang, Q.; Wilson, A.; et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis. 2018, 9, 620. [Google Scholar] [CrossRef]
- Ortiz, M.; Soto-Alarcón, S.A.; Orellana, P.; Espinosa, A.; Campos, C.; López-Arana, S.; Rincón, M.A.; Illesca, P.; Valenzuela, R.; Videla, L.A. Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydroxytyrosol co-administration. Dig. Liver Dis. 2020, 52, 895–904. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, T.W.; Lee, D.R.; Jung, W.H.; Lim, J.H.; Jung, J.Y.; Yang, S.H.; Suh, J.W. A polymethoxy flavonoids-rich Citrus aurantium extract ameliorates ethanol-induced liver injury through modulation of AMPK and Nrf2-related signals in a binge drinking mouse model. Phytother. Res. 2015, 29, 1577–1584. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of Natural Products on Fructose-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell Mol. Med. 2019, 23, 4063–4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, J.; Yu, F.; Tang, Y.; Ding, G.; Yang, Z.; Sun, Y. Protective Effect of Meretrix meretrix Oligopeptides on High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. Mar. Drugs 2018, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Yu, F.; Zhang, G.; Yang, Z.; Huang, F.; Ding, G. A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules 2017, 22, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Groups | T-AOC (U/mg prot) | CAT (U/mg prot) | SOD (U/mg prot) | MDA (nmol/mg prot) | GSH-Px (U/mg prot) |
|---|---|---|---|---|---|
| ND | 5.22 ± 0.07 ## | 126.11 ± 1.37 ## | 121.42 ± 0.97 ## | 5.02 ± 0.28 ## | 140.12 ± 1.63 ## |
| HFD | 2.14 ± 0.47 ** | 50.26 ± 1.33 ** | 58.13 ± 1.01 ** | 9.03 ± 0.99 ** | 67.78 ± 1.67 ** |
| SIL | 5.10 ± 0.13 ## | 123.03 ± 1.04 ## | 111.79 ± 1.05 **,## | 5.44 ± 0.14 # | 163.83 ± 2.39 **,## |
| LP-50 | 3.75 ± 0.13 **,# | 73.22 ± 1.13 **,## | 76.27 ± 0.88 **,## | 7.66 ± 0.68 * | 88.64 ± 2.27 **,## |
| LP-100 | 4.38 ± 0.17 **,## | 95.55 ± 1.76 **,## | 97.24 ± 0.84 **,## | 6.16 ± 0.18 *,# | 109.07 ± 1.12 **,## |
| LP-200 | 5.07 ± 0.15 ## | 126.04 ± 1.97 ## | 113.10 ± 0.47 **,## | 4.92 ± 0.69 # | 135.41 ± 2.04 *,## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Tian, X.; Wang, Q.; Zheng, J.; Yang, Y.; Xu, B.; Zhang, S.; Yuan, F.; Yang, Z. Monkfish Peptides Mitigate High Fat Diet-Induced Hepatic Steatosis in Mice. Mar. Drugs 2022, 20, 312. https://doi.org/10.3390/md20050312
Ye J, Tian X, Wang Q, Zheng J, Yang Y, Xu B, Zhang S, Yuan F, Yang Z. Monkfish Peptides Mitigate High Fat Diet-Induced Hepatic Steatosis in Mice. Marine Drugs. 2022; 20(5):312. https://doi.org/10.3390/md20050312
Chicago/Turabian StyleYe, Jiena, Xiaoxiao Tian, Qiongfen Wang, Jiawen Zheng, Yanzhuo Yang, Baogui Xu, Shuai Zhang, Falei Yuan, and Zuisu Yang. 2022. "Monkfish Peptides Mitigate High Fat Diet-Induced Hepatic Steatosis in Mice" Marine Drugs 20, no. 5: 312. https://doi.org/10.3390/md20050312
APA StyleYe, J., Tian, X., Wang, Q., Zheng, J., Yang, Y., Xu, B., Zhang, S., Yuan, F., & Yang, Z. (2022). Monkfish Peptides Mitigate High Fat Diet-Induced Hepatic Steatosis in Mice. Marine Drugs, 20(5), 312. https://doi.org/10.3390/md20050312





